Abstract
Natural killer (NK) cells keep viral infections under control at the early phase by directly killing infected cells. Influenza is an acute contagious respiratory viral disease transmitted from host-to-host in the first few days of infection. The evasion of host innate immune defenses including NK cells is important for its success as a viral pathogen of humans and animals. NK cells encounter influenza virus within the microenvironment of infected cells. It therefore is important to investigate the direct effects of influenza virus on NK cell activity. Recently we demonstrated that influenza virus directly infects human NK cells and induces cell apoptosis to counter their function (H. Mao, W. Tu, G. Qin, H. K. W. Law, S. F. Sia, P.-L. Chan, Y. Liu, K.-T. Lam, J. Zheng, M. Peiris, and Y.-L. Lau, J. Virol. 83:9215-9222, 2009). Here, we further demonstrated that both the intact influenza virion and free hemagglutinin protein inhibited the cytotoxicity of fresh and interleukin-2 (IL-2)-activated primary human NK cells. Hemagglutinin bound and internalized into NK cells via the sialic acids. This interaction did not decrease NKp46 expression but caused the downregulation of the ζ chain through the lysosomal pathway, which caused the decrease of NK cell cytotoxicity mediated by NKp46 and NKp30. The underlying dysregulation of the signaling pathway involved ζ chain downregulation, leading to decreased Syk and ERK activation and granule exocytosis upon target cell stimulation, finally causing reduced cytotoxicity. These findings suggest that influenza virus developed a novel strategy to evade NK cell innate immune defense that is likely to facilitate viral transmission and also contribute to virus pathogenesis.
Natural killer (NK) cells are key effector cells in innate immunity, and they play a critical role in the first line of host defense against viral infections by killing infected cells without prior antigen stimulation (52). NK cells have a complex receptor repertoire, either in activating or inhibitory form (22). The “missing self” hypothesis explains how NK cells distinguish target cells from self cells (51). Upon NK cell stimulation, the activating signaling event leads to a downstream cascade of kinase activation with the final exocytosis of cytotoxic granules, which results in the killing of target cells (38, 47, 49). The cytotoxicity of NK cells, however, is tightly controlled by the balance of inhibitory and activating receptors, as well as various costimulatory molecules (13, 22, 46). A critical threshold of signaling must be achieved for NK cells to mount a productive response (13, 14, 47).
For a virus to be a successful pathogen, it must counter many host defense mechanisms, including both innate and adaptive immunity (32). During viral infections, viruses and NK cells are in a constant battle. NK cells respond to eliminate the invading viruses via the recognition of the missing self or the increased activating signals. However, many viruses have developed a variety of strategies to modulate NK cell activity (53, 68). These NK cell evasion strategies fall into distinct mechanistic categories, including direct viral effects on NK cells (42, 62). Although NK immunoevasion has been intensively investigated for viruses causing chronic infections, like herpesviruses, it is likely that even acute viral infections modulate NK cell functions as part of their anti-immune strategies (32).
Influenza is an acute respiratory virus infection that continues to pose seasonal, zoonotic, and pandemic threats to human health (50). As the illness and virus transmission usually occur in the first few days of infection, the virus has to devise strategies to evade host innate immune responses. NK cells are key effector cells in host innate defense against acute viral infections by killing infected cells (22, 52). Virus-infected respiratory epithelial cells release inflammatory chemokines that recruit NK cells to the site of infection (35). It therefore is not surprising that, for their survival, viruses have developed numerous strategies to evade NK immunity (42, 53). Patients with severe influenza infection were shown to have diminished NK cells in peripheral blood and an almost complete absence of pulmonary NK cells (40, 79). In addition, during influenza infection, the decrease in NK cell activity also was demonstrated in mice (24, 37, 61). These data suggest that influenza virus directly target NK cells as part of its immunoevasion strategies.
Indeed, as a lytic virus, numerous influenza viral particles are released from infected epithelial cells during infection (26). Free hemagglutinin (HA) protein also is released from disrupted cells or during the assembly of virus particles (18, 48). In the infected microenvironment, NK cells undoubtedly encounter these virions and HA protein. It therefore is important to investigate the direct interaction of NK cells with influenza virions and viral protein. Recently, we demonstrated that influenza virus infects human NK cells and induces marked cell apoptosis (57), providing the first evidence that influenza virus directly targets NK cells to counter their function. Thereafter an in vivo study further confirmed our in vitro findings (37). Apart from the direct infection and subsequent induction of cell death by influenza virus, in vivo studies also revealed that the decrease of NK cell activity after influenza virus infection is dose dependent (24, 61). However, the underlying mechanisms remain to be investigated.
In the present study, we demonstrated that both the intact influenza virion and HA protein inhibited primary human NK cell cytotoxicity in a dose-dependent manner. HA was bound and internalized into NK cells. This interaction caused the downregulation of the ζ chain via lysosomal pathway, which led to decreased Syk and ERK phosphorylation and granule exocytosis upon target cell stimulation, resulting in reduced cytotoxicity. These findings suggest that influenza viruses have developed a novel strategy to evade NK cell innate immune defense, which is likely to facilitate viral survival and transmission.
MATERIALS AND METHODS
Isolation of primary human NK cells.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Pharmacia) gradient centrifugation from whole-blood samples obtained from the Hong Kong Red Cross, as described in our previous study (83). NK cells were magnetically separated from PBMC by negative selection with NK cell isolation kit II (Miltenyi Biotec). The purity of isolated NK cells was constantly >97%, as determined by flow cytometry. NK cells were cultured in RPMI 1640 (Invitrogen) medium supplemented with 10% autologous serum. The research protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority, Hong Kong West Cluster.
Influenza virus preparation and HA treatment of NK cells.
As described previously (83), influenza virus A/Hong Kong/54/98 (H1N1) was cultured in Madin-Darby canine kidney (MDCK) cells and was purified by adsorption to and elution from turkey red blood cells. The virus titer was determined by titration in MDCK cells, with daily observation of cytopathic effect, and confirmed by hemagglutination assay. MDCK cells were routinely maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (FBS) (Invitrogen).
Influenza virus H1N1 was inactivated by UV with energy of 0.2 J or heat at 100°C for 15 min and then incubated with NK cells at the indicated multiplicity of infection (MOI). After 1 h of viral adsorption, unadsorbed virions were washed away by excess phosphate-buffered saline (PBS). Mock-treated cells were treated in parallel, except that the inactivated virions were not added. For HA treatment, NK cells first were incubated with recombinant A/New Caledonia/20/99 (H1N1) HA protein (Protein Sciences) with a cell density of 10 million per milliliter. After 2 h, the culture was diluted 10-fold with the final concentration of HA protein as indicated. According to the manufacturer, HA is a full-length glycosylated recombinant protein with biological activity and tertiary structure (Protein Sciences).
In some experiments, NK cells were preactivated by stimulation with 500 U/ml recombinant human interleukin-2 (IL-2) (Invitrogen) as previously described (57). For HA immobilization, 6-well plates were coated with HA protein or bovine serum albumin (as a mock treatment) overnight. The plates were carefully washed to remove the unbound protein and then incubated with NK cells. Cells were cultured for 24 h. To determine whether the HA binding of NK cells was dependent on sialic acid, the cells first were treated with Arthrobacter ureafaciens sialidase (Sigma) for 30 min and then extensively washed prior to treatment as previously described (57). In some experiments, NK cells were preincubated with 1 μM filomycin or with 30 μM E-64 and 100 μM pepstatin A (Calbiochem) for 30 min prior to HA treatment. The agents were present during the whole process (17, 76).
