Abstract
Dengue virus (DENV) infects human immune cells in vitro and likely infects dendritic cells (DCs) in vivo. DENV-2 productive infection induces activation and release of high levels of chemokines and proinflammatory cytokines in monocyte-derived DCs (moDCs), with the notable exception of alpha/beta interferon (IFN-α/β). Interestingly, DENV-2-infected moDCs fail to prime T cells, most likely due to the lack of IFN-α/β released by moDCs, since this effect was reversed by addition of exogenous IFN-β. Together, our data show that inhibition of IFN-α/β production by DENV in primary human moDCs is a novel mechanism of immune evasion.
Dengue virus (DENV) belongs to the family Flaviviridae and has great importance in the areas of medicine and biodefense (3, 17). Transmitted by Aedes aegypti, DENV is the most prevalent arthropod-borne human virus (34). Generally, infected patients experience dengue fever, but 2 to 20% of cases manifest dengue hemorrhagic fever (DHF), a severe and often lethal illness (34). Dendritic cells (DCs) initiate immune responses and become activated upon contact with pathogens (2), with upregulation of costimulatory molecules and release of proinflammatory cytokines (2). Secretion of type I interferon (alpha/beta interferon [IFN-α/β]) by DCs contributes to the generation of antiviral innate and adaptive immune responses (7, 16, 18, 32). DCs, together with other cell populations, have been suggested as target cells for DENV infection (12, 14, 15, 35).
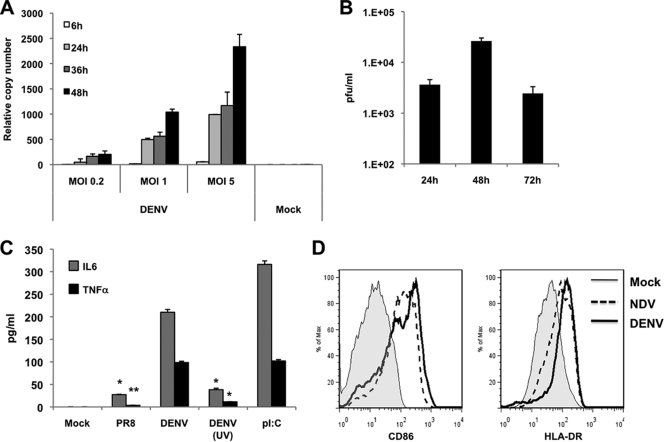
We investigated the replication of DENV-2 in monocyte-derived DCs (moDCs) obtained by culturing CD14+ cells, isolated from blood of healthy donors, for 6 days in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 4 (IL-4), and human serum (9). The purity obtained by flow cytometry was routinely 96 to 99% CD14− CD11c+ HLA-DRlow moDCs (7). DENV-2 (16681 and NGC strains) and DENV-3 (Hawaii strain) were grown in C6/36 insect cells (5) and were titrated by plaque assay (4). moDCs were infected with DENV-2 at different multiplicities of infection (MOIs), and viral replication was tested by quantitative reverse transcription-PCR (qRT-PCR) (9, 28). All infections in this study were performed in moDCs from at least three independent donors with three replicates per sample. DENV-2 replication peaked at 48 h after infection (Fig. 1A), and release of newly synthesized infectious particles had a similar kinetics (Fig. 1B), indicating productive infection of moDCs. As a measure of moDC activation, proinflammatory cytokine levels (IL-6 and tumor necrosis factor alpha [TNF-α]) were quantified by enzyme-linked immunosorbent assay (ELISA) 24 h after DENV-2 infection (Fig. 1C). We observed high expression of those cytokines, comparable to cytokine expression after poly(I:C) treatment of moDCs. Interestingly, UV-inactivated DENV-2 did not induce the moDC activation, suggesting that DENV-2 replication is required to activate moDCs. Infection with influenza virus A/PR8/33 (PR8) showed low levels of cytokine induction due to the activity of the NS1 protein (6). Flow cytometry analysis showed high upregulation of CD86 and HLA-DR markers on DENV-2-infected moDCs, comparable to that observed with Newcastle disease virus (NDV) infection (Fig. 1D). NDV (Hitchner strain) and PR8 viruses, used as controls, were grown in 9-day-old embryonated chicken eggs (8, 24, 27). Together our data demonstrate that DENV-2 is able to productively infect and strongly activate human moDCs.
