Abstract
Hantavirus infections are noted for their ability to infect endothelial cells, cause acute thrombocytopenia, and trigger 2 vascular-permeability-based diseases. However, hantavirus infections are not lytic, and the mechanisms by which hantaviruses cause capillary permeability and thrombocytopenia are only partially understood. The role of β3 integrins in hemostasis and the inactivation of β3 integrin receptors by pathogenic hantaviruses suggest the involvement of hantaviruses in altered platelet and endothelial cell functions that regulate permeability. Here, we determined that pathogenic hantaviruses bind to quiescent platelets via a β3 integrin-dependent mechanism. This suggests that platelets may contribute to hantavirus dissemination within infected patients and provides a means by which hantavirus binding to β3 integrin receptors prevents platelet activation. The ability of hantaviruses to bind platelets further suggested that cell-associated hantaviruses might recruit platelets to the endothelial cell surface. Our findings indicate that Andes virus (ANDV)- or Hantaan virus (HTNV)-infected endothelial cells specifically direct the adherence of calcein-labeled platelets. In contrast, cells comparably infected with nonpathogenic Tula virus (TULV) failed to recruit platelets to the endothelial cell surface. Platelet adherence was dependent on endothelial cell β3 integrins and neutralized by the addition of the anti-β3 Fab fragment, c7E3, or specific ANDV- or HTNV-neutralizing antibodies. These findings indicate that pathogenic hantaviruses displayed on the surface of infected endothelial cells bind platelets and that a platelet layer covers the surface of infected endothelial cells. This fundamentally changes the appearance of endothelial cells and has the potential to alter cellular immune responses, platelet activation, and endothelial cell functions that affect vascular permeability. Hantavirus-directed platelet quiescence and recruitment to vast endothelial cell beds further suggests mechanisms by which hantaviruses may cause thrombocytopenia and induce hypoxia. These findings are fundamental to our understanding of pathogenic-hantavirus regulation of endothelial cell responses that contribute to vascular permeability.
Hantaviruses cause two human diseases with prominent effects on vascular permeability, hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (54, 55). Hantaviruses predominantly infect endothelial cells and cause acute thrombocytopenia in both HPS and HFRS patients (9, 17, 35, 37, 45, 64, 65). Endothelial cells line the vasculature and form a fluid barrier that is the primary determinant of capillary integrity and permeability (3, 19). Platelets also maintain hemostasis through thrombus formation, and platelet adherence and activation are normally inhibited by endothelial cell signals (2, 8, 12, 52). As a result, hantavirus infections affect both platelets and endothelial cells which dynamically regulate vascular permeability. Since hantaviruses do not lyse infected endothelial cells, alternative pathogenic mechanisms need to be considered in order explain the vascular leakage observed in HPS and HFRS patients (35, 37, 45, 65). Although pathogenesis is likely to be a multifactorial process, responses of hantavirus-infected endothelial cells are central to understanding vascular permeability deficits of hantavirus diseases.
Platelets and endothelial cells commonly display β3 integrins on their surfaces, and β3 integrins play prominent roles in regulating vascular integrity (6, 8, 11, 12, 30, 53). β3 integrins are receptors for pathogenic HPS- and HFRS-causing hantaviruses, while nonpathogenic hantaviruses use α5β1 integrins (24, 26, 40). Pathogenic hantaviruses bind to plexin, semiphorin, integrin (PSI) domains present on basal, bent conformations of β3 integrins (39, 58, 67) and inhibit endothelial cell migration on the αvβ3 integrin ligand vitronectin days after infection (23, 49). αvβ3 integrins normally enhance capillary integrity by regulating endothelial cell responses to vascular endothelial growth factor (VEGF) (6, 30, 51, 53). In fact, knocking out β3 integrins results in enhanced endothelial cell permeability in response to VEGF (30, 51, 53). Similarly, pathogenic hantaviruses block β3 integrin functions and enhance the permeability of endothelial cells in response to VEGF, but only at late times postinfection (25). Hantaviruses have been shown to cover the surface of infected VeroE6 cells (28), and the presence of cell-associated hantavirus provides a potential explanation for the loss of β3 integrin function and enhanced endothelial cell permeability days after infection (23, 25).