Flow cytometry.
The following monoclonal antibodies were used in this study: anti-CD56 (NCAM16.2), anti-CD3 (UCHT1), anti-NKp46 (9E2), anti-CD69 (FN50), anti-perforin (G9), anti-granzyme B (GB11), anti-CD107a (H4A3), anti-phospho-Syk (pY348), and anti-phospho-ERK1/2 (T202/Y204) (all from BD Biosciences); anti-NKp46 (9E2), anti-NKp30 (p30-15), and anti-CD3-ζ (6B10.2) (all from BioLegend); and anti-NKp46 (BAB281; Beckman Coulter) and anti-CD3-ζ (6B10.2; Santa Cruz). For surface staining, NK cells were stained with specific antibody. For intracellular staining, cells were fixed, permeabilized, and then labeled with the indicated antibodies. To avoid nonspecific staining, cells were preincubated with FcR blocking reagent (Miltenyi Biotec) prior to specific staining. NK cell apoptosis was determined with the annexin V-fluorescein isothiocyanate (FITC) kit according to the instructions (Beckman Coulter). All data were acquired on a BD FACSAria with FACS Diva (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.).
To examine phosphokinase expression, after being incubated with HA for 24 h, NK cells were collected and stimulated with or without K562 cells for 5 min. Cells then were fixed by Fix Buffer I, permeabilized by Perm Buffer III (both from BD Biosciences), and stained for phospho-Syk or phospho-extracellular signal-regulated kinase (ERK). Samples were analyzed for the percentage of phosphokinase-positive population in NK cells by flow cytometry. The increase in the percentage of phosphokinase expression in stimulated NK cells relative to resting cells was calculated.
Cytotoxicity assay.
The treated NK cells were collected and determined for their cytotoxicity with the LIVE/DEAD Cell-Mediated Cytotoxicity kit (Molecular Probes) as previously reported (75, 82). Briefly, target cells were stained with DioC18 (Dio) and then cocultured with NK cells at specific effector cell/target cell (E/T) ratios in the presence of propidium iodide (PI) for 2 h. After incubation, the cytotoxicity was analyzed by flow cytometry and calculated as the percentage of Dio+ PI+ cells out of total Dio+ cells. In the case of virus-infected cells as target cells, THP-1 macrophages were infected with H1N1 virus. The infection of the THP-1 cells was essentially the same as that of monocyte-derived macrophages described in our previous study (66, 83). For the redirected cytotoxicity assay, murine FcγR-positive P815 cells first were incubated with anti-NKp46 or anti-NKp30 monoclonal antibody and then cocultured with NK cells to assay cell cytotoxicity (71, 77).
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM). Statistical analysis was performed by Student's t test or one-way analysis of variance (ANOVA) with Tukey's multiple-comparison test using Prism 5 (GraphPad Software). A P of <0.05 was considered significant.
RESULTS
Influenza virion inhibits primary human NK cell cytotoxicity.
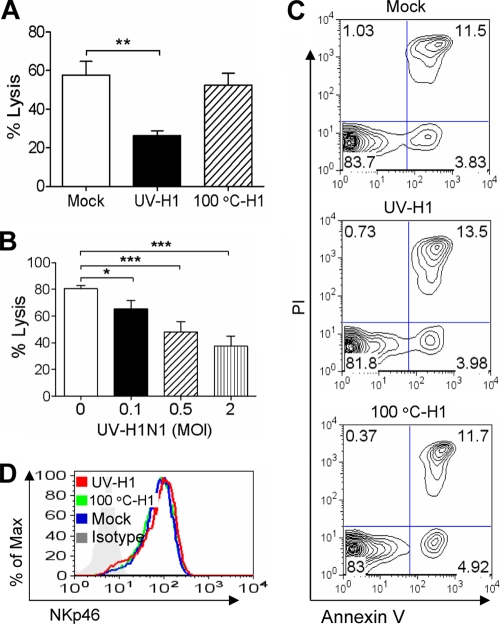
We first determined whether influenza virus could modulate NK cell activity. Since the live virus can directly infect and kill NK cells (57), the effects of inactivated virus on NK cell activity were studied to exclude the effects of direct infection-induced apoptosis. Freshly isolated primary human NK cells were incubated with inactivated virus at the indicated MOI for 1 h, washed, and then cultured for 24 h. NK cells were collected and determined for their cytotoxicity against K562 target cells. As shown in Fig. 1A, the virion inactivated by heat at 100°C did not reduce NK cell cytotoxicity; however, UV-inactivated influenza virion inhibited NK cell cytotoxicity in a dose-dependent manner. The virion at an MOI of 2 reduced NK cytotoxicity by about half (Fig. 1B).
FIG. 1.
Influenza virion inhibits primary human NK cell cytotoxicity. Influenza H1N1 virus was inactivated by UV or heat at 100°C. Fresh primary human NK cells were treated with the inactivated virus at an MOI of 2 (A, C, and D) or the indicated MOI (B) for 1 h, extensively washed, and then cultured for 24 h. (A and B) NK cells were collected and examined for their cytotoxicity against K562 cells. Samples were analyzed by flow cytometry for the percentage of specific lysis. (C and D) After being treated by inactivated influenza virus, NK cells were examined by flow cytometry for annexin V binding and PI uptake (C) or surface NKp46 expression (D). The data shown are means ± SEM from four different donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Images are representatives of at least three independent experiments.
The reduced NK cell cytotoxicity was not due to NK cell death, as both the UV- and heat-inactivated virions did not induce NK cell apoptosis (Fig. 1C). Consistently with previous reports (77), we found that NKp44 was absent from fresh NK cells (data not shown). NKp46 is the major lysis receptor for fresh NK cells and plays a central role in cell cytotoxicity (70, 71). Thus, we examined NKp46 expression on NK cells and found that NKp46 expression was not altered by the inactivated influenza virions (Fig. 1D).
Influenza virus HA binds NK cells through sialic acid.
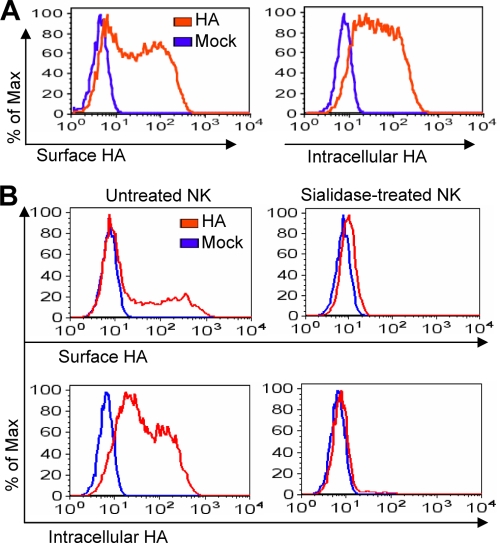
Since UV does not alter viral proteins of fusion activity, while 100°C heat treatment denatures HA protein (15), we were prompted to investigate the regulation of NK cell activity by free HA protein. The interaction of HA and NK cells was determined first. After incubation with influenza virus HA, NK cells were stained by anti-HA antibody. Flow cytometry analysis showed that HA bound to NK cell surface (Fig. 2A), which is consistent with previous reports (5, 56). Intracellular staining also demonstrated the presence of HA in NK cells (Fig. 2A).
