Abstract
Sialylated lipids serve as cellular receptors for polyomaviruses. Using pharmacological inhibitors and cell lines derived from knockout mice, we demonstrate that Abl family tyrosine kinases are required for replication of mouse polyomavirus and BK virus, a human polyomavirus associated with allograft failure following kidney transplantation. We show that decreasing Abl family kinase activity results in low levels of cell surface ganglioside receptors for mouse polyomavirus and that inhibition of sialidase activity promotes virion binding in the absence of Abl family kinase activity. These data provide evidence that Abl family kinases reduce ganglioside turnover in the plasma membrane by inhibiting host cell sialidase activity. Thus, Abl family kinases regulate the susceptibility of cells to polyomavirus infection by modulating gangliosides required for viral attachment.
Polyomaviruses (PyVs) are ubiquitous silent pathogens that persist lifelong in a variety of vertebrate hosts, including humans, but that can become opportunistic pathogens in the setting of depressed immune function (21). Nephritis associated with human PyV BK infection is an important cause of dysfunction and loss of kidney transplants (44), and JC virus (JCV) is the etiologic agent for progressive multifocal leukoencephalopathy (PML), a demyelinating disease in AIDS patients and in patients receiving immunotherapies for autoimmune and inflammatory diseases (21) that is usually fatal. Recently, two new human PyVs have been discovered in respiratory tract infections and clonally integrated sequences of another novel PyV have been detected in an aggressive cutaneous malignancy (4). There are currently no effective antiviral therapeutics for PyV infection.
PyVs are nonenveloped double-stranded DNA viruses that bind to cell surface sialylated ganglioside glycolipids and glycoproteins (11, 14-16, 29, 46, 51). Following virion adsorption to host cell receptors, the various PyV family members utilize similar intracellular trafficking pathways during infection. Virions are endocytosed via clathrin- or caveolin-dependent mechanisms, then traffic to endolysosomes before trafficking to the endoplasmic reticulum (ER), where virion disassembly is initiated (32, 39, 40). For mouse PyV (MPyV), the ganglioside GD1a serves as a receptor for viral binding but also for sorting virions from late endosomes and/or lysosomes to the ER (42). Retrotranslocation from the ER to the cytosol results in further disassembly, with the viral protein-minichromosome complex then being transported across nuclear pores to the nucleus, where viral gene transcription, viral DNA replication, and progeny assembly occur.
Host tyrosine kinases have been implicated in PyV infection. Virus uptake activates protein tyrosine kinase(s) and induces a transient reorganization of the actin network (15, 17, 40). Experiments with the relatively nonspecific inhibitor genistein suggest that tyrosine kinases are required for internalization of simian virus 40 (SV40) PyV and for development of “actin tails” associated with vesicle-encased virions (40). In addition to SV40, genistein has also been shown to inhibit entry by JCV and BK virus (BKV) (12, 43), suggesting a role for tyrosine kinases in entry of many species of PyVs. However, little information regarding which tyrosine kinases mediate entry is available. Finally, the middle T (MT) antigen of MPyV is an integral membrane protein that binds to and is phosphorylated by Src family kinases, (9) thereby creating docking sites for SH2 domain-containing enzymes and adaptor proteins (18).
Abl family tyrosine kinases, which include Abl1 and Abl2, regulate cytoskeletal and trafficking functions in cells (50). Abl family kinases are mutated in human cancers such as chronic myelogenous leukemia (CML), and drugs such as STI-571 (imatinib mesylate; Gleevec), which inhibit Abl family kinases, have been developed as cancer therapeutics (19). Abl family kinases are also involved in the life cycles of several viral and bacterial pathogens (2). For example, Abl family tyrosine kinases contribute to entry into mammalian cells of Pseudomonas aeruginosa, Shigella flexneri, and Chlamydia trachomatis (5, 13, 41), although the precise mechanisms remain unclear. In addition, Abl family kinases mediate formation of actin-filled membranous protrusions, which are required for motility of pathogens such as poxviruses and enteropathogenic Escherichia coli on and between cells (45, 52), facilitating the spread of the infection. Our recent studies demonstrate that STI-571 can be used both as a prophylactic and as a therapeutic for orthopoxvirus infections (45; P. Reeves and D. Kalman, unpublished data).
The requirement for Abl family kinases in pathogenesis of diverse microbes led us to investigate their potential role in PyV infection. Using cell lines lacking Abl family kinases, together with the specific Abl inhibitor STI-571, we report here that Abl family kinases control the levels of ganglioside receptors in the plasma membrane by negatively regulating a plasma membrane sialidase.
MATERIALS AND METHODS
Cell culture, antibodies, and reagents.
3T3 cells and 3T3 cells derived from Abl1−/− Abl2−/− mice (25) were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) as previously described (52). For BKV infections, primary human renal proximal tubule epithelial (RPTE) cells were cultured as described previously (22). Antibodies used were as follows: T-antigen (TAg) monoclonal antibody (MAb) F4, which recognizes all three MPyV TAg proteins; large T (LT), middle T (MT), and small T (ST) (38) for Western analysis of TAg production; polyclonal rat anti-TAg ascites fluid (kindly provided by T. L. Benjamin, Harvard Medical School) for immunofluorescent detection of MPyV LT+ nuclei; polyclonal antibody PAb416 for BKV TAg detection (20); and rat VP1 MAb for neutralization of extracellular virus and immunofluorescent detection of MPyV virions. VP1 MAb was generated by immunizing rats with MPyV VP1 virus-like particles provided by R. Garcia (University of Colorado, Denver, CO), and anti-GD1a and anti-GT1b (Millipore) were used for analysis of cell surface gangliosides. The salt of the Abl family kinase inhibitor STI-571 was synthesized as described previously (48) and was used at 20 μM unless otherwise indicated. Sialidase inhibitor 2,3-didehydro-2-deoxy-N-acetylneuraminic acid (DANA) was purchased from Sigma and was used at 100 μM unless otherwise indicated. Due to instability, a new DANA stock solution was prepared just prior to each experiment. Exogenous gangliosides GD1a, GD1b, GT1b, and GM1 were purchased from Matreya. Biotinylated Sambucus nigra lectin (SNA) and Maackia amurensis lectin II (MAL II) were purchased from Vector Labs.
Infections and drug treatments.
