Abstract
Cyclosporine (CsA) decreases HIV-1 infectivity by blocking HIV-1 capsid (CA) interaction with target cell cyclophilin A (CypA). Yet, HIV-1 virions produced in the presence of CsA also exhibit decreased infectivity that was previously shown to be independent of the well-characterized HIV-1 CA-CypA interaction. Here, we demonstrate that CsA decreases gp120 and gp41 incorporation into HIV-1 virions and that the fusion of these virions with susceptible target cells is impaired. This effect was not observed with HIV-1 virions pseudotyped with the vesicular stomatitis virus glycoprotein or with the amphotropic envelope protein of murine leukemia virus. It was independent of calcineurin signaling, the endoplasmic reticulum luminal protein cyclophilin B, and the long cytoplasmic tail of gp41. Thus, cyclosporine blocks HIV-1 infectivity via two independent mechanisms, the first involving HIV-1 CA in target cells and the second involving HIV-1 Env in producer cells.
The cellular protein cyclophilin A (CypA) interacts with the capsid protein (CA) of HIV-1 (24). This interaction is disrupted by specific CA mutations or by the competitive inhibitor cyclosporine (CsA) (11, 12, 24). The finding that both of these interventions prevent CypA incorporation into virions (11, 12, 33) and, correspondingly, inhibit infection (5) suggested that incorporation of producer cell CypA into nascent virions contributes to virion infectivity. The importance of CypA for HIV-1 replication was formally proven by experiments in CypA knockout cells (7). Subsequently, it was demonstrated that target cell CypA is important for HIV-1 infectivity and, unexpectedly, that the interaction of producer cell CypA with CA has no discernible effect on HIV-1 replication (16, 30).
Though CsA disrupts HIV-1 infectivity by competing with invading virion CA for binding to target cell CypA, it has an independent inhibitory effect on HIV-1 infectivity that is exerted during virion production (30). This second effect of CsA within the virion producer cell is additive to the effect in the target cell and, like the effect in the target cell, is independent of the inhibitory effects of CsA on calcineurin signaling (30). Surprisingly, unlike the effect in the target cell, the effect in the producer cell is independent of producer cell CypA as well as CA determinants for CypA binding (30). Previous attempts to identify biochemical or ultrastructural defects when HIV-1 virions were produced in the presence of CsA were unsuccessful (5, 7, 12, 20, 33, 35). Nonetheless, subtle effects of the drug on virion-associated, env-encoded proteins had been detected (5), but only at CsA concentrations significantly higher than those required to inhibit spreading infection of HIV-1 (6) or to inhibit cyclophilin A interaction with CA (5). In the interest of parsimony, most investigators had focused on the effects of the drug at the lower concentrations.
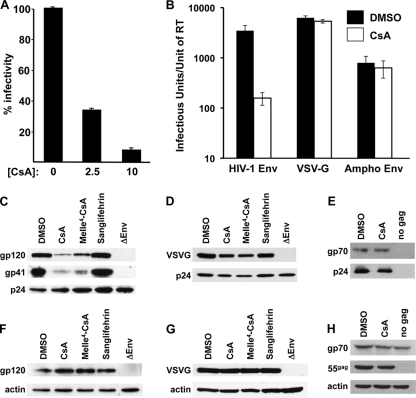
We began by attempting to confirm the antiviral phenotype that occurs when virions are produced in the presence of CsA. HIV-1 virions were produced by calcium phosphate transfection of 293T cells with the complete infectious provirus pNL4-3 (1), as previously described (5). CsA was added to the medium during virion production, and at 48 h posttransfection, the supernatant was filtered (0.45-μm pore size). Virions were concentrated by centrifugation through a 25% sucrose cushion, normalized by reverse transcriptase (RT) activity, and used to infect Jurkat T cells. As previously described, dextran sulfate was added to prevent secondary infection and infectivity was determined by intracellular staining and flow cytometry for p24 (30). Concentration-dependent inhibition of HIV-1 infectivity was observed when CsA was added during virion production (Fig. 1A).
FIG. 1.