αIIbβ3 integrins are abundantly present on the surface of platelets, where they mediate platelet adherence to fibrinogen and platelet activation (8, 12). Endothelial cells produce ADPase and prostacyclin, which normally inhibit platelet activation and prevent platelet adherence to the endothelium (34, 44). However, once activated, platelets are highly adherent to each other and the endothelium, resulting in rapid regulation of vascular leakage (5, 8, 12, 42, 43). There is little information about the interaction of hantaviruses with platelets, the mechanism of hantavirus-induced thrombocytopenia, or the role of thrombocytopenia in hantavirus disease (13-15, 17, 45, 65). Cosgriff et al. demonstrated that platelets from HFRS patients have a defect in platelet activation, suggesting that thrombocytopenia results from platelet inactivation rather than from excessive platelet activation and aggregation (14). Combining this finding with the ability of pathogenic hantaviruses to bind inactive β3 integrins (49), we rationalized that hantaviruses may bind quiescent platelets and that platelets could be a vehicle for hantavirus dissemination. These data further suggest that cell-associated hantaviruses (28) might recruit quiescent platelets to the surface of infected endothelial cells and fundamentally alter normal endothelial cell interactions. In this report, we investigate the ability of hantaviruses and hantavirus-infected endothelial cells to bind platelets. Our findings provide a vital understanding of hantavirus-platelet interactions, suggest potential mechanisms for hantavirus-induced thrombocytopenia, and demonstrate that hantaviruses alter endothelial cell properties that are likely to contribute to hantavirus disease.
Pathogenic hantaviruses bind platelets.
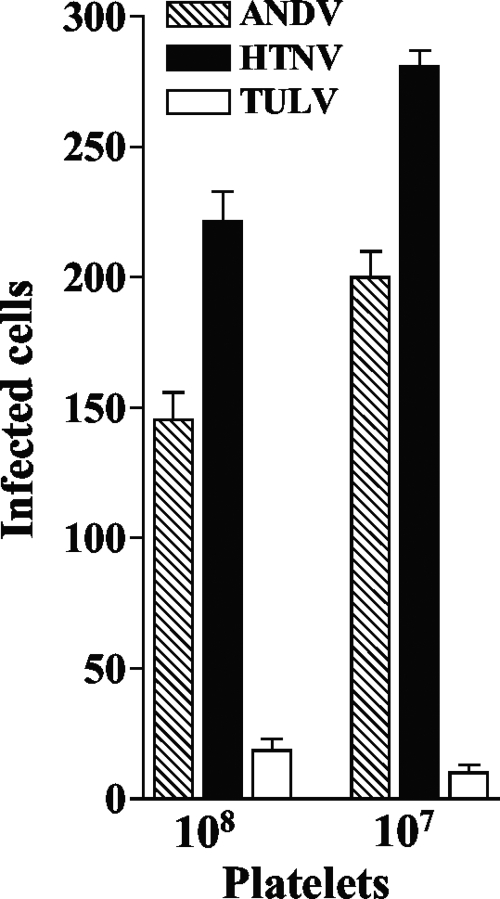
β3 integrins are receptors for pathogenic hantaviruses, and platelets express αIIbβ3 integrins on their surface (12, 24, 26). Despite this, there is little information on the interaction of hantaviruses with platelets. We initially determined whether pathogenic hantaviruses are capable of binding to platelets, using an infectivity assay to detect viral adherence. Platelets were harvested in citrate phosphate buffer, separated by centrifugation in the presence of 1 μM prostaglandin E1 (PGE1) (5), and further purified using Sepharose 2B chromatography as previously described (5, 27). Platelet viability was 95% in all experiments, as determined by aggregometer (Chrono-log Corporation) or calcein AM (Invitrogen) uptake. A constant amount of pathogenic Andes virus (ANDV), pathogenic Hantaan virus (HTNV), or nonpathogenic Tula virus (TULV) (104 focus-forming units [FFU]) (25) was incubated with freshly purified platelets for 1 h at 37°C. Platelets were pelleted, washed 3 times to remove unbound virus, and resuspended prior to adsorption to VeroE6 monolayers (1 h, 37°C). Monolayers were washed 3 times with Dulbecco's modified Eagle's medium (DMEM) supplemented with 2% fetal calf serum (FCS), and at 1 day postinfection, cells were methanol fixed (100%, −20°C, 10 min) and the presence of the hantavirus nucleocapsid protein within infected VeroE6 cells was quantitated by immunoperoxidase staining (26). Using infectivity as a measure of binding, Fig. 1 demonstrates that significantly more ANDV and HTNV (7- to 27-fold) were bound to platelets than TULV (P < 0.01). These findings demonstrate that platelets selectively bind pathogenic ANDV and HTNV and suggest the potential for platelets to harbor infectious pathogenic hantaviruses. Using a [35S]methionine labeling approach, we observed no nucleocapsid protein synthesis following adsorption of ANDV or HTNV to platelets.