Virus stocks of MPyV strain A2 or purified BKV strain Dunlop were prepared and titered as previously described (22, 31). For MPyV infections, cells were incubated with virus at a multiplicity of infection (MOI) of 5 PFU/cell for 1 h at 37°C. Virus was removed, and extracellular virions were neutralized by the addition of the VP1 MAb in DMEM-2% calf serum for 30 min at 37°C. Following neutralization of extracellular virions, the medium was exchanged with DMEM-10% FBS, and internalized virus was allowed to replicate for 24 h. For BKV infections, RPTE cells were infected with purified BKV at a MOI of 0.5 infectious unit (IU)/cell as previously described (22) and allowed to replicate for 48 h.
For pretreatment with STI-571 and/or DANA, cells were incubated in medium containing the drug for 16 to 24 h prior to infection. Drug-containing medium was removed, and cells were washed prior to addition of virus. Drug added during viral adsorption was added directly to the viral lysate for the 1-h incubation period and was removed prior to neutralization of extracellular virions. Drug added posttreatment was added to medium only after the neutralization of extracellular virions and was maintained for the subsequent infection period. Previous reports suggest that inhibitors of Abl family kinases act within minutes after addition to tissue culture media and are effective for days without replenishment (3, 10, 45, 52). Following the various treatments, the level of infection was determined by assaying for TAg production in infected cells by immunofluorescence microscopy or Western analysis as described below.
TAg analysis and quantitation.
TAg production was assessed by both Western blotting and immunofluorescence microscopy. For Western analysis of MPyV-infected cells, samples were lysed and processed as previously described (30) and probed with F4 antibody. Equivalent loading of samples was verified by detection of a non-MPyV-related protein that is nonspecifically recognized by the F4 antibody and that is present in equal amounts in all samples, both infected and uninfected. For BKV-infected cells, total cellular protein was harvested for Western analysis as previously described (22) and data were quantified using a Typhoon imaging system and the ImageQuant software (GE Healthcare). A cell metabolism WST-1 assay was performed according to the manufacturer's instructions (cell proliferation reagent WST-1; Roche Applied Sciences) to measure the cytotoxicity of STI-571.
For immunofluorescence detection of TAg following MPyV infection, cells plated on glass coverslips were fixed and stained as described elsewhere (23) using a MAb to LT to detect LT+ nuclei. Images were acquired with a scientific-grade cooled charge-coupled device (Cool-Snap HQ with ORCA-ER chip) on a multiwavelength, wide-field, three-dimensional microscopy system (Intelligent Imaging Innovations, Denver, CO), based on a 200 M inverted microscope (Carl Zeiss, Thornwood, NY). For each condition or experiment, image data were collected on the same day using identical exposure times. LT+ nuclei were counted, as were total nuclei, as determined by DAPI (4≪,6-diamidino-2-phenylindole) staining, and data are presented as the percentage of total nuclei counted that were LT+. Duplicate samples were counted for each condition, a minimum of 300 nuclei per coverslip per experiment. On average, approximately 35% of total nuclei were TAg positive per coverslip under control conditions. In all cases, data were normalized to represent the percentage of TAg-positive nuclei in control 3T3 cells as 100%.
Cell surface GD1a analysis.
The cell surface GD1a ganglioside was visualized using GD1a MAb and immunofluorescence microscopy as previously described (49). Briefly, cells were incubated with GD1a antibody (1:250 dilution) in Hanks balanced salt solution (HBSS) and 3% bovine serum albumin (BSA) for 30 min at 37°C prior to fixation. Cells were then washed and fixed, and subsequent fluorescent staining was performed under nonpermeabilizing conditions to recognize only the cell surface ganglioside. Images were acquired as described for TAg analysis, and expression of GD1a was quantitated using the MASK function in the Intelligent Imaging Innovations software package. Details regarding use of the MASK function to determine the quantitation of colocalization of overlapping fluorescent signals have been described elsewhere (53). Briefly, eight images were taken per slip, under identical acquisition parameters, and intensity and contrast parameters were adjusted to be equivalent across all images. Next, the area of GD1a-positive signal that overlapped with actin cytoskeletal staining, a representation of total cell surface area, was calculated for each image. Data are presented as the percentage of total cell surface area (in μm2) that was GD1a positive. Duplicate coverslips were assessed for each condition. In all cases, data were normalized such that the percentage of GD1a expression in control 3T3 cells was represented as 100%.
To verify the specificity of anti-GD1a staining, 3T3 cells were treated prior to addition of antibody with sialidase, which cleaves terminal sialic acid moieties, thus converting GD1a to GM1, which is not recognized by the GD1a antibody. 3T3 cells treated with neuraminidase no longer showed any signal following staining with anti-GD1a (data not shown). In addition, 3T3 cells supplemented with exogenous ganglioside GD1a (the method is described below) showed an ∼2-fold increase in GD1a-positive surface area compared to mock-treated 3T3 cells, whereas GM1-supplemented cells showed no increase in GD1a staining compared to mock-treated cells. Taken together, these data confirm the specificity of the GD1a antibody for ganglioside GD1a.
MPyV binding assay.
Cells were incubated with virus in HEPES buffered medium for 30 min at 4°C to allow viral binding but inhibit endocytosis. Following binding, cells were washed extensively with cold phosphate-buffered saline (PBS) to remove unbound virions and fixed at 4°C. Cells were subsequently stained with VP1 MAb under nonpermeabilizing conditions to detect bound virions on the surface of the cells and assessed by immunofluorescence microscopy.
Supplementation with exogenous gangliosides.
Cells were supplemented with exogenous gangliosides at 50 μg/ml for 16 to 24 h in serum-free DMEM. Unincorporated ganglioside was removed by washing extensively with DMEM-20% FBS prior to analysis or infection.
Flow cytometry and lectin analysis.
Cells were stained with biotinylated lectins (either MAL II or SNA at 0.1 μg/ml) and streptavidin-allophycocyanin (APC)-conjugated tetramers as previously described (24). Samples were immediately acquired on a FACSCalibur (BD Biosciences), and data were analyzed using CellQuest software (BD Biosciences).
RESULTS
Abl family kinases are necessary for PyV infection.
