Abstract
The Ebolavirus matrix protein VP40 is essential for virion assembly and egress. Recently, we reported that the coat protein complex II (COPII) transport system plays an important role in the transport of VP40 to the plasma membrane. Here, we show that dominant-negative mutants of the GTPase Rab1b interfere with VP40-mediated particle formation. Rab1b activates GBF1 (Golgi-specific BFA [brefeldin A] resistance factor 1), a critical factor in the assembly of COPI vesicles. Activated GBF1 stimulates ARF1 (ADP ribosylation factor 1), which recruits coat protein to cellular membranes for the assembly of COPI vesicles. Here, we demonstrate that GBF1 and ARF1 are involved in Ebolavirus virion formation, suggesting that both the COPII and COPI transport systems play a role in Ebolavirus VP40-mediated particle formation. These findings provide new insights into the cellular pathways employed for Ebolavirus virion formation.
Ebolavirus, a member of the Filoviridae in the order Mononegavirales (4), causes hemorrhagic fever with extremely high mortality rates in humans and nonhuman primates. Expression of the viral matrix protein (VP40) in mammalian cells results in the formation of virus-like particles (VLP) that resemble authentic Ebolavirus in size and shape (6, 8, 18, 22), demonstrating a critical role for VP40 in Ebolavirus virion formation.
The intracellular pathways employed for Ebolavirus VP40 transport to the plasma membrane, the budding site of Ebolaviruses, have yet to be fully defined. Transport in the early secretory pathway (i.e., from the endoplasmic reticulum [ER] to the Golgi compartment) is carried out by coat protein complex I (COPI)- and II-coated vesicles (19) (Fig. 1A). COPI complexes are involved in both anterograde and retrograde transport between the ER and the Golgi compartment (16); while COPII complexes transport from the ER to the Golgi compartment (11). We recently found that Sec24C, a component of the COPII vesicular transport system, interacts with VP40, indicating that the COPII transport system plays a role in VP40 intracellular transport (26). The transport of COPII-coated vesicles is also regulated by the Rab GTPase Rab1, which tethers COPII vesicles to the ER-Golgi intermediate compartment (ERGIC) or the cis-Golgi membrane (2, 20). To further assess the role of the early secretory pathway in Ebolavirus virion formation, here we analyzed the role of Rab1 in Ebolavirus VP40-induced VLP formation.
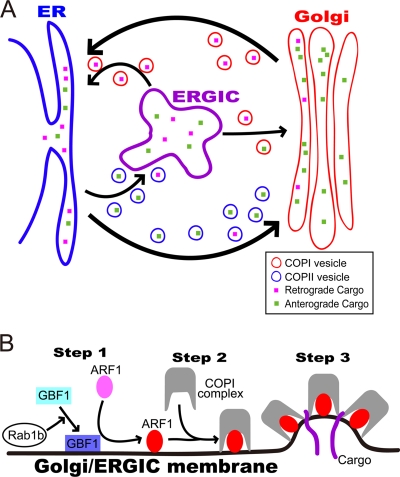
FIG. 1.
Schematic diagram of COPI and COPII transport systems. (A) COPI and COPII transport systems. COPII vesicles transport cargo from the ER to the Golgi compartment (anterograde transport). COPI vesicles transport cargo from the Golgi compartment to the ER (retrograde transport), but also play a role in anterograde cargo transport from the ERGIC to the Golgi compartment. (B) COPI vesicle formation. Rab1b activates GBF1, which then activates ARF1 (step 1). Activated ARF1 recruits preassembled COPI complexes to the Golgi/ERGIC membrane (step 2). Assembled COPI complexes recruit cargo, followed by membrane curvature (step 3).
Dominant-negative mutants of Rab1b reduce Ebolavirus VLP production.
