Abstract
Vasoactive intestinal peptide (VIP) is a potent anti-inflammatory neuropeptide that, by inhibiting Th1-driven responses and inducing the emergence of regulatory T cells (Treg), has been proven successful in the induction of tolerance in various experimental models of autoimmune disorders. Here, we investigate the molecular mechanisms involved in VIP-induced tolerance. VIP treatment in the presence of T-cell receptor (TCR) signaling and CD28 costimulation induced cell cycle arrest in human T cells. VIP blocked G1/S transition and inhibited the synthesis of cyclins D3 and E and the activation of the cyclin-dependent kinases (CDKs) cdk2 and cdk4. This effect was accompanied by maintenance of threshold levels of the CDK inhibitor p27kip1 and impairment of phosphatidylinositol 3-kinase (PI3K)-Akt signaling. Inhibition of interleukin 2 (IL-2) transcription and downregulation of signaling through NFAT, AP-1, and Ras-Raf paralleled the VIP-induced cell cycle arrest. Noteworthy from a functional point of view is the fact that VIP-treated T cells show a regulatory phenotype characterized by high expression of CD25, cytotoxic-T-lymphocyte-associated protein 4 (CTLA4), and Forkhead box protein 3 (FoxP3) and potent suppressive activities against effector T cells. CTLA4 appears to be critically involved in the generation and suppressive activities of VIP-induced Treg. Finally, cyclic AMP (cAMP) and protein kinase A (PKA) activation seems to mediate the VIP-induced cell cycle arrest and Treg generation.
Regulatory T cells (Treg) have emerged as a unique population of suppressor T cells orchestrating peripheral immune tolerance (54). Two major populations of Treg, with complementary and overlapping functions in the control of immune response in vivo, have been characterized: naturally occurring, thymus-generated CD4+ CD25+ Forkhead box protein 3 (FoxP3)-expressing Treg and peripherally induced Treg (38, 54). Numerous studies have demonstrated the successful therapeutic use of antigen-specific Treg in various experimental models of autoimmune diseases and allogeneic transplantation, providing long-term tolerance by active and specific regulation of self-antigen- and alloantigen-specific T cells (7, 38). However, the translation of important biological findings about Treg-based immunotherapy to the clinic has been limited mainly by the inability to define their antigenic specificities and by the scarcity of circulating Treg. To solve this problem, two different strategies have been proposed, either facilitating in vivo Treg function or infusing Treg isolated and manipulated/expanded ex vivo. Several approaches have been used to expand naturally occurring human CD4+ CD25+ Treg, mainly by T-cell receptor (TCR)-CD28 stimulation in combination with interleukin 2 (IL-2) and/or IL-15 (36, 47). An alternative approach consists of the conversion of CD4+ CD25+ Treg from conventional CD4 T cells with inducible factors. Whereas a large body of literature has been dedicated to describing how Treg control ongoing immune responses and tolerance, especially regarding their phenotype, ontogeny, and mechanisms of suppression (38, 54), the endogenous molecules controlling the peripheral expansion or de novo generation of Treg remain largely unknown. For example, the suppressive cytokine transforming growth factor β1 (TGF-β1) or immunosuppressive drugs, such as FK778, generate CD4+ CD25+ Treg from the CD4+ CD25− T-cell compartment (14, 19, 35, 53, 66, 67). The identification of additional Treg-inducing factors should extend the applicability of immunotherapy based on Treg in human patients.
Vasoactive intestinal peptide (VIP) is an immunosuppressive neuropeptide with potent anti-inflammatory effects (16). VIP is produced by Th2 cells upon antigenic stimulation and mediates regulatory actions on both innate and adaptive immunity (16). Indeed, VIP-based therapy has been proven successful in the treatment of various experimental models of inflammatory and autoimmune disorders (25). Beside its inhibitory effect on inflammatory and Th1-driven responses, VIP induces the emergence of Treg in animals with experimental autoimmune encephalomyelitis and arthritis (20, 26). The in vivo VIP-induced Treg seem to consist of two populations: a major population of FoxP3+ CD4+ CD25+ Treg, the suppressive mechanism for which is mediated through direct cellular contact that is mainly dependent on cytotoxic-T-lymphocyte-associated protein 4 (CTLA4), and a minor CD4+ T-cell population, which uses IL-10 and/or TGF-β1 as a suppressive molecule (29). We have recently reported the potential of VIP to promote immune tolerance for alloantigens by generating Treg displaying suppressive functions against allospecific effector T cells and protecting against acute graft-versus-host disease in a mouse model of allogeneic bone marrow transplantation (48). The aim of this study was to investigate the molecular mechanisms involved in the immunosuppressive activity of VIP on human T cells and on the generation of human Treg.
MATERIALS AND METHODS
Antibodies and reagents.
VIP, secretin, pituitary adenylate cyclase (AC)-activating polypeptide (PACAP), VIP10-28, VIP1-12, okadaic acid, forskolin, 8-Br-cyclic AMP (cAMP), H-89 {N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide}, histone H1, and anti-laminin B monoclonal antibody (MAb) were from Calbiochem. Rp-cAMP was from Boehringer Mannheim GmbH. Antibodies (Abs) against phospho-AktSer473, phosphoinositide-dependent protein kinase 1 (PDK-1), Akt, ERK1/2, MEK1, phospho-MEK1, phospho-lckTyr394, FoxO1, and phospho-FoxO1Ser256, as well as glutathione-S-transferase (GST)-retinoblastoma protein (Rb)-(769-921) fusion protein, were from Cell Signaling Technology. Fluorescein isothiocyanate (FITC)-conjugated anti-CTLA4, phycoerythrin (PE)-conjugated anti-CD25, Alexa-conjugated anti-phospho-STAT5, peridinin chlorophyll protein (PerCP)-conjugated anti-CD4, anti-pRb, anti-CD3, and anti-IL-10 were from BD PharMingen. Anti-phospho-Raf1Ser259 and anti-phospho-Raf1Ser43 Abs were from New England Biolabs and Millipore, respectively. Protein A/G agarose and antibodies against CD3ζ chain, cyclin E, cyclin A, cdk2, cyclin D2, cyclin D3, cdk4, cdk6, Rap1, p27kip1, phospho-ERK1/2, p38, phospho-p38, c-Jun, and phospho-c-Jun were from Santa Cruz Biotechnology. Phytohemagglutinin (PHA), enolase, and anti-β-tubulin Ab were from Sigma. Staphylococcal enterotoxin B (SEB) was from Toxin Technology. Anti-p27kip1 and anti-NFATp MAbs were from BD Transduction Laboratories. Anti-Raf1 Ab was from Transduction Laboratories, and anti-Ras Ab was from Oncogene. Human granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 were from R&D Systems and human IL-2 from Roche Biomedical. FITC- and Alexa-conjugated anti-human FoxP3 (clones PCH101 and 236A/E/) and anti-human programmed-death 1 (anti-PD-1) Abs were from eBioscience. The phosphatidylinositol 3-kinase (PI3K) activator 740 Y-P was obtained from Tocris Bioscience. Peroxovanadate was prepared fresh by incubating sodium vanadate and H2O2 (12 mM each) at 20°C in 40 mM HEPES (pH 7.4) for 15 min.
Cell isolation.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat preparations from healthy volunteers by density sedimentation on Ficoll-Hypaque gradients (20 min; 700 × g; 20°C). Cells recovered from the gradient interface were washed twice in RPMI 1640 medium and immediately used for culture or further purification. To isolate T cells, PBMCs were depleted of adherent cells by incubation in plastic dishes and then with anti-CD8, -CD14, -CD19, -CD20, and -CD56 MAbs (Coulter Immunotech) for 1 h at 4°C, followed by incubation with anti-mouse IgG-coated magnetic beads (45 min; 4°C). Bead-bound cells were removed from the PBMCs with a magnetic device. To minimize stimulation of the cells, all the purification steps were carried out in the absence of serum. The purity of the T cells was >96% as assessed by flow cytometry. CD4+ T cells were isolated by negative selection from the total PBMCs using a CD4 isolation kit (Miltenyi Biotec), yielding a population of CD4+ cells with purities of 94 to 98%. Positive selection on anti-CD25 magnetic microbeads was then used to separate the negative fraction containing CD4+ CD25− T cells from the CD4+ CD25+ T-cell fraction, using the CD4+ CD25+ T Regulatory Cell Isolation kit (Miltenyi Biotec). This procedure led to the complete positive selection of CD4+ CD25+ T cells (purity, >96%) and negative depletion of CD4+ CD25− T cells, as measured by flow cytometry. In some experiments, the different T-cell populations (CD4+, CD4+ CD25+, and CD4+ CD25−) were isolated by sorting using a FACS-Calibur flow cytometer (Becton Dickinson) after the cells were labeled with PE-anti-CD25 and PerCP-anti-CD4 Abs as described below.
To generate human dendritic cells (DCs), monocytes (2 × 106) were isolated from the PBMC fraction by adherence to plastic and cultured in RPMI 1640 medium containing GM-CSF (800 U/ml) and IL-4 (500 U/ml). After 6 days, nonadherent cells were collected and negatively selected with anti-CD2 and anti-CD19 MAb-coated beads. The resultant cells were cultured for 48 h with lipopolysaccharide (LPS) (1 μg/ml) to induce activation/maturation.
Cell cultures and stimulation.
Purified human T cells or CD4+ cells (106/ml) were cultured in complete medium consisting of RPMI 1640 medium supplemented with human AB serum (8%), l-glutamine (200 mM), sodium pyruvate (1%), nonessential amino acids (1%), penicillin-streptomycin (1%), and 2-mercaptoethanol (1%) at 37°C and 5% CO2. The cells were stimulated with anti-CD3/anti-CD28 MAb-coated magnetic beads (Invitrogen; one bead per cell), PHA (1 μg/ml), or the superantigen SEB (1 ng/ml) for different times. VIP was added at different concentrations with the different stimuli. In some experiments, VIP was added at different times after cell stimulation or at the same time as CD3/CD28 stimulation and removed by extensive cell washing at various times after initiation of culture. The proliferative response was determined by incubating human T cells (105/well) or CD4+ CD25− T cells (2.5 × 105/well) in complete medium in 96-well flat-bottom plates (Costar) with the indicated factors, pulsing them with 0.5 μCi (0.0185 MBq)/well [3H]thymidine for the last 8 h of the culture, and harvesting them onto membranes; the incorporated [3H]thymidine was measured in a liquid scintillation counter.
In some experiments, cells were harvested after 4 days of primary stimulation, washed, and rested for 2 days. The viable cells (5 × 104) were recovered by centrifugation in density gradient (Lymphoprep; Nycomed Pharma AS) and restimulated in a secondary culture with HLA-mismatched DCs (104), IL-2 (20 to 50 U/ml), anti-CD3 (2 μg/ml), and/or anti-CTLA4 antibody (10 μg/ml). When indicated, PBMCs (2 × 106) were activated with tetanus toxoid (TT) (1 μg/ml) in the absence or presence of VIP. After 4 to 6 days, T cells (2 × 104) were isolated from the primary culture as described above, rested for 2 days, and restimulated on a secondary culture with mature DCs (104) from the same donor in the absence or presence of TT (1 μg/ml).
T-cell suppression assays.