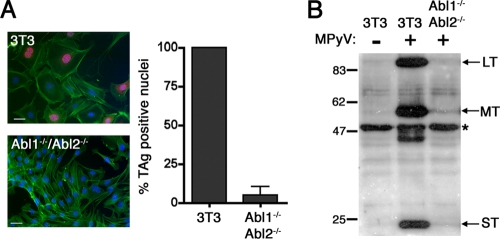
Abl family tyrosine kinases have recently been found to play a crucial role in a wide variety of viral and bacterial infections (2). To determine whether Abl family kinases are involved in MPyV infection, we utilized cell lines derived from mice lacking both c-Abl1 and c-Abl2 (Abl1−/− Abl2−/−). At 24 h postinfection, indirect immunofluorescence microscopy showed that ∼10-fold fewer Abl1−/− Abl2−/− cells had LT+ nuclei than did wild-type 3T3 cells (Fig. 1A). This difference in levels of T-antigen expression was confirmed by Western analysis (Fig. 1B). Bands representing the three T-antigen proteins LT, MT, and ST were barely detectable in infected Abl1−/− Abl2−/− cells (Fig. 1B). This dramatic decrease in T-antigen production in infected Abl1−/− Abl2−/− fibroblasts suggests that Abl family kinases play a role in MPyV infection. Using 3T3 cells derived from mice lacking either c-Abl1 or c-Abl2 individually, we found that either of these Abl family kinases is sufficient to support MPyV infection (data not shown). These findings indicate that Abl1 and Abl2 act redundantly to support MPyV infection.
FIG. 1.
Abl1−/− Abl2−/− cells are nonpermissive to infection with MPyV. (A) Immunofluorescence images and quantitation of LT+ nuclei in 3T3 cells and Abl1−/− Abl2−/− cells infected with MPyV and assessed for TAg production 24 h postinfection. Cells are stained with TAg antibody (Ab) to visualize infected cells (red), DAPI to visualize cell nuclei (blue), and Alexa-488-phalloidin to recognize actin (green). Scale bars, 50 μm. Quantitation of LT+ nuclei was determined as outlined in Materials and Methods, and all values are normalized to infected 3T3 control cells as 100%. (B) Western analysis of 3T3 cells and Abl1−/− Abl2−/− cells infected with MPyV or mock infected and assessed for T-antigen proteins LT, MT, and ST at 24 h postinfection. Equivalent loading of samples was verified by detection of a non-PyV-specific band (*), present in all samples, that was recognized by the F4 TAg Ab.
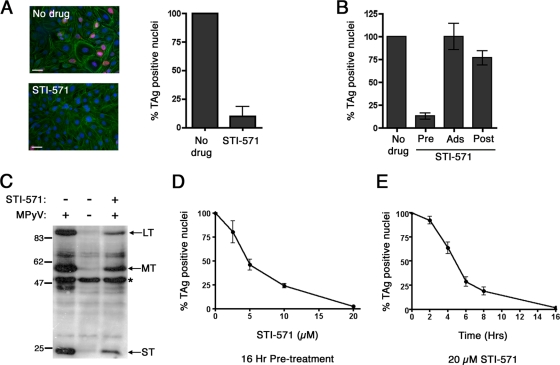
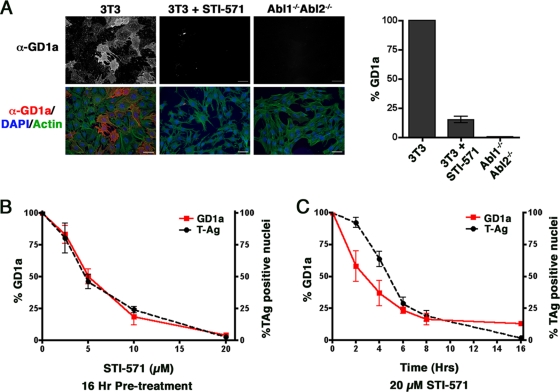
To confirm these results, we next assessed the effect of STI-571, an inhibitor of Abl family kinases, on MPyV infection. Treatment of 3T3 cells with STI-571 16 h before and throughout the 24-h infection period decreased numbers of LT-expressing cells ∼10-fold compared to numbers of mock-treated 3T3 cells (Fig. 2A). Similar effects were evident in primary baby mouse kidney epithelial cells (data not shown). To determine whether Abl family kinases act during early or late steps in PyV infection, 3T3 cells were pretreated with STI-571 for 16 h prior to adsorption of virus, only during the 1-h adsorption period, or only after removal of the viral lysate. As shown in Fig. 2B, the infectivity of MPyV was decreased only in those cells exposed to STI-571 using the pretreatment protocol compared to that in mock-treated control cells. Western analysis of pretreated cells confirmed this result (Fig. 2C). By contrast, STI-571 did not effectively inhibit MPyV infection when added to cells during or after virion adsorption.
FIG. 2.
3T3 cells pretreated with STI-571 are less permissive to infection by MPyV. (A) Immunofluorescence images and quantitation of LT+ nuclei in 3T3 cells continuously exposed to 20 μM STI-571 for 16 h before infection with MPyV and fixed 24 h after infection. Cells are stained as described for Fig. 1. Scale bars, 50 μm. (B) Quantitation of LT+ nuclei in cells pretreated with STI-571 for 16 h prior to adsorption of virus (Pre), only during viral adsorption (Ads), or only during the 24-h infection period following removal of the viral lysate (Post). (C) Western analysis of 3T3 cells pretreated with STI-571 for 16 h prior to infection or left untreated and assessed for TAg production 24 h postinfection. Equivalent loading of samples was verified by detection of a non-PyV-specific band (*), present in all samples, that was recognized by the F4 TAg Ab. (D) Quantitation of LT+ nuclei in 3T3 cells pretreated for 16 h with increasing concentrations of STI-571 (0 to 20 μM) prior to infection with MPyV. (E) Quantitation of LT+ nuclei in 3T3 cells pretreated with 20 μM STI-571 for increasing periods of time (0 to 16 h) prior to infection with MPyV. Quantitation of LT+ nuclei in panels A, B, D, and E was determined as outlined in Materials and Methods, and all values are normalized to infected 3T3 control cells as 100%.