FIG. 1.
Infection and activation of moDCs with DENV-2. (A) DENV-2 RNA levels were measured by specific qRT-PCR of the NS5 in moDCs infected with the indicated MOIs of DENV-2 at different times after infection (6, 24, 36, and 48 h). (B) Titration by plaque assay of the supernatants from moDCs at different times after infection with DENV at an MOI of 1. (C) Quantification 24 h after infection of the levels of IL-6 and TNF-α in the supernatants of moDCs that were either mock infected, infected with DENV-2, UV-treated DENV-2, or influenza virus A/PR8/33 at an MOI of 1, or treated with poly(I:C) (1 μg/ml). Data in panels A, B, and C are the means and standard deviations of three replicates from a representative donor. (D) CD86 and HLA-DR surface expression on moDCs 24 h after infection with DENV-2 at an MOI of 1 (thick line) or with NDV at an MOI of 0.5 (dotted line) or mock treatment (thin line). Differences between data for PR8 and DENV-2 UV and those for DENV-2 were statistically significant. *, P < 0.05; **, P < 0.01.
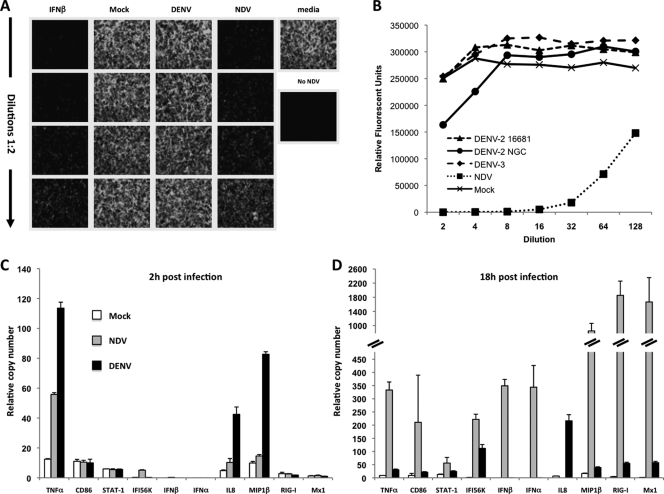
Type I IFN plays an important role in DENV infection, as low levels of the IFN-α/β producing plasmacytoid DCs (pDCs) have been observed in DHF patients (29). Also, the ability of DENV to inhibit IFN-α/β signaling in cells has been recognized (21) and attributed to several DENV proteins (1, 13, 20, 22, 23). In human moDCs, DENV-2 antagonizes IFN-α/β but not IFN-γ signaling (10). Because of the importance of IFN-α/β in the generation of antiviral immune responses (7, 16, 18, 32), we measured functional IFN-α/β by performing a bioassay in Vero cells using green fluorescent protein-tagged NDV (NDV-GFP) (27). Supernatants from mock- and DENV-2-infected moDCs did not impair NDV-GFP infection (Fig. 2A). However, supernatants from NDV-infected moDCs considerably reduced the number of GFP-positive cells, achieving about a 50% reduction at the highest dilution. When we used different strains or serotypes of DENV, New Guinea C (DENV-2 NGC) and DENV-3 (Hawaii strain) (Fig. 2B), neither one was able to induce significant IFN-α/β production. These results strongly indicate that moDCs do not produce functional IFN-α/β after DENV infection. We used qRT-PCR to extensively analyze the expression profile of genes of the IFN-α/β pathway as well as moDC activation genes. We observed a marked chemokine profile (IL-8, MIP1β) as early as 2 h after DENV-2 infection (Fig. 2C), as has also been observed in patients' sera (30), suggesting a possible mechanism of DENV-2 to attract cells for virus amplification. This is in contrast with the cytokine/chemokine profile observed in NDV-infected moDCs (Fig. 2C and D), where no IL-8 or MIP1β production was observed at early times after infection (Fig. 2C) and high levels of TNF-α were induced. According to the bioassay data, there was no expression of IFN-α/β after DENV-2 infection as opposed to NDV infection. However, some IFN-inducible genes, like Mx1, were upregulated by DENV-2 18 h after infection (Fig. 2D). This finding has been previously observed in Rhesus macaques after DENV infection (31) and has also been detected in human moDCs after infection with influenza viruses that have the intact IFN antagonist NS1 protein (9). It is likely that traces of IFN-α/β under our level of detection produced by infected and/or surrounding cells may induce the expression of those interferon-stimulated genes (ISGs). Nevertheless, the induction levels of Mx1 were significantly lower after DENV than after NDV infection (Fig. 2D).