FIG. 2.
Influenza virus HA binds NK cells through sialic acid. (A) Fresh primary human NK cells were incubated with or without 1 μg/ml recombinant HA for 6 h. The cells were stained for surface HA binding (left) or fixed and permeabilized for the intracellular staining of HA (right). Samples were analyzed by flow cytometry. (B) NK cells were pretreated with 0.5 U/ml Arthrobacter ureafaciens sialidase for 30 min at 37°C, extensively washed, and then treated with 1 μg/ml recombinant HA protein. After 6 h, samples were examined by flow cytometry for the presence of surface or intracellular HA. Images are representatives of samples from three independent experiments.
Influenza virus infection and entry into target cells are mediated through the sialic acid on cell surface. Upon viral HA binding to the sialic acid, the virus is internalized by receptor-mediated endocytosis. We then examined the role of sialic acid in the free HA protein binding of NK cells by treating cells with the sialidase. As shown in Fig. 2B, the expression levels of both surface and intracellular HA were significantly decreased by sialidase treatment. These data suggested that the free HA binding and internalization into NK cells also were mediated through the sialic acid. The viability of NK cells was not affected by sialidase treatment, as previously described (57).
Influenza virus HA inhibits primary NK cell cytotoxicity.
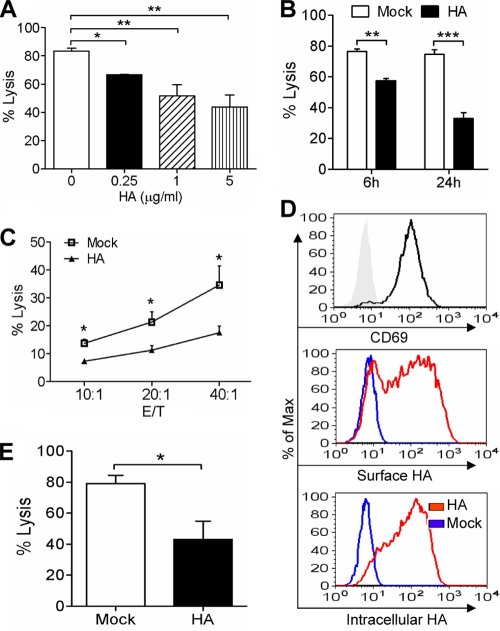
The effect of HA protein on NK cell activity was examined next. After incubation with HA at the indicated concentration for 24 h, NK cells were collected and determined for their cytotoxicity against K562 cells. Like the inactivated influenza virion, HA also inhibited NK cell cytotoxicity in a dose-dependent manner (Fig. 3A). In addition, we determined the time span required for the inhibition of NK cells. As shown in Fig. 3B, a significant decrease in NK cell cytotoxicity was observed already at 6 h after HA treatment. NK cells have been reported to be capable of killing influenza virus-infected cells (56). We then further examined the effect of HA on NK cell cytotoxicity against virus-infected cells. Macrophages are susceptible to influenza virus infection, and the inflammation produced from these cells is associated with the immunopathogenesis of influenza (20, 66, 83); thus, macrophages were selected as target cells here. The THP-1 macrophages were infected with influenza virus and then cocultured with HA-treated NK cells to examine NK cell cytotoxicity. As shown in Fig. 3C, HA-treated NK cells showed significantly lower cytotoxicity against influenza virus-infected macrophages than mock-treated cells.
FIG. 3.
Influenza virus HA inhibits primary NK cell cytotoxicity. (A) After being treated with HA at the indicated concentration for 24 h, NK cells were collected and analyzed for their cytotoxicity against K562 cells at an E/T ratio of 20:1. Samples were analyzed by flow cytometry for the percentage of specific lysis. (B) NK cells were treated with 1 μg/ml HA for 6 or 24 h, and then their cytotoxicity was determined. (C) NK cells were treated with 1 μg/ml HA or left untreated (Mock) for 24 h. The cells were collected and determined for the cytotoxicity against influenza virus-infected THP-1 macrophages at the indicated E/T ratios. (D) IL-2-activated NK cells were examined for the expression of CD69 on cell surface. After being treated with 1 μg/ml HA for 6 h, the IL-2-activated NK cells were examined by flow cytometry for the presence of surface or intracellular HA. (E) Activated NK cells were incubated with 1 μg/ml HA. After 24 h, the cells were collected and determined for their cytotoxicity against K562 cells at an E/T ratio of 10:1. The data shown are means ± SEM from four different donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We further determined whether the cytotoxicity of activated NK cells could be modulated by HA. The activated NK cells were obtained by stimulation with recombinant IL-2 as previously described (57) and expressed the activation marker CD69 on the cell surface (Fig. 3D). After incubation with HA protein, the activated cells showed the presence of HA both on the cell surface and within cells by surface and intracellular staining (Fig. 3D). The effect of HA on cell cytotoxicity was examined. As shown in Fig. 3E, HA-treated activated NK cells had a significantly lower cytotoxicity than mock-treated cells. Taken together, these data indicated that HA protein inhibited the cytotoxicity of both fresh and IL-2-activated primary human NK cells.
Influenza virus HA inhibits granule exocytosis of NK cells upon target cell stimulation.
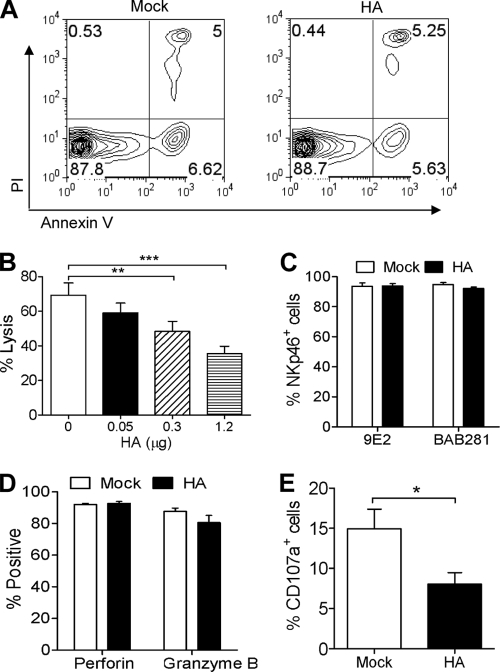
We next investigated the mechanisms underlying the reduced NK cell killing by HA protein. As with inactivated virions, HA did not increase NK cell apoptosis (Fig. 4A). The possibility that the reduced killing was due to the masking of NKp46 by HA was excluded by using immobilized HA. The plate was precoated with HA, and unbound protein was washed away. NK cells then were incubated in this plate. After 24 h, the cells were collected and examined for their cytotoxicity. As shown in Fig. 4B, the immobilized HA also decreased NK cell cytotoxicity in a dose-dependent manner. In addition, by staining with anti-NKp46 monoclonal antibodies 9E2 and BAB281, both of which inhibit NK cell cytotoxicity by masking NKp46 binding of its ligand on target cells (30, 64), we showed that HA-treated NK cells still had NKp46 expression levels similar to those of mock-treated cells (Fig. 4C). Therefore, mechanisms other than masking led to the reduced NK cell cytotoxicity by HA.