CsA disrupts HIV-1 Env glycoprotein incorporation into virions if the drug is present during virion production. HIV-1 virions were produced in 293T cells by transfection of pNL4-3 (A), with pNL4-3-env− and one of the indicated Env proteins (B), or with pNL4-3-env− and either HIV-1 Env (C and F), VSV G (D and G), or ampho-MuLV Env (E and H). Drugs were added to the medium during virion production. For panel A, CsA was added at the indicated concentrations. For panels B to H, the indicated drugs were added at 10 μM CsA. Virion-containing supernatant was filtered, and virions were enriched by ultracentrifugation through a 25% sucrose cushion. Virion preparations were normalized by reverse transcription and tested for infectivity (A and B) or subjected to Western blotting using antibodies against the indicated proteins (C to E). At 48 h posttransfection, the producer cells were harvested using 5 mM EDTA, normalized by cell number, and subjected to Western blotting using antibodies against the indicated proteins (F to H).
To check the Env specificity of the effect, single-cycle virions were produced by 293T cell transfection with env-deleted pNL4-3, and plasmids encoding either HIV-1 Env, vesicular stomatitis virus (VSV) glycoprotein (G), or 4070-A (amphotropic) murine leukemia virus (MuLV) envelope glycoprotein. Transfected cells were placed in medium containing 10 μM CsA or an equivalent volume of solvent (dimethyl sulfoxide [DMSO]). Virus-containing supernatants were collected and normalized as described above, and single-cycle infectivities were determined by challenging TZM-bl indicator cells in triplicate with 10-fold serial dilutions of each virus sample. Infectivity was evaluated by staining infected cells with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and by counting blue-stained foci under a light microscope, as described previously (26). When produced in the presence of 10 μM CsA, the infectivity of HIV-1 virions pseudotyped with HIV-1 Env was reduced 22-fold (Fig. 1B). In contrast, virions pseudotyped with VSV G or amphotropic Env were inhibited to roughly 25% of control levels. The same results were obtained when Jurkat target cells were used (data not shown). Thus, the inhibitory effect of CsA was specific for HIV-1 Env.
To determine if CsA does indeed have effects on Env incorporation into HIV-1 virions, pseudotyped HIV-1 virions were produced as described above, concentrated by centrifugation through a 25% sucrose cushion, and normalized by reverse transcriptase (RT) activity. After removal of the supernatant, the transfected cells were harvested from the plates with 5 mM EDTA and normalized by cell number. Virions and cell lysates were subjected to Western blotting using monoclonal antibodies against gp120 (Intracell), gp41 (NEN), p24 (NEN), anti-VSV G (Sigma), anti-RLV gp70 (Quality Biotech, Inc.), and β-actin (Sigma).
As previously reported (5, 33), CsA had no effect on the yield of virion particles in the transfection supernatant, as determined by RT activity and particulate CA (Fig. 1C to E). In contrast, CsA caused a significant reduction in virion-associated gp120 and gp41 (Fig. 1C). CsA caused no reduction in steady-state levels of env-encoded protein in the cell lysate (Fig. 1F); if anything, cell-associated Env protein was increased by CsA, indicating that the effect of the drug was not to block protein synthesis but to block secretion or incorporation of Env protein into extracellular virions. Consistent with the single-cycle infectivity data in Fig. 1B, and with the effects of CsA on spreading of infection of HIV-1 or amphotropic MuLV (ampho-MuLV) (6), CsA had minimal effect on the incorporation of VSV G or ampho-Env into virions (Fig. 1D and E).
As a complex with CypA, CsA binds and inhibits the calcium-dependent phosphatase calcineurin (14, 22). MeIle4-CsA is a CsA analogue that binds CypA as tightly as the parent compound but does not form a complex with calcineurin (11, 33). Previously, it was shown that MeIle4-CsA disrupted HIV-1 virion infectivity almost as potently as the parent compound (30). Correspondingly, MeIle4-CsA blocked gp120 and gp41 incorporation into virion particles almost as potently as the parent compound (Fig. 1C), but again without effects on steady-state levels of these proteins in the producer cell (Fig. 1F). Thus, disruption of Env protein incorporation into HIV-1 virions by CsA is independent of calcineurin signaling.
The effect of Sanglifehrin, a cyclophilin-binding compound structurally unrelated to CsA (10, 28), was tested next. Sanglifehrin blocks the CypA-CA interaction and prevents CypA incorporation into virions as efficiently as CsA but, when tested side-by-side with CsA, is found to have minimal effect on the production of infectious virions (30). Here, Sanglifehrin was found to have minimal effect on gp120 or gp41 incorporation into virions (Fig. 1C). This result confirms that the effect of CsA on Env glycoprotein incorporation into virions is likely to be independent of CypA or calcineurin.