FIG. 2.
Type I IFN bioassay and gene expression profile of moDCs after DENV-2 infection. Vero cells were infected with NDV-GFP after incubation for 24 h with serial dilutions of supernatants collected from moDCs that had been infected with DENV-2 or NDV or mock infected. (A) Representative pictures of the GFP expression 18 h after NDV-GFP infection. As a positive control of IFN-β activity, Vero cells were incubated with serial dilutions of recombinant IFN-β (starting at 1,000 U/ml). Proper positive (medium) and negative (no NDV-GFP) controls for NDV infection were also included. (B) Quantification of the type I IFN bioassay. Vero cells were incubated with supernatants from moDCs infected with the indicated viruses and then infected with NDV-GFP. All infections were performed at an MOI of 1. (C and D) Expression levels of the indicated genes were analyzed by specific qRT-PCR 2 h (C) or 18 h (D) after infection of moDCs with DENV-2 at an MOI of 1 or NDV at an MOI of 0.5 or with no infection. Data are the means and standard deviations of three sample replicates from a representative donor. In all cases, the variability within relative values between the samples in the different donors was lower than 15%.
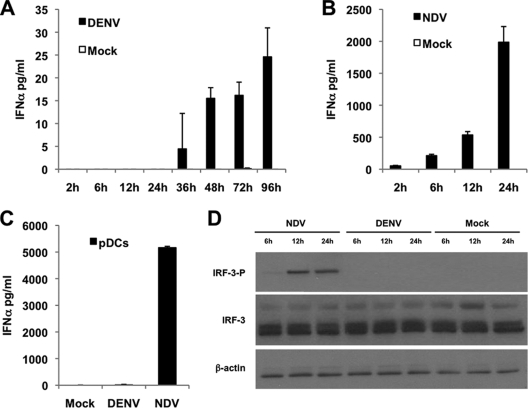
Additionally, we measured the IFN-α protein levels in the supernatant of moDCs and pDCs after DENV-2 or NDV infection (Fig. 3) using a panspecific human IFN-α ELISA. We observed extremely low IFN-α levels in supernatants of DENV-2-infected moDCs and only later than 36 h after infection (Fig. 3A). UV-inactivated DENV-2 did not induce IFN-α production (data not shown), indicating that binding to the receptor is not sufficient to induce an antiviral response. However, NDV-infected moDCs showed a fast and high IFN-α production (Fig. 3B). pDCs, in spite of DENV replication (data not shown), did not show any significant IFN-α production 8 h after infection, in contrast with the strong induction after NDV infection (Fig. 3C). Our results clearly demonstrate that DENV-2 is able to block or may not induce IFN-α/β in human primary immune cells. Although some reports showed low levels of IFN-α/β production after DENV infection (11, 25, 26, 33), the measurements were performed 24 h or later after infection and were performed using a high MOI, which does not reflect IFN induction by viruses and may overestimate the ability of DENV to induce this cytokine. To identify a possible mechanism of the absence of IFN-α/β induction, we analyzed the phosphorylation levels of the IFN regulatory factor 3 (IRF-3), an important element in the IFN-α/β induction pathway. We could not detect IRF-3 phosphorylation in DENV-2 infected moDCs (Fig. 3D) by Western blotting, although 58% of moDCs were infected by DENV-2 at an MOI of 1. In contrast, NDV infection induced strong IRF-3 phosphorylation in moDCs 12 h after infection, with 63% of the moDCs being infected with this virus. This observation indicates that DENV-2 inhibits IFN-α/β production in moDCs upstream or at the level of IRF-3 phosphorylation, while the NF-κB pathway seems unaffected, since we observed high levels of proinflammatory cytokines and chemokines after DENV infection (Fig. 1C and 2C). Taken together, these results indicate that DENV-2 is able to induce a strong activation of the moDCs, with the notable exception of IFN-α/β production.