FIG. 4.
Influenza virus HA inhibits granule exocytosis of NK cells upon target cell stimulation. (A) After either being left untreated or treated with 1 μg/ml influenza virus HA for 24 h, NK cells were examined by flow cytometry for annexin V binding and PI uptake. (B) Six-well plates precoated with HA protein were incubated with NK cells for 24 h. Cells were collected and examined for cytotoxicity against K562 cells at an E/T ratio of 20:1. Samples were analyzed by flow cytometry for the percentage of specific lysis. (C to E) Primary NK cells were treated with 1 μg/ml HA or left untreated (Mock) for 24 h. The cells were examined for the expression of surface NKp46 with two antibody clones, 9E2 and BAB281 (C), and of intracellular perforin and granzyme B (D) by flow cytometry. (E) The treated NK cells were collected and cocultured with K562 cells in the presence of anti-CD107a antibody for 2 h. The surface expression of CD107a on NK cells then was analyzed by flow cytometry. The data shown are means ± SEM from four different donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NK cell cytotoxicity usually is mediated by the complementary action of cytolytic granules, including granzymes and perforin (41, 55). Although the granules are constitutively expressed in the secretory lysosomes, upon target cell stimulation, they must fuse with the cell membrane, allowing the release of perforin and enzymes (granule exocytosis) to exert the killing (13, 25). CD107a is a lysosomal membrane protein and redistributes to the cell surface upon granule exocytosis. Thus, the surface expression of CD107a is used as a marker for the exocytosis of NK cells (3, 12, 13). We therefore determined whether these events were involved in the inhibition of NK cell cytotoxicity. Although HA did not alter the expression levels of intracellular perforin and granzyme B (Fig. 4D), upon target cell stimulation, HA-treated NK cells showed significantly lower levels of the surface expression of CD107a than mock-treated cells (Fig. 4E), indicating that influenza virus HA inhibited the granule exocytosis of NK cells.
Influenza virus HA downregulates the ζ chain of NK cells through lysosomal pathway.
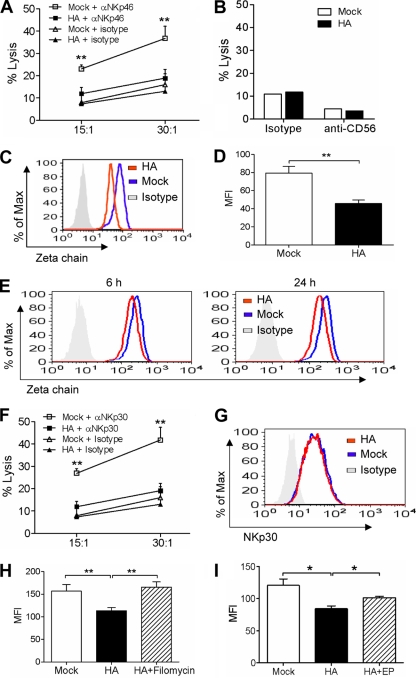
The killing of K562 cells by fresh human NK cells is mediated mainly by the NKp46 receptor (59, 70, 71). To define the specificity of the effect of HA on NK cell activity, we further examined the redirected cytotoxicity of NK cells, which is induced by the monoclonal antibody against a specific receptor and therefore reflects the specific receptor-mediated cytotoxicity. Murine FcγR-positive P815 cells first were incubated with anti-NKp46 monoclonal antibody and then cocultured with NK cells to determine the cell cytotoxicity. As shown in Fig. 5A, anti-NKp46 antibody induced marked lysis against P815 cells by mock-treated NK cells. However, the HA treatment of NK cells significantly inhibited their redirected lysis induced by anti-NKp46 antibody. As a control, anti-CD56 monoclonal antibody did not induce the redirected lysis of NK cells against P815 cells (Fig. 5B).
FIG. 5.
Influenza virus HA downregulates the ζ chain of NK cells through the lysosomal pathway. (A to D) Primary NK cells were left untreated or were treated with 1 μg/ml HA for 24 h. The cells were collected and then determined for their redirected lysis against P815 cells in the presence of anti-NKp46 (A), anti-CD56 (B), or isotype control antibody (A, B) at the indicated E/T ratios (A) or an E/T ratio of 30:1 (B), and for the intracellular expression of the ζ chain (C) with the mean fluorescence intensity (MFI) being determined (D). (E) After incubation with or without 1 μg/ml HA for 6 or 24 h, the cells were fixed, permeabilized, and examined for ζ chain expression by flow cytometry. (F, G) NK cells were left untreated or were treated with 1 μg/ml HA for 24 h. The cells then were determined for their redirected lysis against P815 cells in the presence of anti-NKp30 or isotype control antibody at the indicated E/T ratios (F) and for the expression of NKp30 on the cell surface (G). (H, I) After being left untreated or being treated with filomycin or E-64 and pepstatin A (EP) for 30 min, NK cells were left unincubated or were incubated with 1 μg/ml H1N1 HA in the presence or absence of these reagents for 24 h. Cells were fixed, permeabilized, and determined for ζ chain expression by flow cytometry, and the MFI was determined. The data shown are means ± SEM from four different donors. *, P < 0.05; **, P < 0.01.
NKp46 is a member of the immunoglobulin superfamily, whose intracellular region does not contain immunoreceptor tyrosine-based activation motifs. It couples with the ζ chain to transduce activating signals upon NK cell stimulation (64, 77). We therefore determined whether ζ chain expression in NK cells was altered by HA treatment. As shown in Fig. 5C and D, flow cytometry analysis demonstrated that the intracellular expression of ζ chain in HA-treated NK cells was significantly lower than that in mock-treated cells at 24 h after HA treatment. In addition, the expression of ζ chain also was reduced at 6 h after HA treatment (Fig. 5E), which was in parallel with the decrease of NK cell cytotoxicity at that time point (Fig. 3B).
Although HA does not recognize NKp30 (6) as a natural cytotoxicity receptor, NKp30 also transduces signals through the association with the ζ chain (9, 63). We therefore hypothesized that the downregulation of the ζ chain by HA also would impair NKp30-specific activity. The HA-treated NK cells then were determined for NKp30-induced lysis against P815 cells. As expected, the HA treatment of NK cells significantly inhibited their redirected cytotoxicity induced by anti-NKp30 antibody (Fig. 5F). Similarly to NKp46, the expression of NKp30 on the cell surface was not altered by HA (Fig. 5G).
As many receptors undergo ligand-induced endocytosis followed by recycling or degradation, we further determined the fate of the ζ chain. It was reported that the acidic luminal environment is important for the activation of endolysosomal enzymes and proteolysis (65). The vacuolar-type H+-ATPase inhibitor filomycin increases endolysosomal pH and affects the protease activity, leading to impaired protein degradation (28, 67, 81). We first determined the effect of filomycin on ζ chain expression. NK cells were cultured with HA in the presence or absence of this reagent. After 24 h, the intracellular expression of the ζ chain was analyzed. As shown in Fig. 5H, the HA-induced downregulation of the ζ chain was fully restored by incubation with the H+-ATPase inhibitor. In addition, the inhibitors of lysosomal protease also partially rescued ζ chain downregulation (Fig. 5I). Therefore, these data indicated that HA induced ζ chain degradation through the lysosomal pathway.