FIG. 1.
Pathogenic hantaviruses bind platelets. Platelets were collected in sodium citrate (final concentration, 0.4%) supplemented with 1 μM prostaglandin E1 (PGE1) (Calbiochem) (27), and platelet-rich plasma (PRP) was prepared by centrifugation for 15 min at 1,800 rpm (700 × g) at 25°C. PRP was supplemented with 10 mM EDTA, and platelets were pelleted by centrifugation (15 min at 1,300 × g at 25°C) and washed with HBMT buffer (HEPES-buffered modified Tyrodes buffer; 10 mM HEPES, pH 7.45, 137 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 12 mM NaHCO3, 1 mM MgCl2, 0.1% dextrose, 0.2% bovine serum albumin [BSA] with 1 μM PGE1,10 mM EDTA) (27). Platelets were purified over a Sepharose 2B column equilibrated with HBMT at room temperature and quantitated using a hemacytometer (27). Platelets (107 or 108 per ml) were incubated with 104 FFU of HTNV, ANDV, or TULV for 1 h in an incubator at 37°C and 5% CO2. Platelets were pelleted and washed three times with 10 volumes of HBMT, resuspended in 100 μl HBMT, and then adsorbed onto VeroE6 cells in duplicate wells of a 96-well plate (1 h at 37°C). Monolayers were washed with Dulbecco's modified Eagle's medium supplemented with 2% fetal calf serum, and VeroE6 cells were incubated (37°C, 5% CO2) for 24 h prior to methanol fixation (100%, −20°C) (24). The hantavirus nucleocapsid protein present in infected cells was detected by immunoperoxidase staining as previously described, and the number of N protein-containing cells was quantitated (26). Error bars represent the means ± standard deviations (n = 12) of results from four independent experiments.
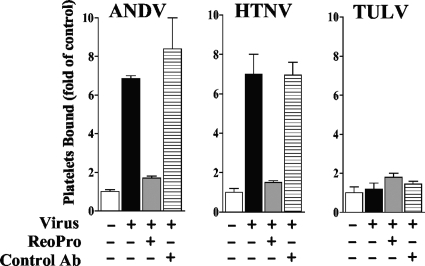
Pathogenic-hantavirus binding to platelets is inhibited by antibodies to β3 integrins.
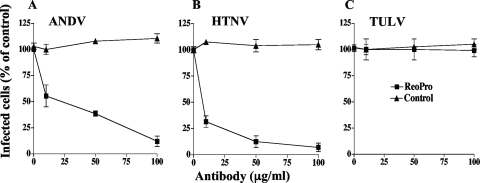
Pathogenic hantaviruses bind inactive conformations of αvβ3 integrin receptors through interactions with the PSI domain present on β3 subunits, while nonpathogenic hantaviruses TULV and Prospect Hill virus (PHV) use discrete α5β1 integrins (49). β3 integrins are present on the surface of endothelial cells, and αIIbβ3 integrins are the most abundant cell surface receptors on platelets (12). In order to determine if ANDV and HTNV binding to platelets is dependent on β3 integrins, we incubated hantavirus (104 FFU) and platelets (106) in the presence of an increasing amount of the β3 integrin-specific Fab fragment c7E3(ReoPro) (10-12) or a control antibody (10 to 100 μg/ml). After a wash, hantaviruses bound to platelets were quantitated using the infectivity assay described above. Figure 2A and B indicate that c7E3 inhibited ANDV and HTNV binding to platelets by >90% while there was no effect of control antibody on viral adherence. Similarly, there was no effect of c7E3 or control antibody on the binding of TULV to platelets. These data demonstrate that pathogenic-hantavirus binding to platelets is dependent on the β3 integrin receptor.
FIG. 2.
Pathogenic-hantavirus binding to platelets is inhibited by β3 integrin antibodies. Platelets (106) were incubated with increasing concentrations of the c7E3(ReoPro) Fab fragment (10) directed at αvβ3 or control antibody (anti-β5 integrin [product no. 1926; Chemicon]) (10 to 100 μg/ml) or mock treated. Subsequently, platelets were incubated with ANDV, HTNV, or TULV (104 FFU) for 2 h at room temperature in HBMT. Following centrifugation and washing 3 times with HBMT to remove unbound virus, platelets were resuspended and adsorbed to VeroE6 cells as described in the legend to Fig. 1. The number of nucleocapsid protein-positive cells is presented as a percentage of the number of mock-treated infected cells (26). Results from two independent experiments are presented.