FIG. 3.
Impaired IFN-α/β interferon production by moDCs after DENV-2 infection. IFN-α levels in cell supernatants were quantified by a panspecific IFN-α ELISA kit at the indicated time points after infection of the moDCs with DENV-2 (A) or NDV (B) at an MOI of 1. (C) IFN-α levels measured similarly 8 h after infection of pDCs with DENV-2 or NDV at MOI of 1 or no infection. Data are the means and standard deviations of three sample replicates from a representative donor. (D) Analysis of IRF-3 phosphorylation in moDCs by Western blotting. Cell lysates were collected 6 h, 12 h, and 24 h after infection of moDCs with NDV or DENV-2 (each at an MOI of 1) or no infection. Total and phosphorylated forms of IRF-3 were detected using specific antibodies.
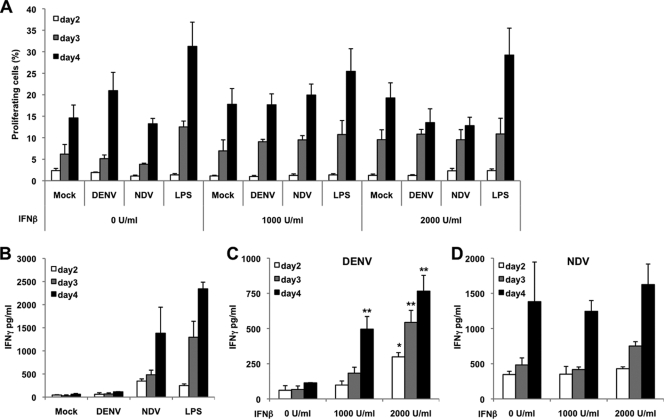
Since production of IFN-α/β is crucial for T-cell priming in virus infections (16, 32), and since exposure of DCs to IFN-α/β prior to infection with influenza virus primes those cells to better responses (28), we analyzed the ability of DENV-2-infected moDCs to prime T cells. We incubated moDCs that were either noninfected, treated with lipopolysaccharide (LPS) (0.5 μg/ml), or infected with NDV (MOI of 0.5) or DENV-2 (MOI of 1) with allogeneic naïve T cells at a 1:1 ratio (105 DCs/105 T cells). T cells isolated from buffy coats were labeled with carboxyfluorescein succinimidyl ester (CFSE; 5 μM) in order to measure their proliferation by flow cytometry. All moDCs, independently of the treatment, were able to induce T-cell proliferation (Fig. 4A), indicating viability and lack of cytotoxicity of DENV-2-infected moDCs. LPS induced a stronger activation of moDCs, resulting in higher T-cell proliferation in the cocultures. Addition of recombinant human IFN-β to the coculture medium had a minimal effect on T-cell proliferation (Fig. 4A). No proliferation was observed in T cells alone, in either the presence or absence of exogenous IFN-β (data not shown). T cells cocultured with DENV-2-infected moDCs produced very small amounts of IFN-γ at day 2 that did not increase with time (Fig. 4B). This observation agrees with the impaired T-cell proliferation seen in patients with acute DENV-2 infection (19). However, T cells cocultured with either NDV-infected or LPS-treated moDCs produced larger amounts of IFN-γ that increased with time. To demonstrate that the lack of IFN-α/β production by DENV-2 infected moDCs was responsible for their impaired ability to prime T cells, we performed cocultures in the presence of recombinant human IFN-β (1,000 and 2,000 U/ml). The results shown in Fig. 4C clearly demonstrate that addition of exogenous IFN-β significantly restored the production of IFN-γ by T cells, indicating that production of IFN-α/β by moDCs is crucial for the priming of T cells. On the other hand, addition of IFN-β to the cocultures of T cells with NDV-infected moDCs did not increase the levels of IFN-γ (Fig. 4D), since the IFN-α/β produced by NDV-infected moDCs might be sufficient to induce T-cell priming. Addition of IFN-β to the cocultures with mock-infected moDCs as well as to the T cells alone did not have any effect on the release of IFN-γ (data not shown). Altogether, these data indicate that infection of moDCs with DENV-2 impairs their ability to prime T cells regardless of the strong activation induced in those cells, due to the lack of IFN-α/β produced in those cells after infection.