The suppressive capacity of VIP-treated cells was analyzed in a coculture assay. Human T cells or CD4+ CD25− T cells isolated from donor A were activated with anti-CD3/CD28 MAb-coated beads with or without 10−7 M VIP. After 4 days, cells were harvested, rested for 2 days, and added at different ratios to cocultures of responder T cells (5 × 104, from donor A) and allogeneic mDCs (104, from donor B). Some cultures were performed in the presence of blocking anti-IL-10 or anti-CTLA4 (10 μg/ml) Abs or of IL-2 (100 U/ml). In similar experiments, responder CD4+ T cells (from donor A) were labeled with 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) prior to setting up cocultures, and proliferating cells were determined by CFSE dilution by fluorescence-activated cell sorting (FACS). To determine the cell contact dependence of the suppressive response, we placed responder T cells (5 × 104) with allogeneic mDCs (104) in the bottom well of a Transwell system (Millipore; 0.4-μm pore) and the recovered T cells (2 × 104) with allogeneic mDCs (104) in the upper Transwell insert. At day 4, the proliferation of the responder cells in the lower compartment was determined.
Cell cycle analysis.
Cells (106) were fixed in ice-cold 70% ethanol for 1 h, washed, incubated with 0.1% RNase-phosphate-buffered saline (PBS) (37°C; 45 min), and stained with 50 μg/ml propidium iodide (37°C; 30 min) prior to FACS analysis for DNA content using CellQuest software. Viability was determined by flow cytometry using an annexin V-based apoptosis detection kit (R&D Systems). Moreover, cell numbers were determined by counting cells excluding trypan blue after 72 h of culture.
Western blot analysis and in vitro kinase assays.
Proteins from whole-cell, nuclear, and cytoplasmic extracts were prepared as described previously (15), separated (70 μg/lane) by SDS-polyacrylamide gel electrophoresis (PAGE) (12%; 8% for phosphorylated Rb [pRb]), and blotted onto polyvinylidene difluoride membranes (Millipore) using a semidry system. The membranes were blocked (Tris-buffered saline-Tween 20-3% nonfat dry milk; 1 h; 22°C), probed with the indicated primary antibody overnight at 4°C, immunodetected with horseradish peroxidase-conjugated secondary antibodies, and visualized by enhanced chemiluminescence (ECL) (Amersham Pharmacia). Equal protein loading was controlled by reprobing with anti-actin, anti-Zap70 (zeta chain-associated protein kinase), anti-β-tubulin, or anti-laminin B antibody. The band intensity was quantitatively determined with ImageJ software (National Institutes of Health) and expressed as densitometric units normalized for the expression of actin, Zap70, β-tubulin, or laminin B in each sample. The levels of phosphorylated proteins were normalized to the total expression of the corresponding protein.
To study the association between cyclin/cyclin-dependent kinase (CDK) complexes and p27kip1, endogenous cdk4, cdk2, cyclin D2, cyclin E, and cyclin D3 were immunoprecipitated from whole-cell lysates (250 μg/sample) by incubation with the corresponding antibody (5 μg; 2 h; 4°C), followed by incubation with protein A/G-Sepharose beads (10 μg; 45 min; 4°C) under constant agitation. The beads were extensively washed with lysis buffer and subjected to immunoblot analysis of cyclins, CDKs, and p27kip1 as described above.
To assay cyclin- and CDK-associated kinase activities, whole-protein extracts (500 μg) were immunoprecipitated with anti-cdk2, anti-cyclin E, anti-cdk4, anti-cyclin D3, and anti-cyclin D2 antibodies as described above. After extensive washing, the kinase activities in the immunoprecipitates were assayed (30 min; 30°C) in 50 mM HEPES (pH 7.5), 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 0.1 mM Na3VO4, 1 mM NaF, 50 μM cold ATP, 10 μCi of [γ-32P]ATP (6,000 Ci/mmol), and 5 μg of histone H1 for cdk2 and cyclin E or 2 μg of Rb-GST for cdk4 and cyclin D2/D3. The phosphorylated proteins were separated by SDS-PAGE, transferred to Immobilon P membranes (Millipore), and analyzed by Western blotting. The optical densities of the bands were determined as described above and expressed as densitometric units relative to values obtained on unstimulated controls.
Phosphorylated p27kip1 was determined by immunoprecipitation of cell lysates with anti-p27kip1, followed by immunoblotting with anti-phospho-Thr antibody. Akt/protein kinase B (PKB) activity in whole lysates was measured using an Akt Kinase Assay kit (Cell Signaling) by immunoprecipitation of cell lysates with an anti-Akt MAb, followed by immunocomplex kinase assay in the presence of GSK-3 fusion protein as a substrate. The Raf1 kinase assay was performed by measuring the phosphotransferase activity toward GST-MEK1 (50). Determination of the active form of CD3ζ was conducted, as previously described (58), by determining the levels of phospho-Tyr by immunoblotting of immunoprecipitates for the ζ chain. Fyn activity was determined by immunoprecipitation with anti-Fyn Abs and measuring the phosphotransferase activity toward enolase using a previously described in vitro kinase assay (56). PKA activity was determined by using the PKA assay kit from Upstate Biotechnology, which measures the phosphotransferase activity toward kemptide (used as a specific substrate). cAMP levels in the lysates were determined by the nonacetylation method using an enzyme immunoassay system (Amersham Pharmacia Biotech).
The activation of Ras and Rap1 was studied by detecting the GTP-bound forms of Ras and Rap1, respectively. Ras-GTP and Rap1-GTP were affinity purified from cell lysates by the use of GST-Raf1-RBD and GST-RalGDS-RBD, respectively, coupled with glutathione-Sepharose beads (30). Complexes were analyzed by 15% SDS-PAGE and immunoblotting with anti-Ras and anti-Rap1 antibodies.
EMSA.
The electromobility shift assay (EMSA) was performed with nuclear extracts using STAT5, AP-1, NF-κB, or NFAT consensus oligonucleotides as described previously (34, 52).
Flow cytometry.
Cells were incubated with various PerCP/Alexa/FITC/PE-labeled antibodies diluted at the optimal concentration for immunostaining, fixed in 1% paraformaldehyde, and analyzed on a FACScalibur cytometer. We used isotype-matched antibodies as controls. For analysis of intracellular CTLA4, FoxP3, and phosphorylated STAT5, cells were first stained for surface CD4 and CD25; fixed with Cytofix/Cytoperm solution (BD PharMingen); incubated with FITC-anti-CTLA4, FITC-Alexa-anti-FoxP3, or Alexa-anti-phospho-STAT5 antibody diluted in 0.5% saponin; and analyzed by FACS.
Intracellular calcium fluxes were determined by flow cytometry using Indo-1 (2 μM; Molecular Probes) as previously described (58).
Cytokine determination.
The cytokine contents of the culture supernatants were determined by specific sandwich enzyme-linked immunosorbent assays (ELISAs) using capture/biotinylated detection Abs from BD Pharmingen. For intracellular-cytokine analysis, viable T cells (106/ml) recovered by gradient centrifugation after stimulation were cultured with phorbol myristate acetate (PMA) (10 ng/ml) plus ionomycin (50 ng/ml) for 6 h. Monensin (1.33 μM) was added for the last 4 h of culture. Cells were stained with PerCP-anti-CD4 MAbs for 30 min at 4°C, fixed/saponin permeabilized with Cytofix/Cytoperm, and stained with FITC-conjugated anti-IL-2, anti-gamma interferon (anti-IFN-γ), anti-IL-4, anti-tumor necrosis factor alpha (anti-TNF-α), or anti-IL-10 MAb (0.5 μg/sample; 45 min;4°C; BD Pharmingen), and analyzed by flow cytometry.
Cell transfection and luciferase assay.
Jurkat T cells were transiently transfected with 20-μg reporter constructs of luciferase driven by the 2-kb IL-2 promoter/enhancer, AP-1, NFAT, or NF-κB (Clontech and Stratagene) as described previously (30). After 48 h of transfection, 5 × 105 cells were cultured for 6 h as indicated in each experiment, and luciferase activity was measured. The transfection efficiency was normalized by cotransfection with pEF-lacZ and assay for β-galactosidase.
mRNA expression.
CTLA4 gene expression was determined by reverse transcription (RT)-PCR as previously described (60). mRNA expression of p27kip1 was determined by Northern blot analysis according to standard methods (15), using a probe for human p27kip1 synthesized by RT-PCR with the primers 5′-GCAACCGACGATTCTTCTAC-3′ and 5′-GTCCATTCCATGAAGTCAGC-3′ under the following PCR conditions: denaturation at 94°C for 5 min, followed by 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. FoxP3 gene expression was quantitated by real-time PCR as previously described (57). mRNA expression of B- and T-lymphocyte attenuator (BTLA) was determined by Northern blot analysis using a probe for human BTLA cDNA synthesized as described previously (62).
Data analysis.
All values are expressed as mean ± standard deviation (SD). Statistical comparisons between experimental groups were done with Duncan's multiple-range test after two-way analysis of variance (ANOVA). P values of <0.05 were considered significant.
RESULTS
VIP induces cell cycle arrest in human activated T cells.
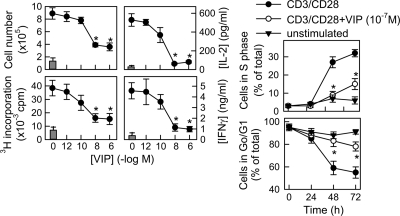
We first established the immunosuppressive effects of VIP on human T cells in vitro by assessing the inhibitory potential of VIP for the proliferation of T cells stimulated by different stimuli, including mitogen PHA, superantigen SEB, or anti-CD3/CD28-coated beads (see Fig. S1A in the supplemental material). Because signaling through TCR/CD3 and CD28 costimulation represents the most physiological condition of antigenic activation of T-cell effector functions, we selected this stimulation (referred to here as CD3/CD28) for further experiments. VIP decreased, in a dose-dependent manner, the clonal expansion and cytokine production of CD3/CD28-activated primary human T cells (Fig. 1). Cell cycle analysis showed that VIP strongly reduced the number of T cells in S phase and prevented the decrease of cells in G0/G1 observed after CD3/CD28 stimulation (Fig. 1). This suggests that VIP prevented exit from G0/G1 and entry into S phase of the cell cycle of activated T cells. The suppressive action of VIP was not due to an effect on survival or apoptosis (Table 1). An initial delay of 2 to 6 h in the addition of VIP after CD3/CD28 stimulation did not alter its effect on cell cycle progression; however, a delay of 12 h reduced its inhibitory effect (Table 2). The converse experiments indicated that a brief treatment (<2 h) with VIP after CD3/CD28 stimulation was sufficient to inhibit exit from G0/G1 (Table 2). These findings indicate that the antiproliferative signal of VIP is fully delivered very early and within a narrow window of time after stimulation, although it is maintained long term.
FIG. 1.
VIP induces cell cycle arrest in human T cells. Primary human T cells were cultured with medium alone (unstimulated; gray bars) or activated with anti-CD3/CD28 MAb-coated beads (CD3/CD28) in the absence or presence of different concentrations of VIP. Cytokine levels in culture supernatants were determined after 48 h by ELISA. Cell numbers were determined by counting cells excluding trypan blue at 72 h. Proliferation was determined by [3H]thymidine incorporation at 96 h. The percentages of cells in G0/G1 and S phases were determined by flow cytometry at different time points. *, P < 0.01 versus CD3/CD28 (n = 4, in duplicate). The error bars indicate SD.