Rab1 has two isoforms, Rab1a and Rab1b, that share about 93% amino acid homology and play an essential role in transport from the ER to the Golgi compartment (13, 27). To evaluate their involvement in VP40 intracellular transport, we cloned the open reading frame (ORF) of Rab1a (NM_004161) or Rab1b (NM_030981) into the eukaryotic protein expression vector pCAGGS (10, 14) with a FLAG tag at the N terminus. Two dominant-negative mutants of Rab1a were generated by replacing asparagine with isoleucine at position 124 (FLAG-Rab1a_N124I) or by replacing serine with asparagine at position 25 (FLAG-Rab1a_S25N) (17, 23). Similarly, two dominant-negative mutants of Rab1b that abrogate GTP binding were generated by an Asn-to-Iso substitution at position 121 (FLAG-Rab1b_N121I) and a Ser-to-Asn substitution at position 22 (FLAG-Rab1b_S22N) (17, 23). We then confirmed the inhibitory effect of these mutants on ER-to-Golgi compartment transport by measuring the efficiency of secreted alkaline phosphatase (SEAP) release (Fig. 2A), as described previously (26). All dominant-negative mutants reduced SEAP secretion, although to different extents.
FIG. 2.
Dominant-negative mutants of Rab1b reduce Ebolavirus VLP production. (A) Inhibition of secretory pathways by dominant-negative mutants of Rab1a or Rab1b. Secreted alkaline phosphatase (SEAP) was coexpressed with Rab1a, Rab1b, or their dominant-negative mutants in 293 cells. Twenty-four hours posttransfection, SEAP activities were measured. For cells expressing wild-type Rab1 proteins, the secretion index (i.e., the ratio of SEAP activity detected in the culture supernatant to the cell-associated SEAP activity) was defined as 100%. Experiments were carried out in triplicate. (B) Ebolavirus VP40-induced VLP production is reduced by dominant-negative Rab1b mutants. VP40 was coexpressed in 293T cells in the absence (“Empty”) or presence of FLAG-Rab1a, FLAG-Rab1b, or their dominant-negative mutants (“N124I” or “S22N” and “N121I” or “S22N,” respectively). Twenty-four hours posttransfection, the released VLPs and total cell lysates were analyzed by Western blot analysis with an anti-FLAG antibody or an anti-VP40 antibody. The intensities of the VP40 double bands were quantified, and VLP release efficiencies were calculated based on the ratio of VP40 in VLPs and cell lysates. The values obtained with wild-type Rab1a and Rab1b proteins were set to 100%. The results shown are representative of three independent experiments. (C) Morphological changes in cells expressing Venus-VP40 fusion protein. The reporter protein Venus or Venus-VP40 fusion protein was expressed in 293 cells. Twenty-four hours posttransfection, the cells were imaged by using confocal microscopy. Arrowheads indicate filamentous cell protrusions. (D) Dominant-negative Rab1b mutants reduce the frequency of VP40-induced formation of cell protrusions. Venus-VP40 was coexpressed with wild-type or dominant-negative Rab1 proteins in 293 cells. The cells were imaged 24 h posttransfection by using confocal microscopy.
Next, we examined the effect of these dominant-negative mutants on Ebolavirus VP40-induced VLP production. VP40 was coexpressed in human embryonic kidney (293T) cells in the absence or presence of FLAG-Rab1a or FLAG-Rab1b or dominant-negative mutants thereof (Fig. 2B). Twenty-four hours posttransfection, cell lysate and VLPs in the cell culture supernatant were prepared, as previously described (25). Samples were resolved on 10% to 20% Tris-glycine gels (Invitrogen, CA), and the resolved proteins were detected with a rabbit anti-VP40 antibody (26) or a mouse anti-FLAG antibody (Sigma, St. Louis, MO) by Western blot analysis. As described previously (8), Ebolavirus VP40 protein was detected as a double band due to an internal start codon. For our analysis, the amount of VP40 was calculated as the sum of both bands by using a CS analyzer, version 2.08d (Atto, Japan). Wild-type Rab1a and Rab1b and their dominant-negative mutants were expressed in transfected cells at similar levels (Fig. 2B, “Cell Lysate, Anti-FLAG”). Ebolavirus VP40-induced VLP formation was not appreciably affected by coexpression of wild-type or dominant-negative Rab1a protein (“N124I” or “S25N”), or by coexpression of wild-type Rab1b protein (Fig. 2B). However, VLP formation was moderately decreased when VP40 was coexpressed with the dominant-negative variants of Rab1b (Fig. 2B, “N121I” and “S22N”).