Using the 16-h pretreatment regimen, we next determined the concentration of STI-571 required to reduce MPyV infection by 50% (IC50). As shown in Fig. 2D, there was an inverse correlation between STI-571 concentration and frequency of LT+ cells, with an IC50 of ∼5 μM. We next determined the duration of pretreatment required to reduce the percentage of LT+ cells by 50% (t1/2) at an STI-571 concentration of 20 μM (Fig. 2E). Although infectivity for MPyV decreased after 2 to 4 h of pretreatment, with a t1/2 of ∼5 h, at least 8 h of pretreatment was required to reduce the percentage of LT+ cells by 75% compared to mock-treated cells. We could find no evidence of toxicity upon treatment of 3T3 cells or other cell types (e.g., RPTE cells [see Fig. 7]) with STI-571 at concentrations as high as 40 μM. Taken together, these data indicate that Abl family kinases affect a cellular process(es) involved in early steps of MPyV infection.
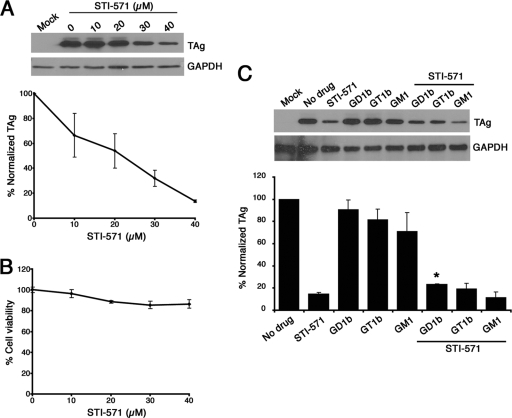
FIG. 7.
Inhibition of Abl family kinases decreases BKV infectivity. (A) Pretreatment with STI-571 blocks BKV infection. Western analysis and quantitation of RPTE cells pretreated with STI-571 for 24 h before infection with BKV. Total protein lysates were prepared at 48 h postinfection and probed for TAg and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) by Western analysis. Quantitation of TAg expression was normalized to GAPDH levels and represents three independent experiments. The no-drug control was set to 100%. (B) Cytotoxicity of STI-571. RPTE cells were treated with STI-571 at the indicated concentrations for 24 h, and a WST-1 assay was performed to measure cell viability. Data were normalized to the no-drug control. (C) GD1b partially rescued the effects of STI-571 on BKV infection. Shown are Western analysis and quantitation of RPTE cells treated with 50 μg/ml of individual ganglioside alone or with 40 μM STI-571 as indicated and then infected with BKV. Quantitation of TAg expression was normalized to GAPDH levels and represents three independent experiments. *, P < 0.001 compared to the STI-571-treated samples.
Abl family kinases regulate binding of MPyV virions to cells.
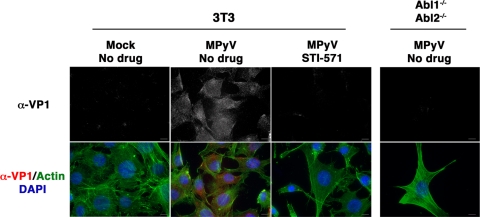
We next determined whether Abl family kinases affected virion adsorption to cells. To do this, we utilized indirect immunofluorescence microscopy together with a monoclonal antibody against VP1, the major PyV capsid protein, to visualize virions at the membrane surface immediately following binding. Cells were held at 4°C during adsorption to inhibit viral uptake and then fixed and stained with VP1 MAb to detect surface virions. As shown in Fig. 3, diffuse VP1 staining was evident on the surfaces of infected, unpermeabilized 3T3 cells. In contrast, little VP1 staining was detected at the surface in either Abl1−/− Abl2−/− cells or 3T3 cells pretreated with STI-571. Together, these data indicate that Abl family kinases are required for binding of virions to the plasma membrane.
FIG. 3.
Reduced binding of MPyV in cells lacking Abl family kinase activity. (A) Immunofluorescence images of MPyV binding to 3T3 cells, 3T3 cells pretreated with 20 μM STI-571, and Abl1−/− Abl2−/− cells. Cells were incubated with virus for 30 min at 4°C or mock infected, and bound virus was visualized with VP1 Ab under nonpermeabilizing conditions. (Top row) Anti-VP1 (α-VP1) staining alone; (bottom row) merged images of the same cells also stained with DAPI to visualize cell nuclei (blue) and Alexa-488-phalloidin to recognize actin (green). Scale bars, 10 μm.
Abl family kinases regulate cell surface levels of ganglioside GD1a.
We next assessed cell surface levels for the two known MPyV receptors, the gangliosides GD1a and GT1b, by indirect immunofluorescence microscopy. In uninfected wild-type 3T3 cells, no signal above background was evident upon staining with anti-GT1b, indicating that these cells likely do not express this ganglioside at detectable levels (data not shown). Although anti-GD1a staining was heterogeneous (Fig. 4A), on average GD1a was detectable on ∼30% of the total cell surface area of 3T3 cell monolayers (normalized to 100% in the graph in Fig. 4A). By contrast, little or no GD1a was expressed by 3T3 cells pretreated with STI-571 or by Abl1−/− Abl2−/− cells (Fig. 4A). To determine the IC50 of STI-571 for GD1a loss, 3T3 cells were exposed to increasing concentrations of STI-571, from 0 to 20 μM, for 16 h and assessed for level of reduction in cell surface GD1a. Notably, the resulting dose-response curve (Fig. 4B, red) nearly matched that generated for T-antigen production in infected 3T3 cells after STI-571 pretreatment (Fig. 4B, black [data reproduced from Fig. 2D]), with an IC50 of ∼5 μM calculated for each curve. Similar profiles of GD1a (Fig. 4C, red) and T-antigen expression (Fig. 4C, black [reproduced from Fig. 2E]) were seen when the duration of STI-571 pretreatment was varied. It is also interesting to note that the loss of surface GD1a induced by STI-571 slightly preceded the decrease in T-antigen production (t1/2s of 3 h for GD1a and 5 h for T antigen).
FIG. 4.