TABLE 1.
Effects of agonists, antagonists and fragments of VIP on activated T cells
| Stimulationa | Proliferationb | IFN-γ (ng/ml)c | % G0/G1d | % S phased | % Apoptosise |
|---|---|---|---|---|---|
| Unstimulated | 7.6 ± 1.1 | 0.4 ± 0.1 | 90 ± 3 | 4 ± 2 | 5 ± 0.8 |
| CD3/CD28 | 42.3 ± 5.2 | 4.7 ± 0.4 | 54 ± 4 | 34 ± 4 | 11 ± 0.8 |
| + VIP | 18.3 ± 2.1f | 1.1 ± 0.3f | 79 ± 3f | 14 ± 3f | 10 ± 1.7 |
| + PACAP | 19.5 ± 1.9f | 1.2 ± 0.4f | 77 ± 4f | 16 ± 3f | 11 ± 1.5 |
| + Gastrin | 39.7 ± 5.2 | 4.6 ± 0.9 | 58 ± 3 | 32 ± 3 | 12 ± 2.3 |
| + Glucagon | 43.5 ± 3.6 | 4.8 ± 0.4 | 53 ± 4 | 35 ± 2 | 10 ± 0.9 |
| + VIP1-12 | 41.9 ± 3.2 | 4.5 ± 0.8 | 55 ± 5 | 35 ± 4 | 12 ± 2.2 |
| + VIP10-28 | 43.5 ± 3.1 | 4.9 ± 0.5 | 53 ± 3 | 36 ± 3 | 12 ± 1.7 |
| + VIP + VPAC1 antagonist | 38.4 ± 4.2g | 4.1 ± 0.6g | 60 ± 5g | 30 ± 4g | 11 ± 2.1 |
Primary human T cells were incubated with medium alone (unstimulated) or activated with anti-CD3/CD28 beads in the presence of VIP (10−7 M), PACAP (10−7 M), glucagon (10−7 M), gastrin (10−7 M), VIP1-12 (10−7 M), or VIP10-28 (10−7 M) or of VIP (10−7 M) plus VPAC1 antagonist (10−6 M).
Proliferation was determined by [3H]thymidine incorporation at 96 h and expressed as 103 cpm.
IFN-γ levels were determined by ELISA in supernatants collected at 48 h.
Percentages of cells in G0/G1 and S phases were determined by FACS at 72 h.
Apoptotic cells were determined at 48 h by FACS after propidium iodide and annexin V staining.
P < 0.01 versus CD3/CD28 (n = 4, in duplicate).
P < 0.05 versus VIP-treated samples (n = 4, in duplicate).
TABLE 2.
Window for VIP effects on cell cycle arrest of human T cells
| Treatmenta | Cells in G0/G1 (% of total)b | Cells in S phase (% of total)b |
|---|---|---|
| CD3/CD28 only | 55 ± 3 | 32 ± 3 |
| CD3/CD28 + VIP added at t = 0 | 78 ± 2c | 14 ± 2c |
| VIP added 2 h after CD3/CD28 | 76 ± 2c | 15 ± 3c |
| VIP added 6 h after CD3/CD28 | 72 ± 3c | 17 ± 2c |
| VIP added 12 h after CD3/CD28 | 67 ± 4d | 24 ± 4d |
| VIP removed 2 h after CD3/CD28 | 77 ± 3c | 18 ± 3c |
| VIP removed 6 h after CD3/CD28 | 76 ± 4c | 16 ± 2c |
| VIP removed 12 h after CD3/CD28 | 77 ± 2c | 17 ± 3c |
Primary human T cells were activated with anti-CD3/CD28 beads (CD3/CD28 only). VIP (10−7 M) was added to cultures at different times after activation. Alternatively, VIP was added with CD3/CD28 stimulation and removed at different times after the initiation of culture by extensive washing.
Percentages of cells in G0/G1 and S phases were determined by FACS at 72 h.
P < 0.01 versus CD3/CD28 only (n = 4 or 5, in duplicate).
P < 0.05 versus CD3/CD28 only (n = 4 or 5, in duplicate).
We next investigated the specificity of the effects of VIP. The immunological actions of VIP are exerted through a family of receptors consisting of VPAC1 and VPAC2 (29). VIP actions on the T-cell cycle and proliferation were reversed by a selective VPAC1 antagonist (Table 1). Moreover, PACAP, a VIP-related neuropeptide that also binds to VPAC1, mimicked the effects of VIP; however, the VIP fragments VIP1-12 and VIP10-28, as well as secretin and glucagon (two peptides of the VIP family), failed to efficiently induce T-cell cycle arrest (Table 1).
VIP generates T cells with suppressive functions.
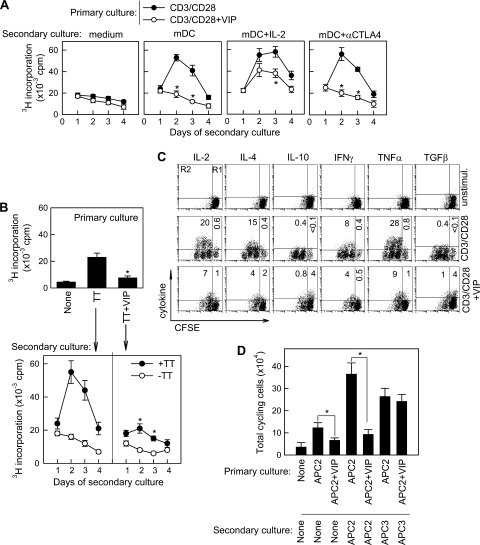
We next investigated the capacity of the VIP-treated T cells to respond to different stimuli in a secondary culture in the absence of the neuropeptide. Whereas restimulation of CD3/CD28-activated T cells with anti-CD3 Abs or with allogeneic mDCs resulted in the induction of functional effector T cells characterized by a fast proliferative response, the cells that were primed in the presence of VIP did not proliferate in response to secondary stimulation to alloantigens (Fig. 2A; see Fig. S1B in the supplemental material) or to a specific antigen (Fig. 2B). This hyporesponsive state was partially reversed by the addition of IL-2, but not blockade of the immunosuppressive molecule CTLA4 (Fig. 2A). Moreover, CD3/CD28-primed T cells showed increased production of the effector cytokines IL-2, IL-4, IFN-γ, and TNF-α upon restimulation, whereas VIP treatment substantially reduced the number of CD4 cells producing all these cytokines (Fig. 2C). In contrast, VIP increased the number of cells producing the suppressive molecules IL-10 and TGF-β, mainly in the nondividing subset, indicating that this effect is not a result of the expansion of preexisting IL-10/TGF-β-producing cells (Fig. 2C). Whether VIP induces the generation of these cells from naïve T cells or converts Th1 or Th2 cells to IL-10/TGF-β-producing cells is still unknown, but VIP did not increase the expression of IL-10 or TGF-β on human Th1 and Th2 clones (unpublished data). The VIP-induced hyporesponsiveness seems to be antigen specific, because T cells from donor 1 primed with allogeneic antigen-presenting cells (APCs) from donor 2 in the presence of VIP did not respond to a secondary restimulation with APCs from donor 2 but did with respond to a secondary restimulation with APCs from donor 3 (Fig. 2D).
FIG. 2.
VIP induces hyporesponsiveness in T cells. (A) Human T cells were CD3/CD28 activated in the absence or presence of VIP (10−7M) for 96 h. Cells were harvested and rested for 48 h, and the recovered viable cells were restimulated in a secondary culture with allogeneic mDCs in the absence or presence of IL-2 or anti-CTLA4 MAb. Proliferation was determined at different times after initiation of secondary culture. *, P < 0.01 versus CD3/CD28 (n = 4, in duplicate). The error bars indicate SD. (B) Human PBMCs (107) were activated with TT in the absence or presence of VIP (10−7 M) for 4 days, and proliferation in this primary culture was determined by [3H]thymidine incorporation (top). T cells (2 × 104) were isolated from the primary culture, rested for 2 days, and restimulated in a secondary culture with syngeneic mDCs (104) in the absence or presence of TT, and proliferation was determined after 4 days (bottom). *, P < 0.01 versus TT-primed cells in the absence of VIP (n = 3, in duplicate). (C) CFSE-labeled human T cells were CD3/CD28 activated in the absence or presence of VIP (10−7 M) for 96 h. Cells were harvested and rested for 48 h, and the cytokine-producing potential of the recovered cells was determined by intracellular cytokine staining on CFSEhigh (nondividing; R1) and CFSEmild/low (cycling; R2) cells using flow cytometry. The numbers represent the percentages of cytokine-positive cells (n = 4). unstimul., unstimulated. (D) T cells from donor 1 (5 × 104) were labeled with CFSE and stimulated with mDCs from donor 2 (APC2; 2 × 104) in the absence or presence of VIP. After 6 days, viable cells (2 × 104) were recovered and restimulated in a secondary culture with medium (None), with APC2 (104), or with mDCs from donor 3 (APC3; 104). The number of CFSElow/mild cycling cells was determined after 4 days of secondary culture. *, P < 0.01 (n = 3 or 4, in duplicate).
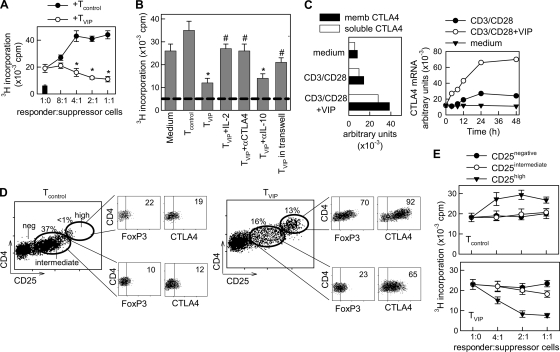
Various reports have closely related T-cell cycle arrest to a regulatory phenotype (10, 11, 37). Moreover, it has been shown that treatment with VIP of mice with autoimmune disorders increases the number of Treg in the periphery (20, 26, 28). Therefore, we investigated whether the human T cells primed in the presence of VIP exerted suppressor activity on effector T cells. To address this issue, human T cells activated with CD3/CD28 in the absence (Tcontrol) or presence (TVIP) of VIP were cocultured at different ratios with syngeneic T cells and allogeneic mDCs. Whereas Tcontrol significantly contributed to the proliferative response of effector T cells, TVIP dose-dependently suppressed their clonal expansion (Fig. 3A). Separation of TVIP from responder T cells in transwells significantly reversed the suppressive effect of TVIP, and blocking of CTLA4, but not of IL-10, fully reversed it (Fig. 3B), suggesting a CTLA4 cell-to-cell dependence. As expected, IL-2 addition to cocultures bypassed their suppressive activity (Fig. 3B). The involvement of CTLA4 in the suppressive effect of TVIP correlated with the fact that VIP induced rapid and sustained expression of both soluble and membrane-bound forms of CTLA4 in CD3/CD28-activated T cells (Fig. 3C; see Fig. S2 in the supplemental material). In contrast to its effect on CTLA4, VIP failed to increase the expression of other molecules involved in inhibitory signals on T cells, including PD-1 and BTLA (see Fig. S2 in the supplemental material), and blockade of PD-1-mediated signaling with anti-PDL1 Abs did not affect the suppressive activity of TVIP on effector T cells (not shown).