The correlation of HA binding, ζ chain expression, and NK cell cytotoxicity.
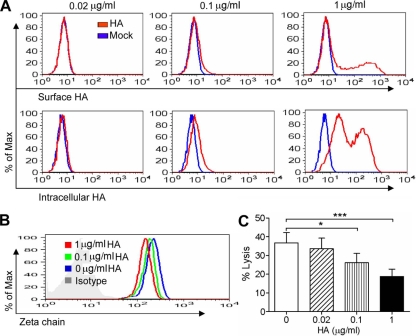
Our present data demonstrated that HA bound to NK cells and induced the downregulation of the ζ chain, which led to the inhibition of the specific cytotoxicity of NK cells. To define the correlation among them, NK cells were cultured with HA at serial concentrations and then determined for HA presence, ζ chain expression, and NKp46-induced cytotoxicity. As shown in Fig. 6A, with the increase of HA concentration, the binding of HA to NK cell surfaces increased. The intracellular presence of HA in NK cells also was increased. Correspondingly, ζ chain expression and redirected cytotoxicity of NK cells induced by anti-NKp46 were decreased in a dose-dependent manner (Fig. 6B and C). Therefore, the more HA binding, the more downregulation of the ζ chain, and the more inhibition of NK cell cytotoxicity.
FIG. 6.
Correlation of HA binding, ζ chain expression, and NK cell cytotoxicity. Primary NK cells were treated with HA at the indicated concentrations for 24 h. The cells were collected and examined for the presence of surface and intracellular HA (A) and for the intracellular expression of the ζ chain (B). The treated NK cells also were determined for their redirected cytotoxicity against P815 cells in the presence of anti-NKp46 antibody at an E/T ratio of 30:1. (C) Samples were analyzed by flow cytometry for the percentage of specific lysis. The data shown are means ± SEM from four different donors. *, P < 0.05; ***, P < 0.001.
Influenza virus HA inhibits Syk and ERK phosphorylation induced by target cell stimulation.
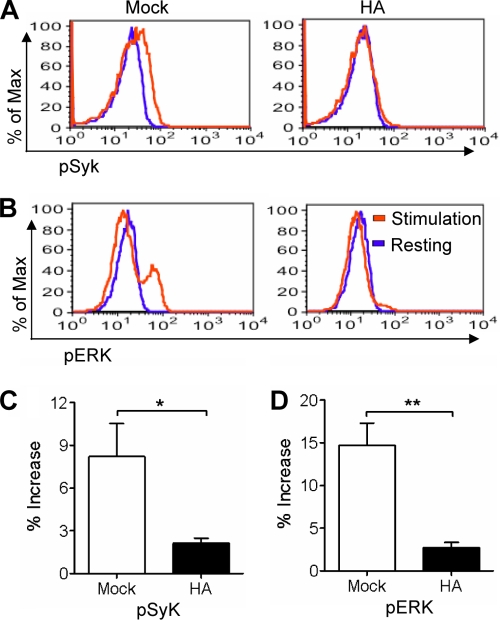
The development of NK cell cytotoxicity against target cells is a multiple-step process. Upon target cell stimulation, the activating signaling event in NK cells leads to a cascade of kinase activation and final exocytosis of cytolytic granules, resulting in the killing of target cells (38, 47, 49). During the process, Syk activation via tyrosine phosphorylation is an early and required signaling event (11). It leads to the activation of downstream signaling pathways through the phosphorylation of signaling proteins, among which ERK is a member, and its activation plays a crucial role and is required for the polarization of cytolytic granules and, therefore, NK cell cytotoxicity (19, 38, 73, 78). To detail the mechanisms underlying the reduced NK cell cytotoxicity by HA, we explored the signaling pathway by flow cytometry. Following target cell stimulation, HA-treated NK cells lost the increase of Syk phosphorylation that was observed in mock-treated cells (Fig. 7A and C). Correspondingly, HA-treated NK cells also showed significantly lower levels of ERK phosphorylation than mock-treated cells (Fig. 7B and D).
FIG. 7.
Influenza virus HA inhibits Syk and ERK phosphorylation induced by target cell stimulation. Primary NK cells were treated with 1 μg/ml HA or left untreated (Mock) for 24 h. The cells were collected and cocultured with (stimulation) or without (resting) K562 cells at a ratio of 1:1 for 5 min. NK cells then were examined by flow cytometry for the expression of intracellular phospho-Syk (A and C) or phospho-ERK (B and D). The increase in the percentage of phosphokinase expression in stimulated NK cells relative to that of resting cells was calculated. Flow figures are representatives of samples from four independent experiments. The data shown are means ± SEM from four different donors. *, P < 0.05; **, P < 0.01.
DISCUSSION
At the early phase of viral infections, NK cells respond to eliminate the invading viruses. For their survival, viruses have developed numerous strategies to evade NK cell immunity (42, 53, 62, 68). In this study, we demonstrated for the first time that both the intact influenza virions and free viral HA markedly inhibited the natural and specific receptor-redirected cytotoxicity of primary human NK cells. These findings suggest that influenza virus developed a novel strategy to evade NK cell innate immune defense that is likely to facilitate viral transmission and also may contribute to virus pathogenesis.
Previous studies have demonstrated that NK cells are involved in host defenses against influenza virus infection. Virus-infected accessory cells, including epithelial cells, dendritic cells, macrophages, and even T cells, induce NK cell activation. The activated NK cells, in turn, kill virus-infected cells (27, 39, 72). However, the activation of NK cells is dependent on the cytokines released from infected cells as well as the interaction between NK cell receptors (NKG2D and NKp46) and the corresponding ligands expressed on the surface of infected accessory cells. Among the cytokines, alpha interferon is responsible for enhanced NK cytotoxicity (27, 39, 69). To dissect the direct effects of influenza virus on NK cells, purified primary human NK cells were used in this study to exclude the effects of accessory cells.
In response to the pressure of NK cells, influenza virus has developed evasion strategies to counter their functions, such as the direct infection of NK cells, the induction of cell apoptosis (37, 57), and the increase in the binding of NK inhibitory receptors to infected cells for the inhibition of NK cell cytotoxicity (1). Although NK cell cytotoxicity was increased at 2 days after influenza infection in mice, the virus was present in the host until almost 1 week, suggesting that in vivo NK cell cytotoxicity is impaired during virus infection. The fact that NK cell activity was decreased with a virus dose-dependent pattern following a transient increase after virus infection in mice further suggested the evasion of NK cell function by influenza virus (24, 61). Contradictory results have been reported for the effects of influenza virus on NK cell activity (increased or decreased cytotoxicity) in studies that used peripheral blood mononuclear cells but not purified NK cells (2, 7). The outcome then would reflect the sum of the effect of accessory cells as discussed above and that of virus itself directly on NK cells. In this study, by using the purified primary human NK cells, we demonstrated that both the intact virion and HA protein inhibited NK cell cytotoxicity. In addition, the inhibition was virus dose dependent, which is compatible with the in vivo findings (24). Therefore, our data provide evidence that influenza virus directly acts on NK cells to modulate their activity.