Abl kinase-deficient cells have reduced levels of plasma membrane-associated GD1a. (A) Immunofluorescence images and quantitation of cell surface GD1a expression in 3T3 cells, 3T3 cells pretreated with 20 μM STI-571, and Abl1−/− Abl2−/− cells. Cells were stained with GD1a Ab under nonpermeabilizing conditions. (Top row) Anti-GD1a (α-GD1a) staining alone; (bottom row) merged images of the same cells also stained with DAPI to visualize cell nuclei (blue) and Alexa-488-phalloidin to recognize actin (green). Scale bars, 50 μm. (B) Quantitation of cell surface GD1a in 3T3 cells pretreated for 16 h with increasing concentrations of STI-571 (0 to 20 μM) prior to infection with MPyV. TAg production under the same conditions (reproduced from Fig. 2D) is included for reference. (C) Quantitation of cell surface GD1a in 3T3 cells pretreated with 20 μM STI-571 for increasing periods of time (0 to 16 h) prior to infection with MPyV. TAg production under the same conditions (reproduced from Fig. 2E) is included for reference. In all cases (A to C), quantitation of GD1a expression was determined as a percentage of total cell surface area as outlined in Materials and Methods and all values are normalized to infected 3T3 control cells as 100%.
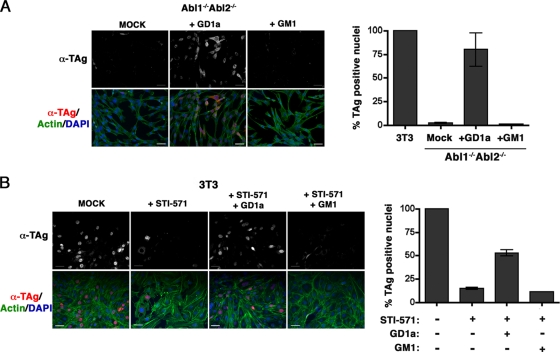
Exogenous GD1a renders Abl kinase-deficient cells susceptible to MPyV infection.
Exogenous gangliosides have been shown to restore infectibility to cell lines lacking the endogenous PyV ganglioside receptor (15, 16, 29, 51). Thus, we asked whether exogenous gangliosides would render Abl1−/− Abl2−/− cells or STI-571-treated wild-type 3T3 cells infectible by MPyV. Abl1−/− Abl2−/− cells or 3T3 cells pretreated with STI-571 were exposed for 16 h to GD1a or, as a negative control, to GM1 and infected with MPyV. Abl1−/− Abl2−/− cells treated with exogenous GD1a supported MPyV infection to almost the same level as 3T3 cells (Fig. 5A). In addition, exogenous GD1a partially restored the infectibility of 3T3 cells pretreated with STI-571 (Fig. 5B). Taken together, these data indicate that Abl1−/− Abl2−/− cells are not intrinsically nonpermissive for MPyV infection and that GD1a is sufficient to restore the susceptibility to infection by MPyV of cells lacking Abl family kinase activity.
FIG. 5.
Exogenous GD1a renders Abl kinase-deficient cells susceptible to MPyV infection. (A) Immunofluorescence images and quantitation of LT+ nuclei in Abl1−/− Abl2−/− cells supplemented with exogenous gangliosides and infected with MPyV. (B) Immunofluorescence images and quantitation of LT+ nuclei in 3T3 cells supplemented with exogenous gangliosides, pretreated with 20 μM STI-571 for 16 h, and infected with MPyV. (Top rows [A and B]) Anti-TAg (α-TAg) staining alone; (bottom rows) merged images of the same cells also stained with DAPI to visualize cell nuclei (blue) and Alexa-488-phalloidin to recognize actin (green). Quantitation of LT+ nuclei (A and B) is normalized to infected 3T3 control cells as 100%. Scale bars (all images), 50 μm.
Abl family kinases regulate cell surface sialidase activity.
Primate, murine, and human PyVs bind to receptors with a terminal sialic acid(s) linked to galactose (37). The sialylation state of plasma membrane glycolipids and glycoproteins is regulated by sialyltransferases within the Golgi apparatus that add sialic acid moieties to precursors and by sialidases in the plasma membrane, lysosome, and cytosol that remove them (33-35, 55, 56). We hypothesized that Abl family kinases might negatively regulate one or more cellular sialidases and thereby control plasma membrane levels of gangliosides. Alternatively, Abl family kinases may activate sialyltransferases. To distinguish between these alternatives, we first assessed whether Abl family kinases regulate sialyltransferases. To assess cell surface sialylation, cells were stained with fluorescently labeled Maackia amurensis lectin II (MAL II) or Sambucus nigra lectin (SNA) and analyzed by flow cytometry. MAL II and SNA recognize α-2,3- and α-2,6-sialic acid linkages, respectively, on both gangliosides and glycoproteins. No significant differences in lectin binding between Abl1−/− Abl2−/− cells and control cells, or between wild-type cells incubated with or without STI-571 for 24 h, were evident (data not shown). Moreover, pretreatment of MDCK cells with STI-571 had no detectable effects on infectivity of influenza virus H1N1 (K. Bradley, D. Steinhauer, and D. Kalman, unpublished data), which binds to sialic acid residues on glycoproteins (7). Together, these data suggest that sialyltransferase activity is not regulated by Abl family kinases.
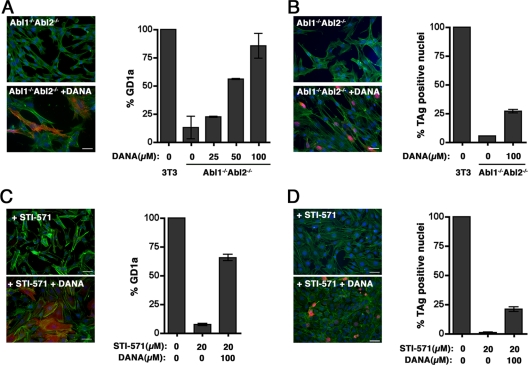
We next assessed whether inhibition of plasma membrane sialidase activity was sufficient to restore GD1a levels in wild-type cells treated with STI-571 or in Abl1−/− Abl2−/− cells. Plasma membrane sialidase preferentially hydrolyzes gangliosides and demonstrates minimal activity against glycoproteins and oligosaccharides (35). 2,3-Didehydro-2-deoxy-N-acetylneuraminic acid (DANA) is a relatively cell-impermeable sialic acid transition state analog that primarily inhibits the activity of plasma membrane sialidase when added to intact cells in culture (26). Exposure of Abl1−/− Abl2−/− cells to DANA increased expression of cell surface GD1a to nearly wild-type levels (Fig. 6A) and increased infectibility by ∼4-fold compared to expression by mock-treated cells (Fig. 6B). The effect was concentration dependent. Notably, although infectibility was restored with DANA, the percentage of LT+ cells was only 25% of that seen in 3T3 cells. Pretreatment of 3T3 cells with 100 μM DANA alone did not lead to any significant increase in either GD1a expression or infectibility over those for untreated cells (data not shown); however, pretreatment of 3T3 cells with DANA and STI-571 together resulted in levels of GD1a on the cell surface that were ∼6-fold higher than seen on cells pretreated with STI-571 alone (Fig. 6C). Moreover, cells exposed to both DANA and STI-571 exhibited ∼7-fold-higher susceptibility to infection by MPyV than cells pretreated with STI-571 alone (Fig. 6D). However, as with Abl1−/− Abl2−/− cells, susceptibility to infection by PyV was only partially restored compared to that of wild-type 3T3 cells. Because treatment with DANA restored plasma membrane GD1a in Abl1−/− Abl2−/− cells and STI-571-treated 3T3 cells to levels similar to those seen in 3T3 cells, these data demonstrate that Abl family kinases regulate plasma membrane sialidase activity. Notably, the ability of Abl1−/− Abl2−/− cells to express cell surface GD1a following inhibition of plasma membrane sialidase offers further evidence that Abl kinases do not regulate sialyltransferase activity.