Previously, it was shown that efficient knockdown (KD) of CypA has no effect on the inhibition of virion infectivity that results when CsA is present during virion production (30). Given that CsA decreases virion incorporation of HIV-1 Env glycoprotein and that gp160 undergoes complex folding reactions within the lumen of the endoplasmic reticulum (ER), we focused our attention on CypB, the only cyclophilin family member known to be within the lumen of the endoplasmic reticulum (27). CypB binds CsA as efficiently as CypA (32) and thus seemed a drug target of relevance to the Env incorporation defect. Additionally, CypB was one of 250 HIV dependency factors recently identified in a large-scale small interfering RNA screen for identifying host factors required by HIV-1 (8).
To test the importance of CypB for Env incorporation into HIV-1 virions, HeLa cells were transduced with pSUPER retroviral vectors targeting either CypB (short hairpin RNA [shRNA] target sequence, 5′-GATGTAGGCCGGGTGATCT-3′) or the luciferase (Luc) gene, as a control (shRNA target sequence, 5′-GTACGCGGAATACTTCGA-3′), as previously described (29). Pools of cells were selected in puromycin. CypB was detected by immunoblot analysis of cell lysates from the Luc knockdown (KD) cells by use of a primary rabbit antibody (Affinity Bioreagents). In contrast, CypB protein was undetectable in the CypB KD cells (Fig. 2A).
FIG. 2.
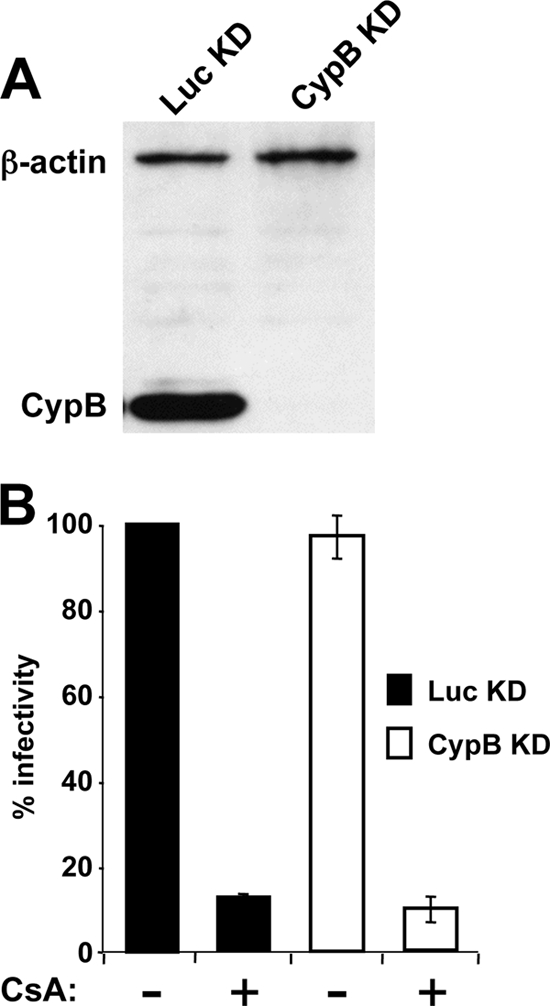
Producer cell CypB has no effect on HIV-1 virion infectivity. Pools of HeLa cells transduced with an shRNA expression construct specific for CypB mRNA or specific for luciferase (Luc), as a control, were normalized by cell number and subjected to immunoblotting with antibodies for CypB and actin (A). Virions were produced by transfection of CypB KD or Luc KD HeLa cells with pNL4-3 in the presence of 10 μM CsA, as indicated (B). Virions were enriched by ultracentrifugation as described in the legend to Fig. 1, normalized by RT activity, and used to infect Jurkat T cells in drug-free medium. Infected cells were identified by flow cytometry after being immunostained for HIV-1 CA. Infectivity is reported relative to the number of virions produced from the control cells in the absence of CsA.
CypB KD and Luc KD cells were then transfected with full-length pNL4-3 (1). Where indicated, 10 μM CsA was included in the media of the transfected cells. Virions were pelleted from the supernatant by ultracentrifugation and used to infect Jurkat T cells, and infected cells were identified by flow cytometry using anti-p24 antibody, as previously described (30). CypB KD in virion producer cells had no effect on the subsequent infectivity of the virions that were produced, and CsA was equally effective at inhibiting the infectivity of virions produced from CypB and Luc KD cells (Fig. 2B). These results indicate that CypB is not required for HIV-1 Env glycoprotein incorporation into virions and that these effects of CsA are independent of CypB.