A previous study reported that the NKp46 recognition of surface HA on influenza virus-infected cells was involved in the NK cell killing of infected cells. However, it is unclear whether the interaction of NKp46 with cell surface HA is sufficient for triggering lysis (6, 56). The receptors other than the natural cytotoxicity receptor and nonviral ligands altered by the infection would be important in regulating the NK cell lysis of influenza virus-infected cells (6, 56, 72). Virally induced changes in the expressions of lymphocyte function-associated antigen-3, transferrin receptor, and intracellular adhesion molecule-1 on cell surfaces have been reported to contribute to the lysis of virus-infected cells by NK cells (29, 33, 54). Indeed, NK cell killing is mediated by the integration of signals from activating and inhibitory receptors, as well as various adhesion and costimulatory molecules (13, 46). A critical threshold of signaling must be achieved for NK cells to mount a productive response (13, 14, 47).
In this study, we demonstrated that influenza virion and HA protein alone markedly inhibited the cytotoxicity of both fresh and IL-2-activated human NK cells. In addition, virus HA not only inhibited the natural cytotoxicity of NK cells but also decreased specific redirected lysis induced by NKp46. This inhibition was not due to NK cell apoptosis, as both the inactivated virion and HA did not increase NK cell death. Neither was this inhibition caused by the masking of NKp46 by HA, as evidenced by the fact that the immobilized HA also inhibited NK cell cytotoxicity. NKp46 associates with the ζ chain to transduce signals (77). Upon target cell simulation, the activation of the ζ chain initiates a cascade signaling pathway, resulting in the final cytotoxicity (11, 49, 73). Here, we found that HA bound to NK cells, which led to the downregulation of the ζ chain in NK cells. Correspondingly, upon target cell stimulation, HA-treated NK cells showed significantly lower activation of Syk and ERK, resulting in decreased granule exocytosis and, finally, reduced NK cell cytotoxicity compared to those of mock-treated cells. Although the enhanced cell apoptosis induced by virus infection (37, 57) could partly contribute to the decreased NK cell activity (24, 37), our present findings of the downregulation of the ζ chain induced by HA provide a specific underlying mechanism to account for the specific inhibition of NK cell cytotoxicity.
It is interesting that influenza virus HA induced ζ chain downregulation in NK cells. Indeed, the downregulation of the ζ chain was described frequently for T cells in cancer, infection, and autoimmune disorders and was associated with impaired cell function (8, 10). For NK cells, the ζ chain downregulation has been reported in patients with a variety of cancers and was related to cell dysfunction (21, 44, 45, 58, 60). NK cells from HIV-infected patients also showed considerably lower levels of the ζ chain and decreased cytotoxicity (34). Here, we found that influenza virus HA induced the downregulation of the ζ chain, which was shown to be mediated through the lysosomal pathway. We further demonstrated the correlation among HA binding, ζ chain expression, and NK cell cytotoxicity. Like NKp46, NKp30 is a natural cytotoxicity receptor and associates with the ζ chain to transduce activating signals (9, 63). Influenza virus HA does not recognize NKp30 (6) and was shown here not to change NKp30 expression on NK cells; however, due to the downregulation of the ζ chain by HA, as expected, HA-treated NK cells presented a significantly decreased redirected lysis induced by anti-NKp30 antibody.
Indeed, the virus-mediated inhibition of NK-activating receptor function is an important NK immunoevasion strategy and has been reported commonly in cancer and virus infections (42, 62). Although the ligand engagement by NKG2D leads to the NK cell killing of target cells, the tumor-derived free major histocompatibility complex (MHC) class I chain-related molecule impaired NK cytotoxicity through the recognition of NKG2D as a tumor immunoevasion mechanism (36). In virus infection, an orthopoxvirus protein was reported to inhibit NKG2D-dependent NK cell killing via binding to NKG2D (16). Human cytomegalovirus pp65 protein is one of the two currently known ligands for natural cytotoxicity receptors and also was demonstrated to inhibit NK cell cytotoxicity via binding the NKp30-activating receptor, acting as an NK evasion mechanism (4). Previous data showed that an envelope protein of hepatitis C virus inhibited NK cell activity (23, 74), while the whole virion did not alter the cell function (80). In investigating the direct effects of influenza virus on NK cells, we demonstrated here the identical impact of UV-inactivated influenza virus particles and viral HA protein. Live influenza virus induces NK cell apoptosis and reduces cell cytotoxicity (57), while the inactivated intact virion and HA inhibited primary human NK cell cytotoxicity. Therefore, considering the constant battle and coevolution between viruses and NK cells, we speculate that our findings here represent a novel strategy of influenza virus to evade NK cell immunity.
The contest between NK cells and invading viruses is crucial in determining the outcome of an acute viral infection (62, 68). Here, we revealed a novel NK cell immunoevasion mechanism of influenza virus. This viral evasion strategy will allow the virus to replicate to the high titers necessary for successful transmission to new hosts before the onset of specific immune responses (42). Such an innate immune evasion strategy is particularly beneficial for virus transmission and also may contribute to virus pathogenesis (31, 42, 43). With the looming threat of current H1N1 influenza virus pandemic, a full investigation of the interaction of influenza viruses and NK cells is important for better understanding influenza pathogenesis and for developing more effective prophylaxis and treatment of this disease.
Acknowledgments
This work was supported in part by the General Research Fund, Research Grants Council of Hong Kong (HKU 777108 M, HKU768108, HKU777407; W.T. and Y.-L.L.); the Area of Excellence program on influenza supported by the University Grants Committee of the Hong Kong Special Administrative Region, China (project no. AoE/M-12/06) (J.S.M.P., Y.-L.L., and W.T.); Seed Funding for Basic Research, University Research Committee, the University of Hong Kong (200611159224; W.T.); postgraduate studentships from the University of Hong Kong (H.M., G.Q., and J.Z.); and the Edward Sai-Kim Hotung Pediatric Education and Research Fund (Y.-L.L.).