FIG. 3.
VIP induces regulatory functions in human T cells. (A) Human T cells isolated from donor A were CD3/CD28 activated in the absence (Tcontrol) or presence (TVIP) of VIP for 4 days. After a 48-h rest period, the suppressive potential of the recovered cells (suppressor Tcontrol or TVIP) was determined by adding increasing numbers of Tcontrol or TVIP to cocultures of responder T cells (from donor A) and allogeneic mDCs (from donor B). The proliferation of responder T cells was determined after 4 days. The black bar represents background proliferation of unstimulated responder T cells. The error bars indicate SD. (B) Tcontrol or TVIP (2.5 × 104) were added to cocultures of responder T cells (donor A; 5 × 104) and allogeneic mDCs (donor B) in the absence or presence of IL-2 (100 U/ml), anti-CTLA4 (10 μg/ml), or anti-IL-10 (10 μg/ml) MAb. When indicated, TVIP-mDC cocultures were separated from responder T-cell-mDC cocultures by a semipermeable membrane in transwells. The proliferation of responder T cells was determined after 4 days. The dashed line represents background proliferation of unstimulated responder T cells. (C) The human T cells isolated were CD3/CD28 activated in the absence or presence of VIP for different times. The mRNA expression of the soluble and membrane (memb) forms of CTLA4 was determined by quantitative PCR and expressed as arbitrary units normalized by β-actin transcripts. The time course corresponds to total mRNA (soluble plus membrane CTLA4). (D) CD4+ CD25− T cells isolated from donor A were CD3/CD28 activated without (Tcontrol) or with (TVIP) VIP for 96 h. Tcontrol and TVIP were analyzed in the CD4+ cell fraction for CD25 expression by flow cytometry. The subsets formed (CD25negative, CD25intermediate, and CD25high) were sorted by flow cytometry and analyzed for FoxP3 and CTLA4 expression. (E) Sorted CD25negative, CD25intermediate, and CD25high populations from Tcontrol and TVIP were added at different cell ratios to cocultures of PBMCs (5 × 104) and allogeneic mDCs, and proliferation was determined. n = 3 or 4, in duplicate, for all panels, except panel C, where n = 2. *, P < 0.01 versus Tcontrol; #, P < 0.01 versus TVIP-treated cocultures.
Similar regulatory activities were obtained when we used purified CD4+ CD25− T cells primed in the presence of VIP (see Fig. S3 in the supplemental material). These data suggest that the induction of regulatory activity by VIP occurs within the CD4+ CD25− T-cell fraction, independent of the presence and expansion of naturally occurring CD4+ CD25+ Treg. FACS analysis indicated that three distinct subsets were distinguished based on the level of CD25 expression (CD25negative, CD25intermediate, and CD25high) after VIP treatment (Fig. 3D). We sorted the three populations and examined FoxP3 and CTLA4 expression as Treg markers, and their functional activities. An elevated percentage of the VIP-induced CD25high population expressed FoxP3 and CTLA4high in comparison to the CD25high cells purified from the untreated controls (Fig. 3D). This was due to an increase in the absolute number of CD4+ FoxP3+ and CD4+ CTLA4+ T-cell subsets, but not to enrichment of these cells due to a decrease in the number of effector cells (not shown). The VIP-induced CD25high subset elicited weak proliferation, did not express markers of activation (they are CD69− CD62Lhigh), and remained hyporesponsive upon allogeneic restimulation with mDCs (see Fig. S4 in the supplemental material). Moreover, the expression of FoxP3 on these cells was confined to the noncycling cells (see Fig. S4A in the supplemental material), supporting the idea that VIP would induce the generation of new Treg rather than a mere enrichment of existing cells. The addition of VIP-induced CD25high cells to cocultures of PBMCs and allogeneic mDCs impaired the proliferation of responder T cells (Fig. 3E). However, the VIP-induced CD25intermediate population showed weak suppressive activity (Fig. 3E) while showing increased expression of CTLA4 and moderate FoxP3 (Fig. 3D). Taken together, these results indicate that stimulation via TCR/CD3-CD28 signaling in the presence of VIP resulted in de novo generation of a CD4+ CD25high CTLA4+ T-cell population with regulatory functions.
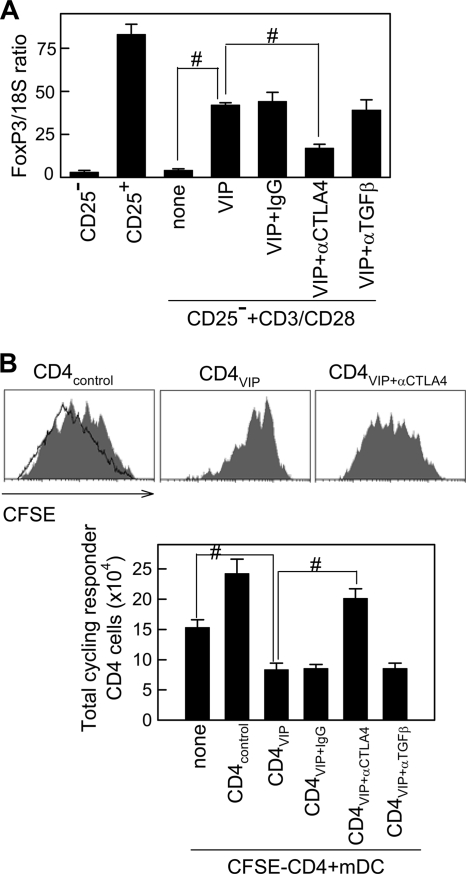
Although the contribution of TGF-β to the suppressive activity of induced Treg is controversial, its critical participation in their generation is clearly established (14, 19, 53, 57, 66). Moreover, a previous study demonstrated that TGF-β1 requires CTLA4 to induce FoxP3 expression and to generate Treg functions (67). By using neutralizing antibodies, we demonstrated that CTLA4 is needed for VIP to induce FoxP3 in CD3/CD28-activated CD4+ CD25− T cells (Fig. 4A) and to generate suppressor T cells (Fig. 4B). However, TGF-β did not participate in any of these VIP effects (Fig. 4A and B).
FIG. 4.
VIP requires CTLA4 to induce FoxP3 and to generate Treg. (A) Purified CD4+ CD25− T cells were CD3/CD28 activated in the absence (none) or presence of VIP, with or without anti-CTLA4 (10 μg/ml), anti-TGF-β (10 μg/ml), or control IgG (10 μg/ml). After 96 h, FoxP3 expression was quantitated by real-time PCR and normalized to 18S rRNA. Unstimulated CD4+ CD25− and CD4+ CD25+ T cells were used as negative and positive controls, respectively. The error bars indicate SD. (B) CD4+ CD25− T cells from donor A were CD3/CD28 activated in the absence (CD4control) or presence (CD4VIP) of VIP, with or without anti-CTLA4, anti-TGF-β, or control IgG. After 96 h, the recovered cells (4 × 104) were cocultured with CFSE-labeled CD4+ cells (2 × 105; donor A) and mDCs (4 × 104; donor B). After 96 h, the total number of cycling cells (percent CFSE-positive cells that had divided × total number of cells) was determined. The shaded histograms represent examples of each experimental group, and the solid-line histogram corresponds to CD4 cells stimulated with allogeneic mDCs. #, P < 0.01. n = 3, in duplicate.
VIP-induced suppressive T cells are arrested at the G1 phase of the cell cycle.
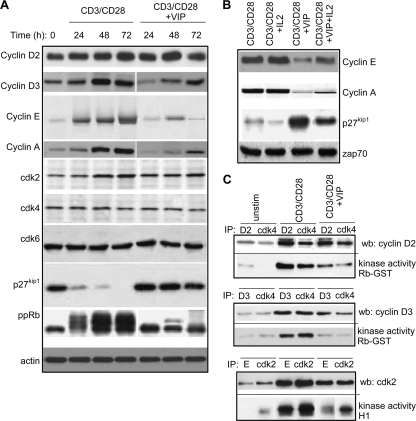
The results described above suggest that VIP must regulate the signals controlling cell cycle progression. Unraveling such signals is critical to understanding how VIP induces T cells with regulatory functions. TCR/CD3 plus CD28 costimulation regulates the entry of T cells into the cell cycle and progression through the G1 phase, leading to downregulation of the CDK inhibitor p27kip1 and activation of cyclin D2-associated cdk4/cdk6 and cyclin E-associated cdk2. These events result in hyperphosphorylation of the retinoblastoma (pRb) gene product, expression of S-phase genes, and cell cycle progression (4, 63). Analysis of the cell cycle regulatory molecules showed that VIP-treated T cells were capable of expressing cyclin D2 and cyclin-associated cdk4 and cdk6, indicating that they could enter the G1 phase (Fig. 5A; see Fig. S5 in the supplemental material). However, VIP blocked progression through the G1 restriction point to the late G1 and S phases. VIP-treated cells showed sustained decrease in the expression of cyclin D3, which is synthesized in the late G1 phase, and cyclin E, which is expressed at the G1 restriction point. Consequently, the levels of S-phase cyclin A were dramatically reduced by VIP (Fig. 5A; see Fig. S5A in the supplemental material). Moreover, whereas T-cell activation via CD3/CD28 downregulated p27kip1 levels, VIP treatment not only avoided the degradation of this CDK inhibitor, but also increased its levels over the background expression found in unstimulated cells. As a result of these events, hyperphosphorylation of pRb was impaired in VIP-treated cells (Fig. 5A; see Fig. S5A in the supplemental material). It is noteworthy that exogenous IL-2 partially, but not totally, reversed the VIP effects on the expression of cyclin E, cyclin A, or p27kip1 (Fig. 5B; see Fig. S5B in the supplemental material), consistent with the hypothesis that IL-2 inhibition by VIP could contribute to cell cycle arrest. In contrast, IL-2 addition to untreated CD3/CD28-activated T cells did not affect the expression of cyclins or p27kip1, suggesting that the IL-2 endogenously produced with T-cell activation is sufficient to induce these molecular events.
FIG. 5.
VIP-induced suppressive T cells are arrested at the early G1 phase of the cell cycle. (A) Effects of VIP on the expression of G1 cyclins and CDK and CDKi proteins. Human T cells were CD3/CD28 activated without or with VIP (10−7 M) for different time intervals. The cell lysates were subjected to Western blot analysis for cyclins, CDKs, p27kip1, phosphorylated pRb (ppRb), or actin. (B) IL-2 partially reverses VIP effects on cyclins and p27kip1. Human T cells were CD3/CD28 activated without or with VIP (10−7 M) and IL-2 (50 U/ml) for 72 h. Western blot analysis was performed on cell lysates for cyclins, p27kip1, and Zap70. (C) VIP inhibits cyclin/CDK activity. Human T cells were CD3/CD28 activated without or with VIP (10−7 M) for 36 h. The cell lysates were immunoprecipitated (IP) with Abs against cyclin D2, cyclin D3, cyclin E, cdk2, or cdk4. Cyclin D2-cdk4, cyclin D3-cdk4, and cyclin E-cdk2 interactions were determined by Western blot (wb) analysis of precipitates with anti-cyclin D2, anti-cyclin D3, and anti-cdk2, respectively. cdk4-cyclin D2/D3 and cyclin E-cdk2 kinase activities were measured using pRb-GST and histone H1, respectively, as substrates. n = 3. (See Fig. S5 in the supplemental material for quantitative analysis.) unstim, unstimulated.