Env incorporation into HIV-1 virions is blocked by particular MA mutants (13, 25). Interestingly, truncation of the long cytoplasmic tail of gp41 restores Env incorporation to virions bearing these MA mutants. The lipid droplet-associated protein TIP47 has been reported to link the cytoplasmic tail of HIV-1 gp41 with gag-encoded MA and to be necessary for incorporation of wild-type (WT) HIV-1 Env into virion particles (23). As with the MA mutants, deletion of the long cytoplasmic tail of gp41 relieves the TIP47 dependence of Env incorporation. It is intriguing that TIP47 possesses a hydrophobic cleft of dimensions comparable to those of the hydrophobic CsA-binding site on CypA (19). Additionally, gp41 cytoplasmic tail truncations rescue HIV-1 replication from the antiviral compound amphotericin B methyl ester (34). Taken together, these observations prompted us to determine if gp41 cytoplasmic tail mutants would rescue HIV-1 from the CsA-induced defects in Env incorporation.
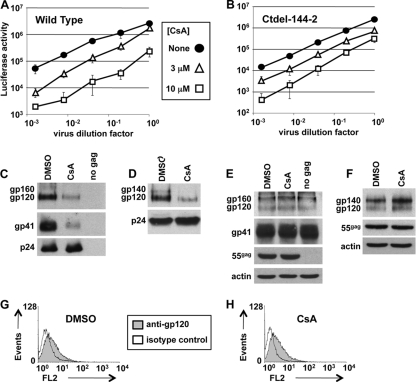
HIV-1 virions were produced by cotransfecting 293T cells with pNL4-3-env−-luc (17) and plasmids encoding Envgp160, either pIIINL4Env/WT or pIIINL4Env/CTdel-144 (34). The latter plasmid encodes gp160 with a deletion of the last 144 amino acids from the cytoplasmic tail. In each case, virions were produced in the presence of 0, 3, or 10 μM CsA, accelerated through 25% sucrose, and used to infect CD4+/CXCR4+/U87 cells (4) in triplicate. At 48 h postinfection, the cells were processed for associated firefly luciferase activity using a standard commercial assay (Promega). As expected, wild-type HIV-1 virion production in the presence of CsA had a dose-dependent inhibitory effect on subsequent infectivity (Fig. 3A). CsA also decreased the infectivity of virions bearing the CTdel-144 mutant Env (Fig. 3B) and decreased the incorporation of CTdel-144 mutant Env into virions (Fig. 3C to F), indicating that the long cytoplasmic tail of gp41 was not a specific target of CsA.
FIG. 3.
Truncation of the gp41 cytoplasmic tail does not rescue HIV-1 infectivity from inhibition by CsA. HIV-1 virions competent for a single cycle of infection were produced by cotransfecting 293T cells with pNL4-3-env−-luc (17) and plasmids encoding Envgp160, either pIIINL4Env/WT (A) or pIIINL4Env/CTdel-144 (B) (34). Virions were produced in the presence of the indicated concentrations of CsA, accelerated through 25% sucrose, and used to infect CD4+/CXCR4+/U87 cells (4) in triplicate. At 48 h postinfection, the cells were processed for firefly luciferase activity using a standard commercial assay (Promega). Immunoblots of virions (C and D) and 293T cell lysates (E and F), produced as in panels A and B, were probed with the indicated antibodies. WT Env is shown in panels C and E. CTdel-144 is shown in panels D and F. The same transfected 293T cells as those in panel B were fixed and stained with anti-gp120 antibody and processed for flow cytometry.
Though no decrease in cell surface HIV-1 Env in response to CsA was detected by flow cytometry, the signal in the absence of CsA was barely above the background level when obtained with any of 4 different antibodies (data not shown). Others reported this technical problem and have solved it by using the CTdel-144 mutant of Env (18). The truncated Env protein is expressed on the cell surface at higher levels because it lacks motifs associated with gp41 downregulation (3, 9, 31). Since CTdel-144 is sensitive to CsA (Fig. 3B), the effect of CsA on cell surface staining of the same 293T cells that were transfected to produce CTdel-144-pseudotyped HIV-1 virions was examined. These cells were stained with anti-gp120 antibody ARP423 (NIBSC, United Kingdom), and it was found that CsA caused no detectable reduction in the cell surface staining of HIV-1 Env (Fig. 3G and H).