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Achdout, H., I. Manaster, and O. Mandelboim. 2008. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J. Virol. 82:8030-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, S. A., R. C. Rees, and J. Oxford. 1984. Modulation of human natural killer cytotoxicity by influenza virus and its subunit protein. Immunology 52:687-695. [PMC free article] [PubMed] [Google Scholar]
- 3.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 4.Arnon, T. I., H. Achdout, O. Levi, G. Markel, N. Saleh, G. Katz, R. Gazit, T. Gonen-Gross, J. Hanna, E. Nahari, A. Porgador, A. Honigman, B. Plachter, D. Mevorach, D. G. Wolf, and O. Mandelboim. 2005. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat. Immunol. 6:515-523. [DOI] [PubMed] [Google Scholar]
- 5.Arnon, T. I., H. Achdout, N. Lieberman, R. Gazit, T. Gonen-Gross, G. Katz, A. Bar-Ilan, N. Bloushtain, M. Lev, A. Joseph, E. Kedar, A. Porgador, and O. Mandelboim. 2004. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood 103:664-672. [DOI] [PubMed] [Google Scholar]
- 6.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. [DOI] [PubMed] [Google Scholar]
- 7.Arora, D. J., M. Houde, D. M. Justewicz, and R. Mandeville. 1984. In vitro enhancement of human natural cell-mediated cytotoxicity by purified influenza virus glycoproteins. J. Virol. 52:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baniyash, M. 2004. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 4:675-687. [DOI] [PubMed] [Google Scholar]
- 9.Biassoni, R., C. Cantoni, M. Falco, D. Pende, R. Millo, L. Moretta, C. Bottino, and A. Moretta. 2000. Human natural killer cell activating receptors. Mol. Immunol. 37:1015-1024. [DOI] [PubMed] [Google Scholar]
- 10.Bronstein-Sitton, N., L. Cohen-Daniel, I. Vaknin, A. V. Ezernitchi, B. Leshem, A. Halabi, Y. Houri-Hadad, E. Greenbaum, Z. Zakay-Rones, L. Shapira, and M. Baniyash. 2003. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat. Immunol. 4:957-964. [DOI] [PubMed] [Google Scholar]
- 11.Brumbaugh, K. M., B. A. Binstadt, D. D. Billadeau, R. A. Schoon, C. J. Dick, R. M. Ten, and P. J. Leibson. 1997. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J. Exp. Med. 186:1965-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryceson, Y. T., M. E. March, D. F. Barber, H. G. Ljunggren, and E. O. Long. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryceson, Y. T., M. E. March, H. G. Ljunggren, and E. O. Long. 2006. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214:73-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryceson, Y. T., M. E. March, H. G. Ljunggren, and E. O. Long. 2006. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussfeld, D., A. Kaufmann, R. G. Meyer, D. Gemsa, and H. Sprenger. 1998. Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactivated influenza A virus. Cell Immunol. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Campbell, J. A., D. S. Trossman, W. M. Yokoyama, and L. N. Carayannopoulos. 2007. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J. Exp. Med. 204:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castino, R., D. Pace, M. Demoz, M. Gargiulo, C. Ariatta, E. Raiteri, and C. Isidoro. 2002. Lysosomal proteases as potential targets for the induction of apoptotic cell death in human neuroblastomas. Int. J. Cancer 97:775-779. [DOI] [PubMed] [Google Scholar]
- 18.Chen, B. J., G. P. Leser, E. Morita, and R. A. Lamb. 2007. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J. Virol. 81:7111-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, X., D. S. Allan, K. Krzewski, B. Ge, H. Kopcow, and J. L. Strominger. 2006. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc. Natl. Acad. Sci. USA 103:10346-10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 21.Ciszak, L., A. Kosmaczewska, B. Werynska, A. Szteblich, R. Jankowska, and I. Frydecka. 2009. Impaired zeta chain expression and IFN-gamma production in peripheral blood T and NK cells of patients with advanced lung cancer. Oncol. Rep. 21:173-184. [PubMed] [Google Scholar]
- 22.Cooper, M. A., T. A. Fehniger, and M. A. Caligiuri. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633-640. [DOI] [PubMed] [Google Scholar]
- 23.Crotta, S., A. Stilla, A. Wack, A. D'Andrea, S. Nuti, U. D'Oro, M. Mosca, F. Filliponi, R. M. Brunetto, F. Bonino, S. Abrignani, and N. M. Valiante. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Gobbo, V., N. Villani, S. Marini, E. Balestra, and R. Calio. 1990. Suppressor cells induced by influenza virus inhibit interleukin-2 production in mice. Immunology 69:454-459. [PMC free article] [PubMed] [Google Scholar]
- 25.Djeu, J. Y., K. Jiang, and S. Wei. 2002. A view to a kill: signals triggering cytotoxicity. Clin. Cancer Res. 8:636-640. [PubMed] [Google Scholar]
- 26.Doherty, P. C., S. J. Turner, R. G. Webby, and P. G. Thomas. 2006. Influenza and the challenge for immunology. Nat. Immunol. 7:449-455. [DOI] [PubMed] [Google Scholar]
- 27.Draghi, M., A. Pashine, B. Sanjanwala, K. Gendzekhadze, C. Cantoni, D. Cosman, A. Moretta, N. M. Valiante, and P. Parham. 2007. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J. Immunol. 178:2688-2698. [DOI] [PubMed] [Google Scholar]
- 28.Dröse, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 29.Eisenthal, A., O. Marder, B. Lifschitz-Mercer, Y. Skornick, R. Tirosh, Y. Irlin, R. Avtalion, and M. Deutsch. 1997. Infection of K562 cells with influenza A virus increases their susceptibility to natural killer lysis. Pathobiology 65:331-340. [DOI] [PubMed] [Google Scholar]
- 30.El-Sherbiny, Y. M., J. L. Meade, T. D. Holmes, D. McGonagle, S. L. Mackie, A. W. Morgan, G. Cook, S. Feyler, S. J. Richards, F. E. Davies, G. J. Morgan, and G. P. Cook. 2007. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 67:8444-8449. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Sesma, A., S. Marukian, B. J. Ebersole, D. Kaminski, M. S. Park, T. Yuen, S. C. Sealfon, A. Garcia-Sastre, and T. M. Moran. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80:6295-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finlay, B. B., and G. McFadden. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767-782. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher, J. M., H. G. Prentice, and J. E. Grundy. 1998. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J. Immunol. 161:2365-2374. [PubMed] [Google Scholar]
- 34.Geertsma, M. F., A. Stevenhagen, E. M. van Dam, and P. H. Nibbering. 1999. Expression of zeta molecules is decreased in NK cells from HIV-infected patients. FEMS Immunol. Med. Microbiol. 26:249-257. [DOI] [PubMed] [Google Scholar]
- 35.Grégoire, C., L. Chasson, C. Luci, E. Tomasello, F. Geissmann, E. Vivier, and T. Walzer. 2007. The trafficking of natural killer cells. Immunol. Rev. 220:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groh, V., J. Wu, C. Yee, and T. Spies. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734-738. [DOI] [PubMed] [Google Scholar]
- 37.Guo, H., P. Kumar, T. M. Moran, A. Garcia-Sastre, Y. Zhou, and S. Malarkannan. 2009. The functional impairment of natural killer cells during influenza virus infection. Immunol. Cell Biol. 87:579-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamerman, J. A., and L. L. Lanier. 2006. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006:re1. [DOI] [PubMed]
- 39.He, X. S., M. Draghi, K. Mahmood, T. H. Holmes, G. W. Kemble, C. L. Dekker, A. M. Arvin, P. Parham, and H. B. Greenberg. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heltzer, M. L., S. E. Coffin, K. Maurer, A. Bagashev, Z. Zhang, J. S. Orange, and K. E. Sullivan. 2009. Immune dysregulation in severe influenza. J. Leukoc. Biol. 85:1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heusel, J. W., R. L. Wesselschmidt, S. Shresta, J. H. Russell, and T. J. Ley. 1994. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76:977-987. [DOI] [PubMed] [Google Scholar]