The effect of VIP in cell cycle progression was accompanied by impaired activation of both cyclin D2/D3-associated cdk4 and cyclin E-associated cdk2 kinase activities (Fig. 5C; see Fig. S5C in the supplemental material). This confirms and extends the observation that VIP affects the functions of the molecular players involved in the G1/S progression.
VIP elevates the levels of p27kip1 in activated T cells through various mechanisms.
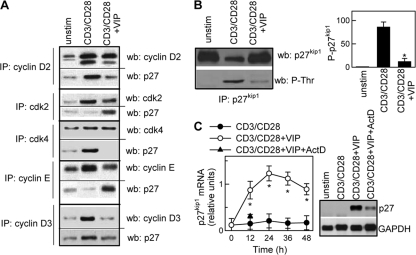
The cell cycle inhibitor p27kip1 acts during late G1 phase by binding and inhibiting cdk2-cyclin E/A complexes. Upon stimulation, T cells can progress through the cell cycle only when p27kip1 is dissociated from the cdk2-cyclin E/A complexes. This is generally achieved by degradation of p27kip1, which is preceded by phosphorylation of p27kip1 (61, 63). VIP increased the p27kip1 that coimmunoprecipitated with cyclin E-cdk2 in comparison to unstimulated and CD3/CD28-stimulated T cells (Fig. 6A; see Fig. S6A in the supplemental material), which is consistent with the VIP-mediated decrease in cyclin E-cdk2 activities. On the other hand, VIP impaired the binding of p27kip1 to cyclin D2/D3-cdk4 complexes (Fig. 6A; see Fig. S6A in the supplemental material). Furthermore, VIP treatment prevented the p27kip1 degradation that occurs upon T-cell activation. VIP-treated T cells showed increased levels of p27kip1 in the nucleus in comparison with untreated CD3/CD28-stimulated cells, which showed nuclear exclusion of p27kip1 (see Fig. S6B in the supplemental material). Increase in p27kip1 could be a consequence of impaired p27kip1 degradation and/or of increased p27kip1 synthesis. Our data indicate that VIP prevented CD3/CD28-induced p27kip1 phosphorylation while increasing total p27kip1 levels (Fig. 6B). Indeed, VIP induced sustained gene expression of p27kip1 (Fig. 6C). Moreover, the induction of p27kip1 mRNA by VIP was blocked by the transcription inhibitor actinomycin D (Fig. 6C). The effect of VIP seems to be selective for p27kip1, since VIP did not affect the levels of other CDK inhibitors, such as p21cip1 and p16ink4a (see Fig. S7A in the supplemental material).
FIG. 6.
VIP maintains threshold levels of p27kip1. Human T cells were CD3/CD28 activated without or with VIP (10−7M). (A) VIP increases the interaction of p27kip1 with cyclin/CDK complexes. Cell lysates obtained after 24 h of culture were immunoprecipitated (IP) with Abs against cyclin D2, cyclin E, cdk2, or cdk4 and analyzed by Western blotting (wb) for cyclins, CDK, and p27kip1 (see Fig. S6A in the supplemental material for quantitative data). (B) VIP inhibits p27kip1 phosphorylation in activated T cells. After 48 h of culture, the cell lysates were immunoprecipitated with anti-p27kip1 Abs and analyzed by Western blotting for p27kip1 (total p27kip1) or phospho-Thr (phosphorylated p27kip1 [P-p27kip1]) and expressed as densitometric units relative to unstimulated samples. The error bars indicate SD. (C) VIP increases p27kip1 mRNA levels. p27kip1 mRNA expression was determined by Northern blotting and expressed as densitometric units relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). When indicated, actinomycin D (ActD) was added during culture (the blot corresponds to 12 h). *, P < 0.001 versus CD3/CD28 (n = 3).
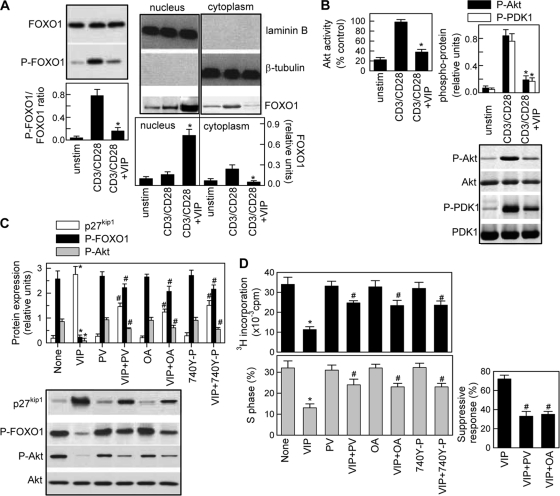
The expression of p27kip1 protein is upregulated at the transcriptional level by the winged-helix family of transcription factors, FOXO1, FOXO3, and FOXO4 (13, 55, 63,). Each of these Forkhead factors contains conserved phosphorylation sites for protein kinase B (PKB/Akt), and Akt-mediated phosphorylation results in translocation of these factors to the cytoplasm and subsequent proteasomal degradation (13, 18, 55). Consistent with previous reports (46), primary T cells express relatively high levels of FOXO1 (Fig. 7A) and very low levels of FOXO3 and FOXO4 (data not shown). VIP inhibited the phosphorylation and the cytoplasmic translocation of FOXO1 promoted by CD3/CD28 stimulation (Fig. 7A).
FIG. 7.
VIP inhibits FOXO1 phosphorylation and the Akt/PI3K pathway. Human T cells were cultured with medium (unstim) or CD3/CD28 activated in the absence or presence of VIP (10−7 M). (A) VIP inhibits phosphorylation and cytoplasmic translocation of FOXO1. Total cell extracts isolated at 4 h of culture (left) or nuclear and cytoplasmic protein extracts isolated at 8 h of culture (right) were analyzed by Western blotting for FOXO1 or phospho-FOXO1 and expressed as densitometric units relative to total FOXO1, laminin B, or β-tubulin. The error bars indicate SD. (B) VIP inhibits Akt/PKB phosphorylation and kinase activity in stimulated T cells. Cell lysates isolated after 2 h of culture were analyzed by Western blotting for phospho-Akt and PDK1 and expressed as densitometric units relative to total Akt or PDK1. Akt kinase activity was determined as described in Materials and Methods and expressed as percentages of the Akt activity of CD3/CD28-activated T cells. (C) The VIP effect on Akt activity is dependent on PI3K and PP2A activities. CD3/CD28-stimulated human T cells were treated with VIP and/or the PI3K activators peroxovanadate (PV) (50 μM) and 740 Y-P (20 μM) or the PP2A inhibitor okadaic acid (OA) (1 μM). Western blot analysis was performed on cell lysates isolated 2 h (for phospho-Akt), 4 h (for phospho-FOXO1), or 48 h (for p27kip1) of culture. The results are expressed as densitometric units relative to total Akt or β-tubulin. (D) Involvement of inhibition of PI3K-Akt signaling in the VIP effect on the T-cell cycle and regulatory functions. CD3/CD28-activated human T cells were treated with VIP and/or PV, OA, or 740 Y-P. The percentage of cells in S phase and proliferation were determined after 72 h. After 96 h in culture, T cells were recovered, and their suppressive potential was determined in cocultures with syngeneic responder T cells (at a suppressor/responder ratio of 1:2) and allogeneic mDCs, as described in the legend to Fig. 3A, and expressed as a percentage of inhibition of proliferation. n = 3 or 4. *, P < 0.01 versus CD3/CD28; #, P < 0.01 versus VIP-treated samples.
Therefore, we next investigated the capacity of VIP to regulate signaling by the PI3K-Akt pathway. T-cell activation through the CD3/TCR-CD28 pathways resulted in increased Akt kinase activity as a consequence of its phosphorylation on Thr308 by PDK-1, which is phosphorylated/activated by PI3K (Fig. 7B). VIP significantly inhibited all these events (Fig. 7B). The inhibitory effect of VIP on Akt could be a consequence of impaired PI3K activity (as the inhibition of PDK-1 phosphorylation suggests) or of increased phosphatase activity on Akt. To test both possibilities, we investigated the effects of peroxovanadate and 740 Y-P, nonselective and selective activators of PI3K, and of okadaic acid, a phosphatase P2 (PP2) inhibitor. The three compounds partially reversed VIP effects on Akt activation, FOXO1 phosphorylation, and p27kip1 levels (Fig. 7C) and the effects of VIP on the inhibition of T-cell proliferation and cell cycle progression and the induction of suppressive T-cell activities (Fig. 7D), suggesting that inhibition of the PI3K/Akt pathway plays a major role in the VIP actions on cell cycle and Treg functions.
Multiple mechanisms are involved in the inhibition of IL-2 production by VIP.
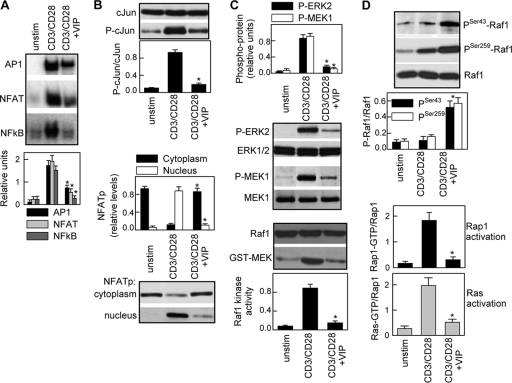
Because IL-2 is a hallmark of T-cell activation and decreased IL-2 production is a mandatory requirement to induce hyporesponsive T cells and suppressive functions by VIP (Fig. 2A and 5B), we investigated the mechanisms related to the VIP-mediated inhibition of IL-2 secretion. By using a luciferase-linked reporter system in Jurkat T cells, we found that VIP downregulated CD3/CD28-induced IL-2 transcription and the transactivation mediated by the transcription factors NF-κB, NFAT, and AP-1 (see Fig. S8 in the supplemental material). In agreement, VIP decreased the nuclear translocation and DNA binding of NF-κB, NFAT, and AP-1 induced by CD3/CD28 signaling in primary T cells (Fig. 8A). AP-1 and NFAT promoter elements are critical for the transcriptional activity of IL-2, and their defective transactivation causes T-cell anergy (10, 39). Upon T-cell activation, the NFAT protein is dephosphorylated and translocates into the nucleus, and AP-1 is activated by phosphorylation of one of its components (c-Jun) by two mitogen-activated protein kinase (MAPK) cascades, finally involving ERK and JNK. VIP impaired the translocation of NFATp from the cytoplasm to the nucleus and decreased the levels of the phosphorylated form of c-Jun (Fig. 8B). Therefore, we investigated whether the ERK-MAP kinase pathway was affected by VIP. ERK1 and ERK2 are activated through phosphorylation by MEK1 and MEK2, which are phosphorylated/activated by Raf1 protein kinase. VIP decreased the levels of phosphorylated ERK1/2 and MEK1 and inhibited Raf1 kinase activity induced by TCR/CD3-CD28 costimulation (Fig. 8C). Interestingly, VIP did not affect the phosphorylation/activation of other MAPKs, such as p38 or JNK (see Fig. S7B in the supplemental material), suggesting a selective effect of VIP on the phosphorylation of c-Jun by the Raf1-MEK1-ERK pathway.