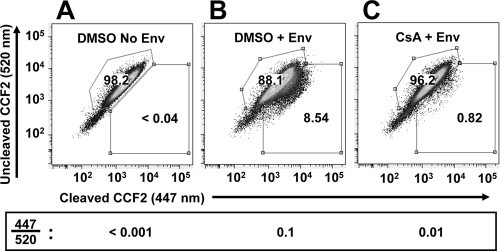
If the reduction in virion-associated gp120 and gp41 by CsA is of sufficient magnitude to disrupt virion infectivity, a functional defect would be expected in virion fusion with target cells. To determine if CsA causes a virion-target cell fusion defect, virions bearing Vpr/β-lactamase (BlaM-Vpr) fusion protein were produced as previously described (15), normalized by RT activity, and used to infect SupT1 target cells. After infection, cells were loaded with the CCF2-AM substrate dye, washed, and incubated overnight. The change in CCF2-AM fluorescence emission after cleavage by the BlaM-Vpr chimera was detected by flow cytometry, and data were analyzed with FlowJo software. Results are reported as the ratio of blue to green fluorescence. Signal from cleavage of the dye was nearly undetectable when virions were produced in the absence of Env (Fig. 4A) and shifted to 8.54% of the cells when Env was present (Fig. 4B). When virions bearing Env were produced in the presence of CsA, dye cleavage was reduced 10-fold (Fig. 4C). This is roughly the magnitude of reduction in Env incorporation onto virion particles (Fig. 1A) and in virion infectivity (Fig. 3A) caused by producing virions in the presence of CsA and indicates that the reduction in Env incorporation by CsA is functionally significant.
FIG. 4.
HIV-1 virions produced in the presence of CsA have decreased ability to fuse with target cells. HIV-1 virions bearing Vpr/β-lactamase (BlaM-Vpr) fusion proteins were produced and purified as described in the legend to Fig. 1, normalized by RT activity, and used to infect SupT1 human T cell targets. After infection, cells were loaded with the CCF2-AM substrate dye, washed, and incubated overnight at room temperature. The change in CCF2-AM fluorescence emission after cleavage by the BlaM-Vpr chimera was detected by flow cytometry using an LSR II (BD). Data were analyzed by FlowJo software, and results are reported as ratios of blue/green fluorescence.
Recently we reported the surprising result that inhibition of virion infectivity by exposure of producer cells to CsA was independent of the CA-CypA interaction (30). Closer examination of the effects of the drug on virion biochemistry in the experiments reported here indicates that the decrease in Env incorporation is of sufficient magnitude to reduce the efficiency of virion fusion. Previous studies indicated that VSV G-pseudotyped virions are resistant to the antiviral effects of CsA (2). Similarly, ampho-MuLV is resistant to CsA (6). We have indeed confirmed that HIV-1 virions pseudotyped with either VSV G or ampho-Env are resistant to the effects of CsA (Fig. 1). HIV-1 Env is distinguished from other viral glycoproteins, like VSV G and influenza virus hemagglutinin, in that it undergoes relatively complex folding reactions within the lumen of the ER with delayed signal sequence cleavage (21). Perhaps the slow metabolism and prolonged residence of gp160 within the ER render HIV-1 Env particularly sensitive to the effects of CsA.
Acknowledgments
We thank Eric Freed for HIV-1 Env expression plasmids, the Centre for AIDS Reagents, NIBSC, United Kingdom, for the antibody ARP423, and the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, for TZM-bl cells provided by John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc.