- 42.Jonjić, S., M. Babic, B. Polic, and A. Krmpotic. 2008. Immune evasion of natural killer cells by viruses. Curr. Opin. Immunol. 20:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katze, M. G., J. L. Fornek, R. E. Palermo, K. A. Walters, and M. J. Korth. 2008. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat. Rev. Immunol. 8:644-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kono, K., M. E. Ressing, R. M. Brandt, C. J. Melief, R. K. Potkul, B. Andersson, M. Petersson, W. M. Kast, and R. Kiessling. 1996. Decreased expression of signal-transducing zeta chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin. Cancer Res. 2:1825-1828. [PubMed] [Google Scholar]
- 45.Lai, P., H. Rabinowich, P. A. Crowley-Nowick, M. C. Bell, G. Mantovani, and T. L. Whiteside. 1996. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 2:161-173. [PubMed] [Google Scholar]
- 46.Lanier, L. L. 2001. On guard—activating NK cell receptors. Nat. Immunol. 2:23-27. [DOI] [PubMed] [Google Scholar]
- 47.Lanier, L. L. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latham, T., and J. M. Galarza. 2001. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 75:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leibson, P. J. 1997. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity 6:655-661. [DOI] [PubMed] [Google Scholar]
- 50.Lewis, D. B. 2006. Avian flu to human influenza. Annu. Rev. Med. 57:139-154. [DOI] [PubMed] [Google Scholar]
- 51.Ljunggren, H. G., and K. Karre. 1990. In search of the missing self: MHC molecules and NK cell recognition. Immunol. Today 11:237-244. [DOI] [PubMed] [Google Scholar]
- 52.Lodoen, M. B., and L. L. Lanier. 2006. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 18:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lodoen, M. B., and L. L. Lanier. 2005. Viral modulation of NK cell immunity. Nat. Rev. Microbiol. 3:59-69. [DOI] [PubMed] [Google Scholar]
- 54.López-Guerrero, J. A., B. Alarcon, and M. Fresno. 1988. Mechanism of recognition of herpes simplex virus type 1-infected cells by natural killer cells. J. Gen. Virol. 69(Pt 11):2859-2868. [DOI] [PubMed] [Google Scholar]
- 55.Lowin, B., M. C. Peitsch, and J. Tschopp. 1995. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 198:1-24. [DOI] [PubMed] [Google Scholar]
- 56.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055-1060. [DOI] [PubMed] [Google Scholar]
- 57.Mao, H., W. Tu, G. Qin, H. K. Law, S. F. Sia, P. L. Chan, Y. Liu, K. T. Lam, J. Zheng, M. Peiris, and Y. L. Lau. 2009. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J. Virol. 83:9215-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuda, M., M. Petersson, R. Lenkei, J. L. Taupin, I. Magnusson, H. Mellstedt, P. Anderson, and R. Kiessling. 1995. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int. J. Cancer 61:765-772. [DOI] [PubMed] [Google Scholar]
- 59.Mavoungou, E., M. K. Bouyou-Akotet, and P. G. Kremsner. 2005. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30). Clin. Exp. Immunol. 139:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakagomi, H., M. Petersson, I. Magnusson, C. Juhlin, M. Matsuda, H. Mellstedt, J. L. Taupin, E. Vivier, P. Anderson, and R. Kiessling. 1993. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 53:5610-5612. [PubMed] [Google Scholar]
- 61.Nogusa, S., B. W. Ritz, S. H. Kassim, S. R. Jennings, and E. M. Gardner. 2008. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech. Ageing Dev. 129:223-230. [DOI] [PubMed] [Google Scholar]
- 62.Orange, J. S., M. S. Fassett, L. A. Koopman, J. E. Boyson, and J. L. Strominger. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006-1012. [DOI] [PubMed] [Google Scholar]
- 63.Pende, D., S. Parolini, A. Pessino, S. Sivori, R. Augugliaro, L. Morelli, E. Marcenaro, L. Accame, A. Malaspina, R. Biassoni, C. Bottino, L. Moretta, and A. Moretta. 1999. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190:1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pessino, A., S. Sivori, C. Bottino, A. Malaspina, L. Morelli, L. Moretta, R. Biassoni, and A. Moretta. 1998. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 188:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillay, C. S., E. Elliott, and C. Dennison. 2002. Endolysosomal proteolysis and its regulation. Biochem. J. 363:417-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin, G., H. Mao, J. Zheng, S. F. Sia, Y. Liu, P. L. Chan, K. T. Lam, J. S. Peiris, Y. L. Lau, and W. Tu. 2009. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J. Infect. Dis. 200:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scalzo, A. A. 2002. Successful control of viruses by NK cells—a balance of opposing forces? Trends Microbiol. 10:470-474. [DOI] [PubMed] [Google Scholar]
- 69.Sirén, J., T. Sareneva, J. Pirhonen, M. Strengell, V. Veckman, I. Julkunen, and S. Matikainen. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J. Gen. Virol. 85:2357-2364. [DOI] [PubMed] [Google Scholar]
- 70.Sivori, S., D. Pende, C. Bottino, E. Marcenaro, A. Pessino, R. Biassoni, L. Moretta, and A. Moretta. 1999. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 29:1656-1666. [DOI] [PubMed] [Google Scholar]
- 71.Sivori, S., M. Vitale, L. Morelli, L. Sanseverino, R. Augugliaro, C. Bottino, L. Moretta, and A. Moretta. 1997. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 186:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spies, T., and V. Groh. 2006. Natural cytotoxicity receptors: influenza virus in the spotlight. Nat. Immunol. 7:443-444. [DOI] [PubMed] [Google Scholar]
- 73.Trotta, R., K. A. Puorro, M. Paroli, L. Azzoni, B. Abebe, L. C. Eisenlohr, and B. Perussia. 1998. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J. Immunol. 161:6648-6656. [PubMed] [Google Scholar]
- 74.Tseng, C. T., and G. R. Klimpel. 2002. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu, W., Y. L. Lau, J. Zheng, Y. Liu, P. L. Chan, H. Mao, K. Dionis, P. Schneider, and D. B. Lewis. 2008. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood 112:2554-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valitutti, S., S. Muller, M. Salio, and A. Lanzavecchia. 1997. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med. 185:1859-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vitale, M., C. Bottino, S. Sivori, L. Sanseverino, R. Castriconi, E. Marcenaro, R. Augugliaro, L. Moretta, and A. Moretta. 1998. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J. Exp. Med. 187:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei, S., A. M. Gamero, J. H. Liu, A. A. Daulton, N. I. Valkov, J. A. Trapani, A. C. Larner, M. J. Weber, and J. Y. Djeu. 1998. Control of lytic function by mitogen-activated protein kinase/extracellular regulatory kinase 2 (ERK2) in a human natural killer cell line: identification of perforin and granzyme B mobilization by functional ERK2. J. Exp. Med. 187:1753-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoon, J. C., M. Shiina, G. Ahlenstiel, and B. Rehermann. 2009. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology 49:12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshimori, T., A. Yamamoto, Y. Moriyama, M. Futai, and Y. Tashiro. 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266:17707-17712. [PubMed] [Google Scholar]
- 82.Zheng, J., Y. Liu, G. Qin, P. L. Chan, H. Mao, K. T. Lam, D. B. Lewis, Y. L. Lau, and W. Tu. 2009. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J. Immunol. 183:3742-3750. [DOI] [PubMed] [Google Scholar]
- 83.Zhou, J., H. K. Law, C. Y. Cheung, I. H. Ng, J. S. Peiris, and Y. L. Lau. 2006. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J. Infect. Dis. 194:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]