FIG. 6.
Inhibition of cell surface sialidase activity in Abl kinase-deficient cells restores surface expression of ganglioside GD1a and increases susceptibility to infection by MPyV. (A) Immunofluorescence images and quantitation of cell surface GD1a in Abl1−/− Abl2−/− cells pretreated with DANA for 16 h. Quantitation represents the increase in cell surface GD1a expression following pretreatment with increasing concentrations of DANA (0 to 100 μM) in Abl1−/− Abl2−/− cells compared to untreated control 3T3 cells. (B) Immunofluorescence images and quantitation of LT+ nuclei in Abl1−/− Abl2−/− cells pretreated with DANA for 16 h prior to infection with MPyV. (C) Immunofluorescence images and quantitation of cell surface GD1a in 3T3 cells pretreated with STI-571 and DANA for 16 h. (D) Immunofluorescence images and quantitation of LT+ nuclei in 3T3 cells pretreated with STI-571 and DANA for 16 h prior to infection with MPyV. Scale bars (all images), 50 μm. Quantitation of GD1a and LT+ nuclei is normalized to infected 3T3 control cells as 100%.
Abl family kinases mediate BKV infectivity.
To test relevance to a human pathogen, we next asked whether Abl family kinases regulate the susceptibility to BKV infection of primary human renal proximal tubule epithelial (RTPE) cells. BKV is known to use gangliosides GD1b and GT1b, both of which contain an α-2,8-linked disialic acid motif, as cellular receptors (29). Expression of BKV LT was reduced by ∼85% upon pretreatment of RPTE cells with up to 40 μM STI-571 (Fig. 7A) without cytotoxic effects (Fig. 7B). Addition of GD1b, but not GT1b or GM1, restored the infectibility of STI-571-treated cells to a limited, but statistically significant extent (up to 2-fold) (Fig. 7C). These data suggest that regulation of ganglioside receptors by Abl family kinases occurs in human cells and can control infection by human PyVs.
DISCUSSION
Human PyV infections are associated with kidney transplant rejection and PML, a demyelinating disease seen in patients immunocompromised by HIV/AIDS or by agents that block T-cell entry into the central nervous system (CNS). The substantial morbidity and mortality of these PyV-associated diseases highlight the pressing need for effective therapeutic strategies. In this study, we provide evidence that Abl family tyrosine kinases modulate levels of cell surface gangliosides, sialylated glycolipids that serve as receptors for PyV. Abl family tyrosine kinases have been implicated in regulating the life cycles of a number of bacterial and viral pathogens, raising the possibility that inhibitors of Abl family kinases (e.g., STI-571) may prove effective in treating PyV-associated diseases in humans.
Gangliosides are amphipathic glycosphingolipids that consist of a ceramide anchor linked to an oligosaccharide chain of variable length and are distinguished by the presence of one or more sialic acid residues (1). The levels and diversity of gangliosides on the cell surface reflect a complex balance between ganglioside biosynthesis and degradation. Ganglioside biosynthesis is regulated by glycosyltransferases and sialyltransferases in the Golgi apparatus and ER, which sequentially add monosaccharides and sialic acids to ceramide to create continuously more complex ganglioside species. Ganglioside degradation occurs by endocytosis and lysosomal degradation, as well as by the catalytic removal of sialic acids by sialidases within the plasma membrane, converting membrane-bound multisialogangliosides, such as GD1a, to less-complex monosialo- or asialogangliosides, such as GM1 (56). Our observations showing that cells lacking Abl1 and Abl2, which do not express GD1a on their surfaces, can be made to do so when sialidase activity is inhibited with DANA (Fig. 6A) suggest that Abl family kinases do not affect ganglioside biosynthesis pathways and likely do not regulate sialyltransferases, which sialylate both glycoproteins and glycolipids (54). The lack of any changes in lectin binding in cells lacking Abl family kinase activity (this report) and the inability of STI-571 to block influenza virus infection (data not shown) also support this conclusion. The data presented here provide evidence that Abl family kinases regulate cell surface ganglioside levels by controlling ganglioside-specific sialidase activity at the plasma membrane. Although a connection between Abl family kinases and regulation of sialidases has not previously been identified, our model is consistent with known activities of gangliosides and Abl family kinases in other systems. For example, inhibition of Abl family kinase activity in BCR-ABL-overexpressing leukemic cells results in increased cell surface expression of ganglioside GM1 (6), the direct downstream product of GD1a desialylation. GM1 is not a substrate for plasma membrane sialidase and therefore can accumulate in some tissues and cells as a result of upregulated sialidase activity at the plasma membrane (27, 47).
To date, four mammalian cell sialidases have been described (designated Neu1, Neu2, Neu3, and Neu4), each having a different subcellular locale and substrate specificity (36). Neu1 and Neu4 are both associated with the lysosome, while Neu2 is localized in the cytoplasm. All three of these enzymes have broad substrate specificity and can desialylate both glycoproteins and gangliosides. By contrast, Neu3 localizes to lipid microdomains in the plasma membrane and has been shown to act specifically on gangliosides, with the exception of GM1 and GM2, while showing little or no activity against glycoproteins (34). Our observation that DANA, which is cell impermeable, restores cell surface GD1a and susceptibility to MPyV infection of STI-571-treated cells and to Abl1−/− Abl2−/− cells without altering global cell surface sialylation of proteins, raises the possibility that Neu3 is a direct or indirect target of Abl family kinases.