To visually evaluate the involvement of Rab1a and Rab1b in Ebolavirus VP40 transport, we examined the morphology of human embryonic kidney (293) cells expressing VP40 fused to the Venus reporter protein (Fig. 2C). Cells were transfected with a Venus-VP40 expression plasmid or a Venus control plasmid and observed 24 h later by confocal microscopy (LSM510 system; Carl Zeiss, Germany). Cells expressing the Venus reporter protein (Fig. 2C, “Venus”) were morphologically indistinguishable from nontransfected cells (data not shown). Expression of the Venus-VP40 fusion protein resulted in the formation of filamentous fibers protruding from the cell body (Fig. 2C, “Venus-VP40,” arrowheads), which may represent sites of Ebolavirus VLP budding. The morphology of cells coexpressing Venus-VP40 and Rab1a, Rab1b, or Rab1a dominant-negative mutants (Fig. 2D) was similar to that of Venus-VP40-expressing cells (Fig. 2C). In contrast, coexpression of Rab1b dominant-negative mutants (Rab1b_N121I and Rab1b_S22N) resulted in fewer protrusions from the cell surface, potentially suggesting less efficient VLP formation. A similar effect was observed with Sar1 dominant-negative mutants, which are the well-characterized COPII transport inhibitors (26). Collectively, these data suggest a role for Rab1b in Ebolavirus VP40-mediated VLP production.
Role of GBF1 in VP40-induced VLP formation.
Both Rab isoforms (Rab1a and Rab1b) are critical for the tethering of COPII-derived vesicles on the ERGIC and cis-Golgi membrane, and the dominant-negative effect of each isoform is antagonized by the other (15). Hence, for COPII transport, the downregulation of one Rab1 isoform can be compensated for by the other. Our finding that dominant-negative Rab1b mutants reduce the efficiency of Ebolavirus VP40-mediated VLP formation thus points to an additional role for Rab1b in Ebolavirus virion formation. In fact, Rab1b also plays a role in COPI complex assembly at the cellular membrane (13) (Fig. 1B). Rab1b activates the guanine nucleotide exchange factor GBF1 (Golgi-specific BFA [brefeldin A] resistance factor 1) (1, 13), which catalyzes the GDP/GTP exchange of the GTPase ARF1 (ADP ribosylation factor 1) (9). Activated ARF1 then recruits coat protein to cellular membranes for the assembly of COPI vesicles (1, 13).
To test whether dominant-negative Rab1b mutants interfere with Ebolavirus virion formation by affecting GBF1, we cloned the N-terminally FLAG-tagged ORF of GBF1 (NM_004193) into pCAGGS/MCS. A dominant-negative mutant of GBF1 which abolishes the ARF1 nucleotide-exchange activity was created by replacing glutamic acid with lysine at position 794 (FLAG-GBF1_E794K) (5, 21). As reported, expression of dominant-negative GBF1 resulted in the relocalization of GM130 (a Golgi marker) to the cytoplasm in a dotted staining pattern (Fig. 3A, arrows) (5). We then asked whether this mutant inhibited VLP production (Fig. 3B). Expression of FLAG-GBF1 (Fig. 3B, lane 2, “Anti-FLAG”) or its dominant-negative variant (Fig. 3B, lane 3, “Anti-FLAG”) did not affect VP40 expression levels in plasmid-transfected 293T cells. However, the efficiency of Ebolavirus VP40-induced VLP formation was reduced upon coexpression of FLAG-tagged, dominant-negative GBF1 (Fig. 3B, lane 3), but not FLAG-GBF1 (Fig. 3B, lane 2). We next visually evaluated the involvement of GBF1 in Ebolavirus VP40 intracellular transport by using a Venus-VP40 fusion protein (Fig. 3C). The dominant-negative GBF1 mutant (GBF1_E794K) reduced the number of cell protrusions induced by Venus-VP40, similar to that seen with the Rab1b dominant-negative mutants (Fig. 2D). These results suggest that GBF1 is required for efficient Ebolavirus VP40 intracellular transport and VP40-induced virion formation.
FIG. 3.