FIG. 8.
Mechanisms of inhibition of IL-2 expression by VIP. (A) Human T cells were cultured with medium (unstim) or CD3/CD28 activated without or with VIP (10−7 M). After 4 h, nuclear proteins were isolated and assayed for DNA binding to NF-κB, AP-1, or NFAT consensus sites by EMSA and expressed as densitometric units relative to the binding of the constitutive nuclear factor NF-Y. The error bars indicate SD. (B) After 3 h, whole-cell extracts were analyzed by Western blotting for c-Jun and phospho-c-Jun expression. NFATp levels were determined in both nuclear and cytoplasmic extracts and expressed as densitometric units relative to laminin B or β-tubulin. (C) After 1 h of culture, cell lysates were prepared, and activation of ERK1/2 and MEK1 was determined by Western blotting with phosphospecific Abs and expressed as densitometric units normalized for total ERK1/2 or MEK1. The kinase activity of Raf1 was determined by assaying the phosphotransferase activity toward GST-MEK1. (D) Activation of Rap1 and Ras was determined as described in Materials and Methods. The inactive forms of Raf1 were measured in cell lysates by Western blotting for phospho-Ser43-Raf1 and phospho-Ser259-Raf1. n = 3 or 4. *, P < 0.01 versus CD3/CD28.
The kinase activity of Raf1 is regulated by the Ras family of small GTP-binding proteins, Ras and Rap1. Ras-Raf1 interaction activates Raf1, and phosphorylation of Rap1 forms Rap1-GTP, which competes with Ras for binding to Raf1 (9, 50). Furthermore, Raf1 activity depends on its phosphorylation status: phosphorylation on Ser43 impairs its kinase activity, whereas phosphorylation on Ser259 reduces binding to Ras (17). VIP increased the levels of both phosphorylated forms of Raf1 (Fig. 8D), suggesting that VIP could directly affect Raf1 kinase activity and reduce its binding to Ras. Interestingly, VIP also affected CD3/CD28-mediated Ras activation (Fig. 8D), suggesting that VIP inhibition of Raf1 by phosphorylation should not be required to inhibit the MEK-ERK1/2 pathway, because Ras is upstream of Raf1. Moreover, contrary to the VIP effects found in other cell types (1, 51), VIP impaired the activation of Rap1-GTP in stimulated T cells (Fig. 8D), indicating that Rap1-GTP competition for Ras is not involved in the VIP effect on Raf1 activation.
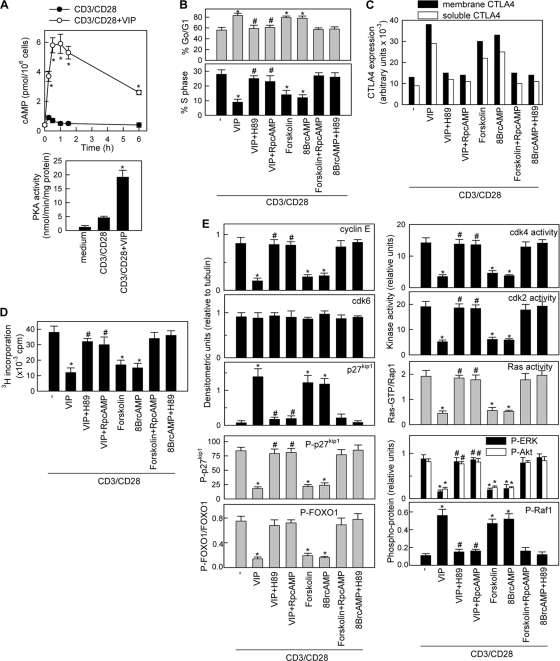
VIP mediates the suppressive effect by elevating cAMP levels.
The immunological actions of VIP are exerted through a family of receptors, consisting of VPAC1 and VPAC2, coupled to AC and the elevation of intracellular cAMP levels and subsequent PKA activation (29). Indeed, VIP rapidly and for a long time increased the cAMP levels and PKA activity in CD3/CD28-activitated T cells (Fig. 9A). However, VIP did not affect the intracellular calcium fluxes (see Fig. S7C in the supplemental material) or the activation of PKC (data not shown) induced by TCR signaling. To determine whether the cAMP/PKA pathway mediates the regulatory actions described in this study for VIP, we assayed the effects of H89 (a PKA inhibitor), forskolin (a cAMP-inducing agent), 8-Br-cAMP (a cell-permeable cAMP analogue), and Rp-cAMP (an AC inhibitor). The VIP effects on cell cycle arrest, CTLA4 expression, T-cell-suppressive activity, cyclin-CDK activity, p27kip1 phosphorylation and stability, FOXO1 phosphorylation, ERK1/2 activation, and phosphorylation of Akt and Raf1 were reversed by both AC and PKA inhibitors (Fig. 9B to E; see Fig. S9 in the supplemental material). Forskolin and 8-Br-cAMP mimicked VIP actions (Fig. 9B to E; see Fig. S9 in the supplemental material). These findings indicate that the regulatory effects of VIP on T cells are mainly mediated through increases in intracellular cAMP and PKA activation.
FIG. 9.
VIP mediates the suppressive effect by elevating cAMP levels. (A) VIP increases cAMP levels and stimulates PKA activity in human activated T cells. Human T cells were CD3/CD28 stimulated with or without VIP (10−7 M). Intracellular cAMP levels were determined at different times (top). PKA activity was assayed by radioimmunoassay in cell lysates obtained 1 h after stimulation (bottom). The error bars indicate SD. (B to E) Human T cells were CD3/CD28 stimulated in the absence (−) or presence of forskolin (10−6 M), 8-Br-cAMP (0.1 mM), or VIP (10−7 M) with or without H89 (50 ng/ml) or Rp-cAMP (0.1 mM). (B) Cell cycle analysis was performed after 72 h. (C) Expression of the soluble and membrane forms of CTLA4 was determined at 96 h. (D) The suppressive activity on effector T cells was determined as described in the legend to Fig. 3A at an effector/suppressor cell ratio of 2:1. (E) The expression and phosphorylation statuses of cyclins, CDK, p27kip1, FOXO1, Akt, Raf1, and ERK1/2 and the activation of cyclin-CDK complexes and Ras were quantified as detailed in the legends to Fig. 5 to 8. The specificity of H89 and Rp-cAMP in our study was confirmed by their blocking effects on the actions of forskolin and Br-cAMP. Representative blots are shown in Fig. S9 in the supplemental material. n = 3 or 4. *, P < 0.001 versus CD3/CD28; #, P < 0.001 versus VIP-treated samples.
DISCUSSION
In this report, we provide evidence that VIP treatment in the presence of TCR signaling and CD28 costimulation induces a regulatory CD4+ CD25high T-cell subset from peripheral human CD4+ CD25− responder T cells. Apparently, VIP directly programs the CD4+ CD25− cell toward a regulatory phenotype independently of the presence of naturally occurring Treg. In fact, VIP failed to promote the growth of existing human Treg in vitro (48) and VIP protected against experimental autoimmune encephalomyelitis and arthritis in CD25-depleted mice by inducing the emergence of new peripheral CD4+ CD25+ Treg (20, 27, 28). Phenotypically, VIP-converted human suppressive T cells retain surface CD25 and express high levels of FoxP3 and CTLA4, but not markers of activation. It remains unknown whether VIP uniformly affects the entire CD4+ CD25− T-cell repertoire or only a subset that differentiates into FoxP3+ CD4+ CD25+ Treg, which subsequently suppresses the clonal expansion to the remaining T cells, and whether these cells are initially committed to differentiate on Treg. The mechanisms through which VIP stimulates this regulatory capacity could be defined by two key events that occur with VIP treatment: induction of cell cycle arrest and increase on CTLA4 expression.
Evidence now exists that T-cell anergy is clearly related to generation of Treg (5, 10, 11, 14, 35, 37). The VIP-converted Treg share a number of biochemical characteristics with anergic T cells generated following other approaches, including CD28 blockade and CD40L costimulation, or treatments with IL-10 plus TGF-β1 or immunosuppressive agents (1, 5, 6, 9, 10, 11, 14, 17, 27, 30, 33, 35, 43, 51). Understanding the molecular players in the cell cycle regulated during the induction of T-cell anergy may provide a powerful tool in quantifying the degree of tolerance induction in individual patients who receive T cells tolerized by different strategies. Our data indicate that VIP-treated T cells are capable of entering the G1 phase but do not progress through the G1 restriction point to the late G1 and S phases. Blockade of cell cycle progression by VIP could be exerted at multiple levels (see Fig. S10 in the supplemental material). VIP impairs the activation of the cdk4-cyclin D3 and cdk2-cyclin E complexes, which are needed to phosphorylate pRb in two different moments of the G1 phase, and thereby deactivates the E2F transcription factor and inhibits the expression of S-phase genes. The inhibition of the G1 CDK-cyclin holoenzymes could be in part a consequence of VIP-induced reduction of cyclin D3 and cyclin E levels, which are synthesized at late G1 phase and the G1 restriction point, respectively. Moreover, VIP keeps the levels of the cell cycle inhibitor p27kip1 elevated on activated T cells. Independently of the amounts of cyclins synthesized during T-cell activation, p27kip1 must be dissociated from the cdk2-cyclin E complexes to permit G1/S progression (63). Whereas cdk2-cyclin E activity is highly sensitive to p27kip1, cdk4-cyclin D complexes are catalytically active even in the presence of high levels of p27kip1. In fact, it has been proposed that cdk4-cyclin D complexes function to sequester p27kip1 away from cdk2-cyclin E (63). Interestingly, VIP favors the interaction of p27kip1 with cdk2-cyclin E complexes, but not with cdk4-cyclin D2 complexes, through a mechanism that is still unknown. However, VIP treatment decreases the activation of the cdk4-cyclin D2 complexes without affecting their expression. Because cdk4 activation depends on its phosphorylation by Akt, VIP-induced Akt inhibition could partially explain the suppressive activity of VIP on cdk4-cyclin D2 activation. It is plausible that the induction of p27kip1 by VIP plays a central role in the effect of VIP on cell cycle arrest, an effect that seems to occur at two levels. VIP inhibits the phosphorylation and subsequent degradation of p27kip1 and also stimulates gene expression of p27kip1. Since p27kip1 is phosphorylated by PKB/Akt and cdk2, among others, VIP inhibition of both kinases could prevent p27kip1 phosphorylation/degradation. Furthermore, as previously described (13, 18, 46, 55), inhibition of Akt-mediated phosphorylation of the transcription factor FOXO1 could increase the transcription of p27kip1, because FOXO1 binds to the p27kip1 promoter only in its dephosphorylated form. Moreover, a recent work using FOXO1-deficient mice has demonstrated the critical role of this factor in T-cell activation and tolerance (44). Regulation of p27kip1 at both the transcriptional and posttranslational levels could explain why the inhibitory signal of VIP on cell cycle dynamics is maintained long term and does not require treatment for extended periods. As in the cases of other antiproliferative treatments (3), inhibition of the PI3K-Akt pathway appears to be critical for VIP regulation of p27kip1. Our data support the involvement of both PP2-mediated Akt dephosphorylation and inhibition of PI3K activity in the VIP effects. Whether VIP affects any factor localized upstream of PI3K is unknown; however, VIP does not regulate initial TCR signaling through Zap70 (data not shown), p59fyn or p56lck tyrosine kinase, or the ζ chain associated with TCR (see Fig. S7D to F in the supplemental material). The capacity of PP2A to deactivate G1 CDKs, including cdk2 and cdk4 (65), could also explain why VIP slightly decreases cdk4-cyclin D2 activity while keeping cyclin D2 and cdk4 levels unaffected. The activation of the cAMP/PKA pathway seems to play a major role in all these VIP actions. Indeed, the effects of other cAMP/PKA-inducing agents in T-cell cycle arrest and p27kip1 upregulation are well known (10, 30, 31, 42, 50, 59).