Several lines of evidence suggest that different PyV family members utilize distinct pathways to infect host cells. For example, SV40 and BKV transit through the caveosome, a specialized pH-neutral endosomal compartment, before trafficking to the ER (8), whereas endocytosed MPyV virions are transported directly to the ER (28). Because gangliosides are involved in virion trafficking to the ER (42), it remains possible that Abl family kinases regulate sialylation of gangliosides following virion uptake. In this regard, our preliminary data suggest that levels of LT were reduced by STI-571 added postinfection to RPTE cells infected with BKV or macrophages infected with MPyV (data not shown). These data raise the possibility that Abl family kinases mediate additional, postadsorption steps in PyV infection in a cell-type-dependent manner.
The question arises as to whether inhibitors of Abl family kinases such as STI-571 will prove useful as therapeutics for human PyVs. STI-571 has somewhat limited side effects, though some cardiotoxicity has been reported with long-term administration. Moreover, STI-571 does not have significant immunotoxicity, and it has been used both prophylactically and in a therapeutic context against poxvirus infections in mice (45; Reeves and Kalman, unpublished). However, it is unlikely that STI-571 will downregulate all ganglioside receptors on all cell types. In preliminary experiments, we have not detected significant reduction in viral load in mice acutely infected by MPyV (data not shown). However, in situations where viral infection is low level, as in the persistent phase of PyV infection, a decrease in ganglioside receptor availability may confer resistance to permissive host cells. Moreover, the capacity of STI-571 to act at a step following adsorption makes it a promising candidate therapeutic for BKV infection of kidney epithelial cells, a major host cell target in BKV-associated nephropathy in transplant patients.
Acknowledgments
We thank Patrick Reeves and Bettina Bommarius for helpful discussions, Annette Hadley for technical assistance, Tony Koleske for Abl−/− Arg−/− cells, and T. L. Benjamin for rat anti-LT ascites fluid.
This work was supported by grants R21AI073317 (to A.E.L. and D.K.), R01AI056067 (to D.K.), R01CA71971 (to A.E.L.), and R01AI060584 (to M.J.I.) and American Heart Association Postdoctoral Fellowship 0825806G (to M.J.).
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Allende, M. L., and R. L. Proia. 2002. Lubricating cell signaling pathways with gangliosides. Curr. Opin. Struct. Biol. 12:587-592. [DOI] [PubMed] [Google Scholar]
- 2.Backert, S., S. M. Feller, and S. Wessler. 2008. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem. Sci. 33:80-90. [DOI] [PubMed] [Google Scholar]
- 3.Bommarius, B., D. Maxwell, A. Swimm, S. Leung, A. Corbett, W. Bornmann, and D. Kalman. 2007. Enteropathogenic Escherichia coli Tir is an SH2/3 ligand that recruits and activates tyrosine kinases required for pedestal formation. Mol. Microbiol. 63:1748-1768. [DOI] [PubMed] [Google Scholar]
- 4.Boothpur, R., and D. C. Brennan. 6 January 2010, posting date. Human polyoma viruses and disease with emphasis on clinical BK and JC. J. Clin. Virol. doi: 10.1016/j.jcv.2009.12.006. [DOI] [PMC free article] [PubMed]
- 5.Burton, E. A., R. Plattner, and A. M. Pendergast. 2003. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 22:5471-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cebo, C., S. Da Rocha, S. Wittnebel, A. G. Turhan, J. Abdelali, S. Caillat-Zucman, J. H. Bourhis, S. Chouaib, and A. Caignard. 2006. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J. Immunol. 176:864-872. [DOI] [PubMed] [Google Scholar]
- 7.Chu, V. C., and G. R. Whittaker. 2004. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 101:18153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilworth, S. M. 2002. Polyoma virus middle T antigen and its role in identifying cancer-related molecules. Nat. Rev. Cancer 2:951-956. [DOI] [PubMed] [Google Scholar]
- 10.Druker, B. J., S. Tamura, E. Buchdunger, S. Ohno, G. M. Segal, S. Fanning, J. Zimmermann, and N. B. Lydon. 1996. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2:561-566. [DOI] [PubMed] [Google Scholar]
- 11.Dugan, A. S., S. Eash, and W. J. Atwood. 2005. An N-linked glycoprotein with alpha(2,3)-linked sialic acid is a receptor for BK virus. J. Virol. 79:14442-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eash, S., W. Querbes, and W. J. Atwood. 2004. Infection of Vero cells by BK virus is dependent on caveolae. J. Virol. 78:11583-11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwell, C. A., A. Ceesay, J. H. Kim, D. Kalman, and J. N. Engel. 2008. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 4:e1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Focosi, D., R. E. Kast, F. Maggi, L. Ceccherini-Nelli, and M. Petrini. 2008. Sialic acid moieties and 5-HT2a: two faces of the same receptor for JC virus? J. Clin. Virol. 43:132-133. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, J., and T. Benjamin. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, J., J. Dahl, C. Riney, J. You, C. Cui, R. Holmes, W. Lencer, and T. Benjamin. 2005. Ganglioside GD1a restores infectibility to mouse cells lacking functional receptors for polyomavirus. J. Virol. 79:615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert, J. M., I. G. Goldberg, and T. L. Benjamin. 2003. Cell penetration and trafficking of polyomavirus. J. Virol. 77:2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb, K. A., and L. P. Villarreal. 2001. Natural biology of polyomavirus middle T antigen. Microbiol. Mol. Biol. Rev. 65:288-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin, J. 2001. The biology of signal transduction inhibition: basic science to novel therapies. Semin. Oncol. 28:3-8. [PubMed] [Google Scholar]
- 20.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, M., J. R. Abend, S. F. Johnson, and M. J. Imperiale. 2009. The role of polyomaviruses in human disease. Virology 384:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, M., J. R. Abend, B. Tsai, and M. J. Imperiale. 2009. Early events during BK virus entry and disassembly. J. Virol. 83:1350-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalman, D., O. D. Weiner, D. L. Goosney, J. W. Sedat, B. B. Finlay, A. Abo, and J. M. Bishop. 1999. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1:389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemball, C. C., E. D. Lee, E. Szomolanyi-Tsuda, T. C. Pearson, C. P. Larsen, and A. E. Lukacher. 2006. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J. Immunol. 176:1814-1824. [DOI] [PubMed] [Google Scholar]