Role of GBF1 in Ebolavirus VP40-mediated VLP formation. (A) Functionality of a dominant-negative GBF1 mutant. 293 cells were transfected with a plasmid encoding FLAG-GBF1 or its dominant-negative mutant. After 24 h, cells were fixed and stained with an anti-GM130 antibody (green; GE Health Care, England) and an anti-FLAG antibody (red). Hoechst 33342 (Invitrogen, CA) was used to stain the nuclei (blue). Arrows indicate the GM130 redistribution in cells expressing dominant-negative GBF1_E794K, confirming the functionality of this mutant. (B) Reduction of Ebolavirus VP40-mediated VLP production by a dominant-negative GBF1 mutant. VP40 was expressed in the absence (lane 1) or presence of wild-type (lane 2) or dominant-negative (lane 3) GBF1 in 293T cells. VLP production efficiency was calculated as described in the legend to Fig. 2B. The results shown are representative of two independent experiments. (C) A dominant-negative GBF1 mutant reduces the frequency of VP40-induced formation of cell protrusions. Venus-VP40 was coexpressed with FLAG-GBF1 or its mutant in 293 cells. The cells were imaged 24 h posttransfection by confocal microscopy.
Role of ARF1 in VP40-induced VLP formation.
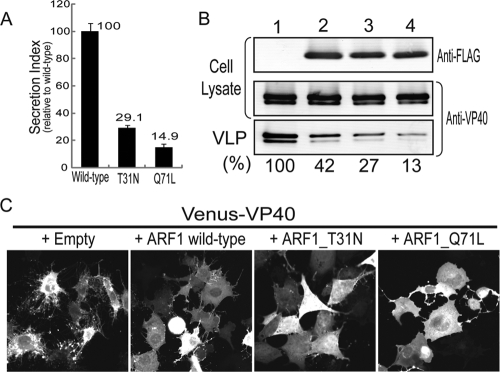
Our findings suggest that efficient Ebolavirus VLP formation relies on functional Rab1b and GBF1. After activation by Rab1b, GBF1 catalyzes the activation of the GTPase ARF1, a critical factor in the recruitment of COPI complexes to the cellular membrane (Fig. 1B). To assess the role of ARF1 in Ebolavirus VP40-induced VLP formation, we generated a FLAG-tagged version of the ORF of ARF1 (NM_001024226) in pCAGGS/MCS. We also generated a dominant-negative mutant by replacing threonine with asparagine at position 31 (FLAG-ARF1_T31N) and a constitutively active mutant by replacing glutamine with leucine at position 71 (FLAG-ARF1_Q71L) (24). Both mutants affected ER-to-Golgi compartment transport, as assessed by use of a SEAP assay (Fig. 4A), although different mechanisms were likely involved: the dominant-negative mutant is enzymatically inactive (21), whereas the constitutively active mutant is locked in the GTP-bound stage and lacks GDP/GTP recycling activity, which likely accounts for its negative effect on membrane trafficking (3). Wild-type and mutant ARF1 proteins were expressed at similar levels in plasmid-transfected 293T cells (Fig. 4B, lanes 2 to 4, “Anti-FLAG”), and their expression did not affect VP40 protein expression levels (compare lanes 2 to 4 with lane 1). Interestingly, overexpression of FLAG-ARF1 (Fig. 4B, lane 2) reduced the efficiency of VLP production to 42% of that of cells expressing endogenous ARF1 only (Fig. 4B, lane 1). ARF1 executes a critical regulatory role in COPI assembly and the regulation of vesicle transport between the ER and Golgi compartment (7); its overexpression may disturb the balance between anterograde and retrograde ER-to-Golgi compartment transport and thereby interfere with the efficient formation of Ebolavirus VLPs. Coexpression of VP40 with the dominant-negative ARF1 mutant (Fig. 4B, lane 3) or the constitutively active variant, which is known to affect membrane trafficking (24) (Fig. 4B, lane 4), further reduced the amounts of Ebolavirus VP40-induced VLPs. Consistent with these findings, overexpression of wild-type or mutant ARF1 reduced the number of VP40-induced cell protrusions relative to control cells (Fig. 4C), similar to the effect observed with dominant-negative mutants of Rab1b or GBF1 (Fig. 2D and 3C). These data demonstrate a role for ARF1 in the efficient formation of Ebolavirus VP40-mediated VLP production.