FIG. 4.
DENV-2-infected moDCs fail to induce Th1 responses in allogeneic T cells. Allogeneic T cells were cocultured for 2, 3, and 4 days with DENV-2 (MOI = 1)- or NDV (MOI = 0.5)-infected moDCs (ratio, 1:1). As positive and negative controls, mock- and LPS (0.5 μg/ml)-treated moDCs were included. Some groups of infected moDCs and T cells were cocultured in the presence of 1,000 or 2,000 U/ml of recombinant human IFN-β. (A) Analysis of T-cell proliferation measured after CFSE labeling of the purified T cells cocultured for several days in the presence of different concentrations of recombinant human IFN-β. Data are the percentages of proliferating labeled T cells after different days of coculturing. (B) Production of IFN-γ by those T cells cocultured with mock-infected, DENV-infected, NDV-infected, or LPS-treated moDCs in the absence of exogenously added recombinant human IFN-β. Quantification of IFN-γ released by T cells cocultured with DENV-infected moDCs (C) or NDV-infected moDCs (D) in the presence of different concentrations of recombinant human IFN-β. Data are the means and standard deviations of three sample replicates per condition from a representative donor. Significant differences were observed between samples treated with recombinant IFN-β and nontreated ones. *, P < 0.05; **, P < 0.01. The variability within relative values between samples in the different donors was lower than 15%.
In conclusion, our results provide new data about how DENV-2 can induce activation of moDCs and release of proinflammatory cytokines, except IFN-α/β. We also demonstrate that DENV-2 infection blocks IRF-3 phosphorylation in moDCs, impairing the subsequent IFN-α/β production in response to the infection. This lack of IFN-α/β production renders DENV-infected moDCs incapable of priming T cells toward antiviral Th1 responses, regardless of other markers of activation observed in those moDCs after DENV-2 infection. These data strongly suggest that lack of IFN-α/β induction in immune cells after DENV infection contributes to the inefficient and cross-reactive adaptive immune responses observed in patients. Since therapeutic options for dengue remain modest due to the lack of vaccine or antiviral treatments available, our findings will help in the design of treatments for dengue disease, as they suggest that targeting IFN-α/β production may be an efficient antiviral therapy for DENV.
Acknowledgments
We thank Jorge Munoz-Jordan, Sujan Shresta, Irene Bosch, and Maudry Laurent-Rolle for protocols and reagents. We also acknowledge Hannah Phipps-Yonas, for help with pDC isolations and Dailia Francis for contributions during early stages of the experiments.
This work was funded by 1R01AI073450-01A2 (A.F.-S.), U19 AI62623 (A.F.-S.), and a Ramon Areces Foundation fellowship (J.R.R.-M.).
We have no competing financial interests.
Footnotes
Published ahead of print on 17 February 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Ashour, J., M. Laurent-Rolle, P. Y. Shi, and A. Garcia-Sastre. 2009. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 83:5408-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Clyde, K., J. L. Kyle, and E. Harris. 2006. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J. Virol. 80:11418-11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Sesma, A. 2007. The influenza virus NS1 protein: inhibitor of innate and adaptive immunity. Infect. Disord Drug Targets 7:336-343. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Sesma, A., S. Marukian, B. J. Ebersole, D. Kaminski, M. S. Park, T. Yuen, S. C. Sealfon, A. Garcia-Sastre, and T. M. Moran. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80:6295-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 9.Haye, K., S. Burmakina, T. Moran, A. Garcia-Sastre, and A. Fernandez-Sesma. 2009. The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. J. Virol. 83:6849-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, L. J., L. F. Hung, C. Y. Weng, W. L. Wu, P. Chou, Y. L. Lin, D. M. Chang, T. Y. Tai, and J. H. Lai. 2005. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J. Immunol. 174:8163-8172. [DOI] [PubMed] [Google Scholar]