- 25.Koleske, A. J., A. M. Gifford, M. L. Scott, M. Nee, R. T. Bronson, K. A. Miczek, and D. Baltimore. 1998. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron 21:1259-1272. [DOI] [PubMed] [Google Scholar]
- 26.Kopitz, J., C. Muhl, V. Ehemann, C. Lehmann, and M. Cantz. 1997. Effects of cell surface ganglioside sialidase inhibition on growth control and differentiation of human neuroblastoma cells. Eur. J. Cell Biol. 73:1-9. [PubMed] [Google Scholar]
- 27.Kopitz, J., C. von Reitzenstein, K. Sinz, and M. Cantz. 1996. Selective ganglioside desialylation in the plasma membrane of human neuroblastoma cells. Glycobiology 6:367-376. [DOI] [PubMed] [Google Scholar]
- 28.Lilley, B. N., J. M. Gilbert, H. L. Ploegh, and T. L. Benjamin. 2006. Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J. Virol. 80:8739-8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low, J. A., B. Magnuson, B. Tsai, and M. J. Imperiale. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 80:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukacher, A. E., R. Freund, J. P. Carroll, R. T. Bronson, and T. L. Benjamin. 1993. Pyvs: a dominantly acting gene in C3H/BiDa mice conferring susceptibility to tumor induction by polyoma virus. Virology 196:241-248. [DOI] [PubMed] [Google Scholar]
- 31.Lukacher, A. E., and C. S. Wilson. 1998. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J. Immunol. 160:1724-1734. [PubMed] [Google Scholar]
- 32.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagi, T. 2008. Aberrant expression of sialidase and cancer progression. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 84:407-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyagi, T., T. Wada, K. Yamaguchi, and K. Hata. 2004. Sialidase and malignancy: a minireview. Glycoconj. J. 20:189-198. [DOI] [PubMed] [Google Scholar]
- 35.Miyagi, T., T. Wada, K. Yamaguchi, K. Hata, and K. Shiozaki. 2008. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J. Biochem. 144:279-285. [DOI] [PubMed] [Google Scholar]
- 36.Monti, E., A. Preti, B. Venerando, and G. Borsani. 2002. Recent development in mammalian sialidase molecular biology. Neurochem. Res. 27:649-663. [DOI] [PubMed] [Google Scholar]
- 37.Neu, U., T. Stehle, and W. J. Atwood. 2009. The Polyomaviridae: contributions of virus structure to our understanding of virus receptors and infectious entry. Virology 384:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pallas, D. C., C. Schley, M. Mahoney, E. Harlow, B. S. Schaffhausen, and T. M. Roberts. 1986. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J. Virol. 60:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelkmans, L. 2005. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 1746:295-304. [DOI] [PubMed] [Google Scholar]
- 40.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 41.Pielage, J. F., K. R. Powell, D. Kalman, and J. N. Engel. 2008. RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization. PLoS Pathog. 4:e1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian, M., D. Cai, K. J. Verhey, and B. Tsai. 2009. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 5:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Querbes, W., A. Benmerah, D. Tosoni, P. P. Di Fiore, and W. J. Atwood. 2004. A JC virus-induced signal is required for infection of glial cells by a clathrin- and eps15-dependent pathway. J. Virol. 78:250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos, E., C. B. Drachenberg, R. Wali, and H. H. Hirsch. 2009. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation 87:621-630. [DOI] [PubMed] [Google Scholar]
- 45.Reeves, P. M., B. Bommarius, S. Lebeis, S. McNulty, J. Christensen, A. Swimm, A. Chahroudi, R. Chavan, M. B. Feinberg, D. Veach, W. Bornmann, M. Sherman, and D. Kalman. 2005. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 11:731-739. [DOI] [PubMed] [Google Scholar]
- 46.Sapp, M., and P. M. Day. 2009. Structure, attachment and entry of polyoma- and papillomaviruses. Virology 384:400-409. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki, A., K. Hata, S. Suzuki, M. Sawada, T. Wada, K. Yamaguchi, M. Obinata, H. Tateno, H. Suzuki, and T. Miyagi. 2003. Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice. J. Biol. Chem. 278:27896-27902. [DOI] [PubMed] [Google Scholar]
- 48.Schindler, T., W. Bornmann, P. Pellicena, W. T. Miller, B. Clarkson, and J. Kuriyan. 2000. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289:1938-1942. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz, A., and A. H. Futerman. 2000. Immunolocalization of gangliosides by light microscopy using anti-ganglioside antibodies. Methods Enzymol. 312:179-187. [DOI] [PubMed] [Google Scholar]
- 50.Sirvent, A., C. Benistant, and S. Roche. 2008. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol. Cell 100:617-631. [DOI] [PubMed] [Google Scholar]
- 51.Smith, A. E., H. Lilie, and A. Helenius. 2003. Ganglioside-dependent cell attachment and endocytosis of murine polyomavirus-like particles. FEBS Lett. 555:199-203. [DOI] [PubMed] [Google Scholar]
- 52.Swimm, A., B. Bommarius, Y. Li, D. Cheng, P. Reeves, M. Sherman, D. Veach, W. Bornmann, and D. Kalman. 2004. Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol. Biol. Cell 15:3520-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swimm, A. I., and D. Kalman. 2008. Cytosolic extract induces Tir translocation and pedestals in EPEC-infected red blood cells. PLoS Pathog. 4:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takashima, S. 2008. Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci. Biotechnol. Biochem. 72:1155-1167. [DOI] [PubMed] [Google Scholar]
- 55.Tettamanti, G. 2004. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj. J. 20:301-317. [DOI] [PubMed] [Google Scholar]
- 56.Tettamanti, G., R. Bassi, P. Viani, and L. Riboni. 2003. Salvage pathways in glycosphingolipid metabolism. Biochimie 85:423-437. [DOI] [PubMed] [Google Scholar]