FIG. 4.
Role of ARF1 Ebolavirus VP40-mediated VLP formation. (A) Inhibition of secretory pathways by ARF1 mutants. SEAP was coexpressed with FLAG-tagged wild-type ARF1 or with dominant-negative (FLAG-ARF1_T31N) or constitutively active (FLAG-ARF1_Q71L) mutants. The secretion index was calculated as described in the legend to Fig. 2A. (B) Ebolavirus VP40-mediated VLP production is reduced by overexpression of ARF1 or its mutants. VP40 was coexpressed in the absence (lane 1) or presence of FLAG-ARF1 (lane 2), FLAG-ARF1_T31N (dominant negative; lane 3), or FLAG-ARF1_Q71L (constitutively active; lane 4) in 293T cells. VLP production efficiency was calculated as described in the legend to Fig. 2B. The results shown are representative of three independent experiments. (C) ARF1 or its mutants reduce the frequency of VP40-induced formation of cell protrusions. Venus-VP40 was coexpressed with ARF1 or its mutants in 293 cells. The cells were imaged 24 h posttransfection by confocal microscopy.
Here, we show that dominant-negative mutants of Rab1b, but not nonfunctional variants of Rab1a, reduce Ebolavirus VP40-mediated VLP production (Fig. 2). Both isoforms are interchangeable in their ability to tether COPII complexes to the ERGIC and cis-Golgi membranes (15), suggesting that the inhibitory effect of Rab1b is mediated through a different pathway. In addition to its role in COPII vesicle transport, Rab1b also plays a role in COPI transport by activating GBF1, which in turn activates ARF1, a GTPase required for COPI recruitment (Fig. 1B). Here, we demonstrate that dominant-negative mutants of GBF1 and ARF1 reduce the efficiency of Ebolavirus VP40-mediated VLP formation—findings that suggest a direct or indirect role for COPI vesicle transport in Ebolavirus VP40 transport to the plasma membrane.
COPI complexes function primarily in retrograde transport from the Golgi compartment to the ER, but also play a role in anterograde transport from the ER to the Golgi compartment (16) (Fig. 1A). COPI-mediated retrograde transport is involved in the “capture” and “recycling” of transport cargo that escapes from the ER (12). Ebolavirus may usurp this mechanism to maximize the efficiency of VP40 transport to the plasma membrane. Alternatively, Ebolavirus may exploit the COPI-mediated anterograde transport system and COPII-mediated transport for efficient VP40 delivery to the plasma membrane.
In a different scenario, the COPI system may play an indirect role in Ebolavirus VP40 transport to the membrane. The up- or downregulation of COPI transport can cause an imbalance between anterograde and retrograde transport between the ER and Golgi compartment, as demonstrated by ARF1 mutants that resulted in the disassembly of Golgi components (3). Hence, downregulation of the COPI-mediated Golgi compartment-to-ER transport may deplete the cell of reshuttled membrane components and, through this mechanism, affect Ebolavirus virion formation. Further studies are needed to assess the exact role of the COPI transport system in the Ebolavirus life cycle.
Here, we present data that further our understanding of the cellular pathways employed by Ebolavirus. Such knowledge may help in the identification of potential targets for the development of antivirals to Ebolavirus infections.
Acknowledgments
We thank Susan Watson for editing the manuscript.
This work was supported by ERATO (Japan Science and Technology Agency) and by grants-in-aid from the Ministries of Education, Culture, Sports, Science, Japan; by National Institute of Allergy and Infectious Diseases Public Health Service research grants; and by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge memberships within and support from the Region V “Great Lakes” RCE (NIH award U54-AI-057153).
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Alvarez, C., R. Garcia-Mata, E. Brandon, and E. Sztul. 2003. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol. Biol. Cell 14:2116-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appenzeller-Herzog, C., and H. P. Hauri. 2006. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 119:2173-2183. [DOI] [PubMed] [Google Scholar]
- 3.Dascher, C., and W. E. Balch. 1994. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269:1437-1448. [PubMed] [Google Scholar]
- 4.Feldmann, H., T. W. Geisbert, P. B. Jahrling, H. D. Klenk, S. V. Netesov, C. J. Peters, A. Shanchez, R. Swanepoel, and V. E. Volchkov. 2004. Filoviridae, p. 645-653. In C. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom.