A decrease in IL-2 production accompanies the effect of VIP on cell cycle arrest. This seems to be critical to reinforce T-cell hyporesponsiveness, since exogenous IL-2 partially reverses this state. As with CD3/CD28 signaling, VIP is able to block the activation of the PI3K-Akt and Ras-Raf1-ERK1/2 cascades induced by IL-2 (see Fig. S7G in the supplemental material). However, IL-2 signaling seems to partially bypass the suppressive action of VIP through the activation of the STAT5 pathway, which is not affected by VIP (see Fig. S7G in the supplemental material). Activation of STAT5 is critically involved in the mitogenic effects of IL-2, because it directly activates crucial genes, including cyclins D2/D3, cdk4/6, and c-Myc (41). However, although the PI3K and Ras-MAPK pathways are crucial for T-cell activation through the TCR (64), its absolute requirement in IL-2-mediated signaling has been more difficult to demonstrate, since it is mainly cooperative with the STAT5 signal (24, 40). Although VIP does not fully block IL-2 signaling, our study clearly demonstrates that VIP deactivates three of the transcription factors, NFAT, NF-κB, and AP-1, critically involved in IL-2 transcription and T-cell activation. It was reported that VIP inhibits NFAT dephosphorylation and nuclear translocation without affecting calcineurin activity (15). Therefore, PKA-mediated phosphorylation of NFAT emerges as a plausible mechanism for the inhibitory effect of VIP on NFAT (32). Regarding NF-κB, various studies clearly demonstrated that VIP regulates NF-κB activation by inhibiting the phosphorylation/degradation of the NF-κB inhibitor IκB (15, 16, 29). Alternatively, because PDK1 integrates TCR-CD28 signaling and NF-κB activation (45), VIP could impair NF-κB-dependent gene activation by inhibiting PDK1. Finally, AP-1 inhibition by VIP is more complex and involves downregulation of the Ras-Raf1-MEK1-ERK cascade. Interestingly, VIP does not affect the activation of JNK and p38 MAPK, which could be more dependent on kinases other than Ras-Raf1. Our results provide evidence that VIP treatment directly inhibits Ras kinase activity and impairs the activation of Raf1 and its binding to Ras. VIP effects on the Ras-Raf1-MEK1-ERK signaling cascade are cell type specific, since, contrary to our findings in T cells, VIP stimulates this pathway in other cells (1, 51). Interestingly, cAMP/PKA signaling is involved in both VIP inhibitory and stimulatory actions. Beside the effects on IL-2 production, VIP inhibition of Ras-Raf1 could affect cell cycle regulation, since interaction of Raf1 with Cdc25 phosphatases activates CDKs during the G1/S transition (22).
On the other hand, our study demonstrates that CTLA4 plays a major role in the generation and regulatory activity of the VIP-induced Treg. Interestingly, although VIP also stimulates the production of IL-10, a cytokine frequently linked to the regulatory activity of induced Treg (38, 54), IL-10 seems to play a minor role, if any, in the suppressive activity of the VIP-tolerized T cells. Moreover, TGF-β, although critical in the generation of other Treg subtypes (10, 14, 19, 53, 66), does not participate in the induction of Treg by VIP. CTLA4 has striking inhibitory effects on T-cell activation and is essential for maintaining immune tolerance (12). Besides its essential role in the suppressive activity of Treg (21), CTLA4 costimulation appears to be needed for generating adaptive CD4+ CD25+ suppressor cells (67). Similarly, VIP requires CTLA4 to induce CD4+ CD25− FoxP3− cells to become CD4+ CD25+ FoxP3+ suppressor cells. VIP not only increases the expression of CTLA4 in T cells, but also accelerates its appearance and maintains elevated levels long term. The presence of CTLA4 early after T-cell activation critically contributes, in an autocrine/paracrine manner, to the tolerizing activity of VIP. However, CTLA4 seems to play a minor role in the cell cycle arrest and hyporesponsive state of the VIP-tolerized T cells, suggesting that this is an effect directly exerted on molecular players in cell cycle progression. In addition, VIP does not induce other molecules involved in negative signaling in T cells, such as BTLA4 and PD-1. The fact that VIP induces both soluble and membrane-bound forms of CTLA4 explains why the suppressive activity of VIP-induced Treg is entirely CTLA4 dependent but only partially cell-to-cell contact dependent. Again, signaling through cAMP/PKA appears to be critical for the VIP effects on CTLA4 increase and subsequently on FoxP3 expression. In agreement with this, other cAMP-inducing agents were previously found to increase FoxP3 and CTLA4 expression in CD4 cells (5, 60). Therefore, it is likely that the sustained elevation of cAMP plays a unique role in the generation and regulatory activity of the VIP-induced Treg. Consistent with these findings, elevated intracellular cAMP was identified as a hallmark of Treg (23), and Treg partially exert the suppressive activity by transferring cAMP to effector T cells through gap junctions (8). Therefore, VIP-generated Treg could partially exert their function through this cell-to-cell contact-dependent mechanism.
In summary, we are proposing an alternative strategy for developing Treg immunotherapy by generating human Treg ex vivo from conventional CD4+ CD25− FoxP3− T cells, a method that would be more convenient than expanding natural Treg and more feasible for generating antigen/organ-specific Treg. Understanding precisely how VIP controls immunoregulatory mechanisms will help to further its use in the clinic. For now, we must wait to see whether the inclusion of VIP-generated antigen-specific Treg ex vivo in future therapeutic regimens will be of value. However, some evidence indicates that the findings reported here are of physiological and clinical relevance. For example, the treatment with aerosolized VIP of patients with sarcoidosis, a systemic autoimmune granulomatous disease, significantly increased the number of functional Treg in bronchoalveolar fluid (49). Moreover, patients suffering from rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosis have less circulating VIP or VIP receptor than healthy subjects (2), suggesting a correlation between susceptibility to autoimmune disorders and defective VIP signaling.
Supplementary Material
Acknowledgments
This work was supported by the Spanish Ministry of Science and Innovation, Junta de Andalucia, and Spanish Collaborative Network on Multiple Sclerosis (ISCIII-RETICS-REEM).
Footnotes
Published ahead of print on 15 March 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alleaume, C., A. Eychène, E. Caigneaux, J. M. Muller, and M. Philippe. 2003. Vasoactive intestinal peptide stimulates proliferation in HT29 human colonic adenocarcinoma cells: concomitant activation of Ras/Rap1-B-Raf-ERK signalling pathway. Neuropeptides 37:98-104. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and M. Delgado. 2008. Endogenous anti-inflammatory neuropeptides and proresolving lipid mediators: a new therapeutic approach for immune disorders. J. Cell Mol. Med. 12:1830-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signalling pathway in primary human T cells. J. Immunol. 168:2729-2736. [DOI] [PubMed] [Google Scholar]
- 4.Appleman, L. J., A. Berezovskaya, I. Grass, and V. A. Boussiotis. 2000. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 164:144-151. [DOI] [PubMed] [Google Scholar]
- 5.Baratelli, F., Y. Lin, L. Zhu, S. C. Yang, N. Heuze-Vourc'h, G. Zeng, K. Reckamp, M. Dohadwala, S. Sharma, and S. M. Dubinett. 2005. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175:1483-1490. [DOI] [PubMed] [Google Scholar]
- 6.Blazar, B. R., P. A. Taylor, R. J. Noelle, and D. A. Vallera. 1998. CD4(+) T cells tolerized ex vivo to host alloantigen by anti-CD40 ligand (CD40L:CD154) antibody lose their graft-versus-host disease lethality capacity but retain nominal antigen responses. J. Clin. Invest. 102:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluestone, J. A. 2005. Regulatory T-cell therapy: is it ready for the clinic? Nat. Rev. Immunol. 25:343-349. [DOI] [PubMed] [Google Scholar]
- 8.Bopp, T., C. Becker, and M. Klein. 2007. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 204:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussiotis, V. A., G. J. Freeman, A. Berezovskaya, D. L. Barber, and L. M. Nadler. 1997. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science 278:124-128. [DOI] [PubMed] [Google Scholar]
- 10.Boussiotis, V. A., G. J. Freeman, P. A. Taylor, A. Berezovskaya, I. Grass, B. R. Blazar, and L. M. Nadler. 2000. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6:290-297. [DOI] [PubMed] [Google Scholar]
- 11.Boussiotis, V. A., Z. M. Chen, J. C. Zeller, W. J. Murphy, A. Berezovskaya, S. Narula, M. G. Roncarolo, and B. R. Blazar. 2001. Altered T-cell receptor + CD28-mediated signaling and blocked cell cycle progression in interleukin 10 and transforming growth factor-beta-treated alloreactive T cells that do not induce graft-versus-host disease. Blood 97:565-571. [DOI] [PubMed] [Google Scholar]
- 12.Chambers, C. A., M. S. Kuhns, J. G. Egen, and J. P. Allison. 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19:565-594. [DOI] [PubMed] [Google Scholar]
- 13.Charvet, C., A. J. Canonigo, S. Becart, U. Maurer, A. V. Miletic, W. Swat, M. Deckert, and A. Altman. 2009. Vav1 promotes T cell cycle progression by linking TCR/CD28 costimulation to FOXO1 and p27kip1 expression. J. Immunol. 177:5024-5031. [DOI] [PubMed] [Google Scholar]
- 14.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198:1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado, M., and D. Ganea. 2001. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J. Immunol. 166:1028-1040. [DOI] [PubMed] [Google Scholar]
- 16.Delgado, M., D. Pozo, and D. Ganea. 2004. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 56:249-290. [DOI] [PubMed] [Google Scholar]
- 17.Dumaz, N., and R. Marais. 2003. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 278:29819-29823. [DOI] [PubMed] [Google Scholar]
- 18.Fabre, S., V. Lang, J. Harriague, A. Jobart, T. G. Unterman, A. Trautmann, and G. Bismuth. 2005. Stable activation of phosphatidylinositol 3-kinase in the T cell immunological synapse stimulates Akt signaling to FoxO1 nuclear exclusion and cell growth control. J. Immunol. 174:4161-4171. [DOI] [PubMed] [Google Scholar]