This work was supported by National Institutes of Health grant RO1AI36199, by Swiss National Science Foundation grant 3100A0-128655, by the EU collaborative project HIV-ACE, and by EU Marie Curie grant 237265.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyö. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV(CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J. Virol. 70:4220-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brass, A. L., D. M. Dykxhoorn, Y. Benita, N. Yan, A. Engelman, R. J. Xavier, J. Lieberman, and S. J. Elledge. 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319:921-926. [DOI] [PubMed] [Google Scholar]
- 9.Bültmann, A., W. Muranyi, B. Seed, and J. Haas. 2001. Identification of two sequences in the cytoplasmic tail of the human immunodeficiency virus type 1 envelope glycoprotein that inhibit cell surface expression. J. Virol. 75:5263-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehr, T., J. Kallen, L. Oberer, J. J. Sanglier, and W. Schilling. 1999. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110. II. Structure elucidation, stereochemistry and physico-chemical properties. J. Antibiot. (Tokyo) 52:474-479. [DOI] [PubMed] [Google Scholar]
- 11.Franke, E. K., and J. Luban. 1996. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology 222:279-282. [DOI] [PubMed] [Google Scholar]
- 12.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 13.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, J., and I. Weissman. 1991. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell 66:799-806. [DOI] [PubMed] [Google Scholar]
- 15.Gurer, C., A. Hoglund, S. Hoglund, and J. Luban. 2005. ATPgammaS disrupts human immunodeficiency virus type 1 virion core integrity. J. Virol. 79:5557-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatziioannou, T., D. Perez-Caballero, S. Cowan, and P. D. Bieniasz. 2005. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J. Virol. 79:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrera, C., P. J. Klasse, E. Michael, S. Kake, K. Barnes, C. W. Kibler, L. Campbell-Gardener, Z. Si, J. Sodroski, J. P. Moore, and S. Beddows. 2005. The impact of envelope glycoprotein cleavage on the antigenicity, infectivity, and neutralization sensitivity of Env-pseudotyped human immunodeficiency virus type 1 particles. Virology 338:154-172. [DOI] [PubMed] [Google Scholar]
- 19.Hickenbottom, S. J., A. R. Kimmel, C. Londos, and J. H. Hurley. 2004. Structure of a lipid droplet protein; the PAT family member TIP47. Structure 12:1199-1207. [DOI] [PubMed] [Google Scholar]
- 20.Kong, L. B., D. An, B. Ackerson, J. Canon, O. Rey, I. S. Chen, P. Krogstad, and P. L. Stewart. 1998. Cryoelectron microscopic examination of human immunodeficiency virus type 1 virions with mutations in the cyclophilin A binding loop. J. Virol. 72:4403-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Land, A., D. Zonneveld, and I. Braakman. 2003. Folding of HIV-1 envelope glycoprotein involves extensive isomerization of disulfide bonds and conformation-dependent leader peptide cleavage. FASEB J. 17:1058-1067. [DOI] [PubMed] [Google Scholar]
- 22.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Verges, S., G. Camus, G. Blot, R. Beauvoir, R. Benarous, and C. Berlioz-Torrent. 2006. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. U. S. A. 103:14947-14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 25.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Gottlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzato, M., E. Popova, and H. G. Gottlinger. 2008. Nef can enhance the infectivity of receptor-pseudotyped human immunodeficiency virus type 1 particles. J. Virol. 82:10811-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price, E. R., L. D. Zydowsky, M. J. Jin, C. H. Baker, F. D. McKeon, and C. T. Walsh. 1991. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc. Natl. Acad. Sci. U. S. A. 88:1903-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanglier, J. J., V. Quesniaux, T. Fehr, H. Hofmann, M. Mahnke, K. Memmert, W. Schuler, G. Zenke, L. Gschwind, C. Maurer, and W. Schilling. 1999. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110. I. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. (Tokyo) 52:466-473. [DOI] [PubMed] [Google Scholar]
- 29.Sokolskaja, E., L. Berthoux, and J. Luban. 2006. Cyclophilin A and TRIM5alpha independently regulate human immunodeficiency virus type 1 infectivity in human cells. J. Virol. 80:2855-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolskaja, E., D. M. Sayah, and J. Luban. 2004. Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J. Virol. 78:12800-12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staropoli, I., C. Chanel, M. Girard, and R. Altmeyer. 2000. Processing, stability, and receptor binding properties of oligomeric envelope glycoprotein from a primary HIV-1 isolate. J. Biol. Chem. 275:35137-35145. [DOI] [PubMed] [Google Scholar]
- 32.Swanson, S. K., T. Born, L. D. Zydowsky, H. Cho, H. Y. Chang, C. T. Walsh, and F. Rusnak. 1992. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc. Natl. Acad. Sci. U. S. A. 89:3741-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 34.Waheed, A. A., S. D. Ablan, M. K. Mankowski, J. E. Cummins, R. G. Ptak, C. P. Schaffner, and E. O. Freed. 2006. Inhibition of HIV-1 replication by amphotericin B methyl ester: selection for resistant variants. J. Biol. Chem. 281:28699-28711. [DOI] [PubMed] [Google Scholar]
- 35.Wiegers, K., G. Rutter, U. Schubert, M. Grattinger, and H. G. Krausslich. 1999. Cyclophilin A incorporation is not required for human immunodeficiency virus type 1 particle maturation and does not destabilize the mature capsid. Virology 257:261-274. [DOI] [PubMed] [Google Scholar]