- 11.Ho, L. J., J. J. Wang, M. F. Shaio, C. L. Kao, D. M. Chang, S. W. Han, and J. H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 12.Jessie, K., M. Y. Fong, S. Devi, S. K. Lam, and K. T. Wong. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411-1418. [DOI] [PubMed] [Google Scholar]
- 13.Jones, M., A. Davidson, L. Hibbert, P. Gruenwald, J. Schlaak, S. Ball, G. R. Foster, and M. Jacobs. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79:5414-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King, A. D., A. Nisalak, S. Kalayanrooj, K. S. Myint, K. Pattanapanyasat, S. Nimmannitya, and B. L. Innis. 1999. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health 30:718-728. [PubMed] [Google Scholar]
- 15.Kou, Z., M. Quinn, H. Chen, W. W. Rodrigo, R. C. Rose, J. J. Schlesinger, and X. Jin. 2008. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 80:134-146. [DOI] [PubMed] [Google Scholar]
- 16.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 17.Lindenbach, B. D., and C. M. Rice. 2003. Molecular biology of flaviviruses. Adv. Virus Res. 59:23-61. [DOI] [PubMed] [Google Scholar]
- 18.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 19.Mathew, A., I. Kurane, S. Green, D. W. Vaughn, S. Kalayanarooj, S. Suntayakorn, F. A. Ennis, and A. L. Rothman. 1999. Impaired T cell proliferation in acute dengue infection. J. Immunol. 162:5609-5615. [PubMed] [Google Scholar]
- 20.Mazzon, M., M. Jones, A. Davidson, B. Chain, and M. Jacobs. 2009. Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J. Infect. Dis. 200:1261-1270. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Jordan, J. L. 2010. Subversion of interferon by dengue virus. Curr. Top. Microbiol. Immunol. 338:35-44. [DOI] [PubMed] [Google Scholar]
- 22.Munoz-Jordan, J. L., M. Laurent-Rolle, J. Ashour, L. Martinez-Sobrido, M. Ashok, W. I. Lipkin, and A. Garcia-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz-Jordán, J. L., G. G. Sánchez-Burgos, M. Laurent-Rolle, and A. García-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. U. S. A. 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaya, T., J. Cros, M. S. Park, Y. Nakaya, H. Zheng, A. Sagrera, E. Villar, A. Garcia-Sastre, and P. Palese. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868-11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nightingale, Z. D., C. Patkar, and A. L. Rothman. 2008. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 84:1028-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer, D. R., P. Sun, C. Celluzzi, J. Bisbing, S. Pang, W. Sun, M. A. Marovich, and T. Burgess. 2005. Differential effects of dengue virus on infected and bystander dendritic cells. J. Virol. 79:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipps-Yonas, H., J. Seto, S. C. Sealfon, T. M. Moran, and A. Fernandez-Sesma. 2008. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 4:e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichyangkul, S., T. P. Endy, S. Kalayanarooj, A. Nisalak, K. Yongvanitchit, S. Green, A. L. Rothman, F. A. Ennis, and D. H. Libraty. 2003. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J. Immunol. 171:5571-5578. [DOI] [PubMed] [Google Scholar]
- 30.Raghupathy, R., U. C. Chaturvedi, H. Al-Sayer, E. A. Elbishbishi, R. Agarwal, R. Nagar, S. Kapoor, A. Misra, A. Mathur, H. Nusrat, F. Azizieh, M. A. Khan, and A. S. Mustafa. 1998. Elevated levels of IL-8 in dengue hemorrhagic fever. J. Med. Virol. 56:280-285. [DOI] [PubMed] [Google Scholar]
- 31.Sariol, C. A., J. L. Munoz-Jordan, K. Abel, L. C. Rosado, P. Pantoja, L. Giavedoni, I. V. Rodriguez, L. J. White, M. Martinez, T. Arana, and E. N. Kraiselburd. 2007. Transcriptional activation of interferon-stimulated genes but not of cytokine genes after primary infection of rhesus macaques with dengue virus type 1. Clin. Vaccine Immunol. 14:756-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 33.Sun, P., S. Fernandez, M. A. Marovich, D. R. Palmer, C. M. Celluzzi, K. Boonnak, Z. Liang, H. Subramanian, K. R. Porter, W. Sun, and T. H. Burgess. 2009. Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology 383:207-215. [DOI] [PubMed] [Google Scholar]
- 34.WHO. 1997. Dengue hemorrhagic fever: diagnosis, treatment and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 35.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]