- 19.Fantini, M. C., C. Becker, F. Monteleone, F. Pallone, P. R. Galle, and M. F. Neurath. 2004. Cutting edge: TGF-b induces regulatory phenotype in CD4+CD25− T cells through FoxP3 induction and down-regulation of Smad7. J. Immunol. 172:5149-5153. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Martin, A., E. Gonzalez-Rey, A. Chorny, D. Ganea, and M. Delgado. 2006. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 36:318-326. [DOI] [PubMed] [Google Scholar]
- 21.Friedline, R. H., D. S. Brown, H. Nguyen, H. Kornfeld, J. Lee, Y. Zhang, M. Appleby, S. D. Der, J. Kang, and C. A. Chambers. 2009. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 206:421-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galaktionov, K., C. Jessus, and D. Beach. 1995. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 9:1046-1058. [DOI] [PubMed] [Google Scholar]
- 23.Gavin, M. A., S. R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2002. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 3:33-41. [DOI] [PubMed] [Google Scholar]
- 24.Gomez, J., A. C. Martinez, B. Fernandez, A. Garcia, and A. Rebollo. 1996. Critical role of Ras in the proliferation and prevention of apoptosis mediated by IL-2. J. Immunol. 157:2272-2281. [PubMed] [Google Scholar]
- 25.Gonzalez-Rey, E., A. Chorny, and M. Delgado. 2007. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 7:52-63. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Rey, E., A. Fernandez-Martin, A. Chorny, and M. Delgado. 2006. Vasoactive intestinal peptide induces CD4+,CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum. 54:864-876. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Rey, E., A. Fernandez-Martin, A. Chorny, J. Martin, D. Pozo, D. Ganea, and M. Delgado. 2006. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am. J. Pathol. 168:1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Rey, E., and M. Delgado. 2007. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol. Med. 13:241-251. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey, E., and M. Delgado. 2007. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol. Sci. 28:482-491. [DOI] [PubMed] [Google Scholar]
- 30.Grader-Beck, T., A. A. van Puijenbroek, L. M. Nadler, and V. A. Boussiotis. 2003. cAMP inhibits both Ras and Rap1 activation in primary human T lymphocytes, but only Ras inhibition correlates with blockade of cell cycle progression. Blood 101:998-1006. [DOI] [PubMed] [Google Scholar]
- 31.Heijink, I. H., H. F. Kauffman, D. S. Postma, J. G. de Monchy, and E. Vellenga. 2003. Sensitivity of IL-5 production to the cAMP-dependent pathway in human T cells is reduced by exogenous IL-2 in a phosphoinositide 3-kinase-dependent way. Eur. J. Immunol. 33:2206-2215. [DOI] [PubMed] [Google Scholar]
- 32.Hermann-Kleiter, N., N. Thuille, C. Pfeifhofer, T. Gruber, M. Schäfer, C. Zitt, A. Hatzelmann, C. Schudt, M. Leitges, and G. Baier. 2006. PKCtheta and PKA are antagonistic partners in the NF-AT transactivation pathway of primary mouse CD3+ T lymphocytes. Blood 107:4841-4848. [DOI] [PubMed] [Google Scholar]
- 33.Hleb, M., S. Murphy, E. F. Wagner, N. N. Hanna, N. Sharma, J. Park, X. C. Li, T. B. Strom, J. F. Padbury, Y. T. Tseng, et al. 2005. Evidence for cyclin D3 as a novel target of rapamycin in human T lymphocytes. J. Biol. Chem. 279:31948-31955. [DOI] [PubMed] [Google Scholar]
- 34.Kang, S. M., B. Beverly, A. C. Tran, K. Brorson, R. H. Schwartz, and M. J. Lenardo. 1992. Transactivation by AP-1 is a molecular target of T-cell clonal anergy. Science 257:1134-1138. [DOI] [PubMed] [Google Scholar]
- 35.Kreijveld, E., H. J. Koenen, L. B. Hilbrands, H. J. van Hooff, and I. Joosten. 2008. The immunosuppressive drug FK778 induces regulatory activity in stimulated human CD4+ CD25− T cells. Blood 109:244-252. [DOI] [PubMed] [Google Scholar]
- 36.Levings, M. K., R. Sangregorio, and M. G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, L., W. R. Godfrey, S. B. Porter, Y. Ge, C. H. June, B. R. Blazar, and V. A. Boussiotis. 2005. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood 106:3068-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4:841-855. [DOI] [PubMed] [Google Scholar]
- 39.Mondino, A., C. D. Whaley, D. R. DeSilva, W. Li, M. K. Jenkins, and D. Mueller. 1996. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB and JunB in anergic T helper 1 cells. J. Immunol. 157:2048-2057. [PubMed] [Google Scholar]
- 40.Moon, J. J., and B. H. Nelson. 2001. Phosphatidylinositol 3-kinase potentiates, but does not trigger, T cell proliferation mediated by IL-2 receptor. J. Immunol. 167:2714-2723. [DOI] [PubMed] [Google Scholar]
- 41.Moriggl, R., D. J. Topham, S. Teglund, V. Sexl, C. McKay, D. Wang, A. Hoffmeyer, J. van Deursen, M. Y. Sangster, K. D. Bunting, G. C. Grosveld, and J. N. Ihle. 1999. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10:249-259. [DOI] [PubMed] [Google Scholar]
- 42.Naderi, S., K. B. Gützkow, J. Christoffersen, E. B. Smeland, and H. K. Blomhoff. 2000. cAMP-mediated growth inhibition of lymphoid cells in G1: rapid down-regulation of cyclin D3 at the level of translation. Eur. J. Immunol. 30:1757-1768. [DOI] [PubMed] [Google Scholar]
- 43.Nourse, J., E. Firpo, W. M. Flanagan, S. Coats, K. Polyak, M. H. Lee, J. Massague, G. R. Crabtree, and J. M. Roberts. 1994. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570-573. [DOI] [PubMed] [Google Scholar]
- 44.Ouyang, W., O. Beckett, R. A. Flavell, and M. O. Li. 2009. An essential role of the Forkhead-Box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30:358-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park, S. G., J. Schulze-Luehrman, M. S. Hayden, N. Hashimoto, W. Ogawa, M. Kasuga, and S. Ghosh. 2009. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signalling to induce NF-kappaB and activate T cells. Nat. Immunol. 10:158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng, S. L. 2008. FoxO in the immune system. Oncogene 27:2337-2344. [DOI] [PubMed] [Google Scholar]
- 47.Peters, J. H., L. B. Hilbrands, H. J. Koenen, and I. I. Joosten. 2008. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4+CD25high T cells for immunotherapy. PLoS One 3:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pozo, D., P. Anderson, and E. Gonzalez-Rey. 2009. Induction of alloantigen-specific human T regulatory cells by vasoactive intestinal peptide. J. Immunol. 183:4346-4359. [DOI] [PubMed] [Google Scholar]
- 49.Prasse, A., G. Zissel, N. Lutzen, J. Schupp, R. Schmiedlin, E. Gonzalez-Rey, A. Rensing-Ehl, G. Bacher, V. Cavalli, D. Bevec, M. Delgado, and J. Muller-Quernheim. Inhaled vasoactive intestinal peptide exerts immuno-regulatory effects in sarcoidosis. Am. J. Respir. Crit. Care Med. (in press). [DOI] [PubMed]
- 50.Ramstad, C., V. Sundvold, K. H. Johansen, and T. Lea. 2000. cAMP-dependent protein kinase (PKA) inhibits T cell activation by phosphorylating ser-43 of raf-1 in the MAPK/ERK pathway. Cell Signal. 12:557-563. [DOI] [PubMed] [Google Scholar]
- 51.Romano, D., K. Magalon, A. Ciampini, C. Talet, A. Enjalbert, and C. Gerard. 2003. Differential involvement of the Ras and Rap1 small GTPases in vasoactive intestinal and pituitary adenylyl cyclase activating polypeptides control of the prolactin gene. J. Biol. Chem. 278:51386-51394. [DOI] [PubMed] [Google Scholar]
- 52.Schade, A. E., G. L. Schieven, R. Townsend, A. M. Jankowska, V. Susulic, R. Zhang, H. Szpurka, and J. P. Maciejewski. 2008. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 111:1366-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shevach, E. M., D. Q. Tran, T. S. Davidson, and J. Andersson. 2008. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol. 38:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shevach, E. M. 2006. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25:195-201. [DOI] [PubMed] [Google Scholar]
- 55.Stahl, M., P. F. Dijkers, G. J. Kops, S. M. Lens, P. J. Coffer, B. M. Burgering, and R. H. Medema. 2002. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168:5024-5031. [DOI] [PubMed] [Google Scholar]
- 56.Tamura, T., H. Nakano, H. Nagase, T. Morokata, O. Igarashi, Y. Oshimi, S. Miyazaki, and H. Nariuchi. 1995. Early activation signal transduction pathways of Th1 and Th2 cell clones stimulated with anti-CD3. J. Immunol. 155:4692-4701. [PubMed] [Google Scholar]
- 57.Tran, D. Q., H. Ramsey, and E. M. Shevach. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110:2983-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vang, T., H. Abrahamsen, S. Myklebust, J. Enserink, H. Prydz, T. Mustelin, M. Amarzguioui, and K. Tasken. 2004. Knockdown of C-terminal Src kinase by siRNA mediated RNA interference augments T cell receptor signaling in mature T cells. Eur. J. Immunol. 34:2191-2199. [DOI] [PubMed] [Google Scholar]
- 59.van Oirschot, B. A., M. Stahl, S. M. Lens, and R. H. Medema. 2001. Protein kinase A regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J. Biol. Chem. 276:33854-33860. [DOI] [PubMed] [Google Scholar]
- 60.Vendetti, S., A. Riccomi, A. Sacchi, L. Gatta, C. Pioli, and M. T. De Magistris. 2002. Cyclic adenosine 5′-monophosphate and calcium induce CD152 (CTLA-4) up-regulation in resting CD4+ T lymphocytes. J. Immunol. 169:6231-6235. [DOI] [PubMed] [Google Scholar]
- 61.Vidal, A., and A. Koff. 2000. Cell-cycle inhibitors: three families united by a common cause. Gene 247:1-15. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe, N., M. Gavrieli, J. R. Sedy, J. Yang, F. Fallarino, S. K. Loftin, M. A. Hurchla, N. Zimmerman, J. Sim, X. Zang, T. L. Murphy, J. H. Russell, J. P. Allison, and K. M. Murphy. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4:670-679. [DOI] [PubMed] [Google Scholar]
- 63.Wells, A. D. 2009. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 182:7331-7341. [DOI] [PubMed] [Google Scholar]
- 64.Wells, A. D. 2003. Cell-cycle regulation of T-cell responses: novel approaches to the control of alloimmunity. Immunol. Rev. 196:25-36. [DOI] [PubMed] [Google Scholar]
- 65.Yan, Y., and M. C. Mumby. 1999. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G1 cyclin-dependent kinases. J. Biol. Chem. 274:31917-31924. [DOI] [PubMed] [Google Scholar]
- 66.Zheng, S. B., J. H. Wang, D. Gray, H. Soucier, and D. A. Horwitz. 2004. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J. Immunol. 172:5213-5221. [DOI] [PubMed] [Google Scholar]
- 67.Zheng, S. G., J. H. Wang, W. Stohl, K. S. Kim, J. D. Gray, and D. A. Horwitz. 2006. TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 176:3321-3329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.