- 5.Garcia-Mata, R., T. Szul, C. Alvarez, and E. Sztul. 2003. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol. Biol. Cell 14:2250-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harty, R. N., M. E. Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. U. S. A. 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu, V. W., S. Y. Lee, and J. S. Yang. 2009. The evolving understanding of COPI vesicle formation. Nat. Rev. Mol. Cell Biol. 10:360-364. [DOI] [PubMed] [Google Scholar]
- 8.Jasenosky, L. D., G. Neumann, I. Lukashevich, and Y. Kawaoka. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamoto, K., Y. Yoshida, H. Tamaki, S. Torii, C. Shinotsuka, S. Yamashina, and K. Nakayama. 2002. GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic 3:483-495. [DOI] [PubMed] [Google Scholar]
- 10.Kobasa, D., M. E. Rodgers, K. Wells, and Y. Kawaoka. 1997. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J. Virol. 71:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, M. C., E. A. Miller, J. Goldberg, L. Orci, and R. Schekman. 2004. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20:87-123. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, M. J., and H. R. Pelham. 1992. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68:353-364. [DOI] [PubMed] [Google Scholar]
- 13.Monetta, P., I. Slavin, N. Romero, and C. Alvarez. 2007. Rab1b Interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol. Biol. Cell 18:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 15.Nuoffer, C., H. W. Davidson, J. Matteson, J. Meinkoth, and W. E. Balch. 1994. A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 125:225-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelham, H. R., and J. E. Rothman. 2000. The debate about transport in the Golgi—two sides of the same coin? Cell 102:713-719. [DOI] [PubMed] [Google Scholar]
- 17.Pind, S. N., C. Nuoffer, J. M. McCaffery, H. Plutner, H. W. Davidson, M. G. Farquhar, and W. E. Balch. 1994. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J. Cell Biol. 125:239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez, A., A. S. Khan, S. R. Zaki, G. J. Nabel, T. G. Ksiazek, and C. J. Peters. 2001. Filoviridae: Marburg and Ebola viruses, p. 1279-1304. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 19.Spang, A. 2009. On vesicle formation and tethering in the ER-Golgi shuttle. Curr. Opin. Cell Biol. 21:531-536. [DOI] [PubMed] [Google Scholar]
- 20.Stenmark, H., and V. M. Olkkonen. 2001. The Rab GTPase family. Genome Biol. 2:REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szul, T., R. Garcia-Mata, E. Brandon, S. Shestopal, C. Alvarez, and E. Sztul. 2005. Dissection of membrane dynamics of the ARF-guanine nucleotide exchange factor GBF1. Traffic 6:374-385. [DOI] [PubMed] [Google Scholar]
- 22.Timmins, J., S. Scianimanico, G. Schoehn, and W. Weissenhorn. 2001. Vesicular release of Ebola virus matrix protein VP40. Virology 283:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Tisdale, E. J., J. R. Bourne, R. Khosravi-Far, C. J. Der, and W. E. Balch. 1992. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119:749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang, Y., J. Seemann, B. Bisel, S. Punthambaker, and Y. Wang. 2007. Active ADP-ribosylation factor-1 (ARF1) is required for mitotic Golgi fragmentation. J. Biol. Chem. 282:21829-21837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamayoshi, S., and Y. Kawaoka. 2007. Mapping of a region of Ebola virus VP40 that is important in the production of virus-like particles. J. Infect. Dis. 196(Suppl 2):S291-S295. [DOI] [PubMed] [Google Scholar]
- 26.Yamayoshi, S., T. Noda, H. Ebihara, H. Goto, Y. Morikawa, I. S. Lukashevich, G. Neumann, H. Feldmann, and Y. Kawaoka. 2008. Ebola virus matrix protein VP40 uses the COPII transport system for its intracellular transport. Cell Host Microbe 3:168-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]