Platelets adhere to hantavirus-infected endothelial cells.
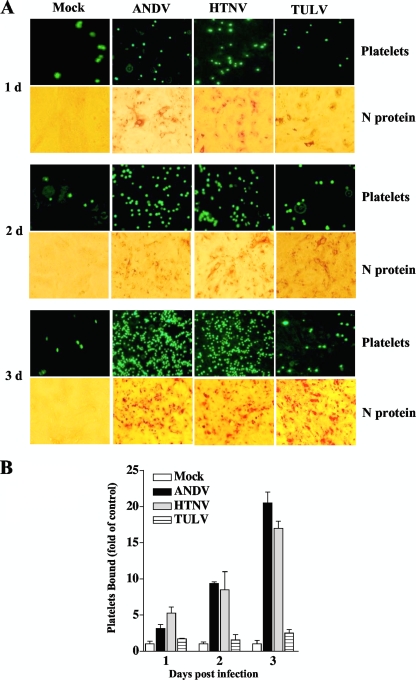
The pathogenic hantavirus Sin Nombre virus (SNV) has been shown to coat the surfaces of infected VeroE6 cells days after infection (28). Since pathogenic hantaviruses bind β3 integrins on endothelial cells and platelets, these findings also suggest that cell surface-displayed hantaviruses may recruit platelets to the surfaces of hantavirus-infected endothelial cells. In order to test this, we labeled purified platelets with calcein AM (Molecular Probes) and evaluated their ability to bind hantavirus-infected endothelial cells. Endothelial cells were infected with HTNV, ANDV, and TULV (multiplicity of infection [MOI] of 0.5) or mock infected for 1 to 3 days. Monolayers were washed, and constant amounts of calcein-labeled platelets were adsorbed to cells. Endothelial cells were washed 3 times to remove nonadherent platelets, and the binding of labeled platelets was visualized and quantitated by fluorescence microscopy (Fig. 3). Monolayers were comparably infected with ANDV, HTNV, and TULV as demonstrated by immunoperoxidase staining of the hantavirus nucleocapsid protein from 1 to 3 days postinfection (Fig. 3A), and platelet aggregation was not observed after binding of platelets to endothelial cells. Platelets are not normally adherent to endothelial cells (12, 34, 44), and levels of platelet binding to TULV-infected cells were nearly identical to those observed for mock-infected controls at all time points (Fig. 3A and B). In contrast, there were dramatic increases in platelet adherence to ANDV- and HTNV-infected cells from 1 to 3 days postinfection, with the highest levels of platelet adherence occurring at 3 days postinfection (Fig. 3A and B). These results demonstrate that quiescent platelets are recruited to the surface of pathogenic-hantavirus-infected endothelial cells and that platelet binding to infected endothelial cells increases from 1 to 3 days postinfection.
FIG. 3.
Platelets adhere to pathogenic-hantavirus-infected endothelial cells. Human umbilical vein endothelial cells were purchased from Cambrex and seeded onto 96-well plates at a cell density of 2 × 104 cells and grown in endothelial basal medium 2 (Cambrex) containing 10% fetal calf serum, as previously described (25). Confluent endothelial cells were infected in duplicate with HTNV, ANDV, and TULV at an MOI of 0.5 or mock infected. Platelets were purified as described in the legend to Fig. 1 and labeled with calcein AM (5 mM, 15 min, 37°C; Molecular Probes) in HBMT (5, 27). Platelets were pelleted and washed 3 times prior to resuspension in HBMT buffer. At the indicated times postinfection, cells were washed with HBMT (calcium free, with 1 mM MgCl2), incubated with calcein-labeled platelets (107 platelets/well) for 30 min at 37°C, and subsequently washed twice with HBMT buffer. (A) Platelet adherence to endothelial cells was visualized using an Olympus IX51 fluorescence microscope. At least four independent experiments were performed, with similar results. Identically infected endothelial cell monolayers were fixed with methanol, immunoperoxidase stained for the hantavirus N protein, and visualized by light microscopy. (B) The number of platelets bound to endothelial cell monolayers was quantitated using the NIH Image program. Results are presented as fold change relative to level of mock-infected cells. Error bars represent the means ± standard deviations (n = 12) of results from four experiments.
Neutralizing antibodies to ANDV and HTNV specifically inhibit platelet adherence to cells.
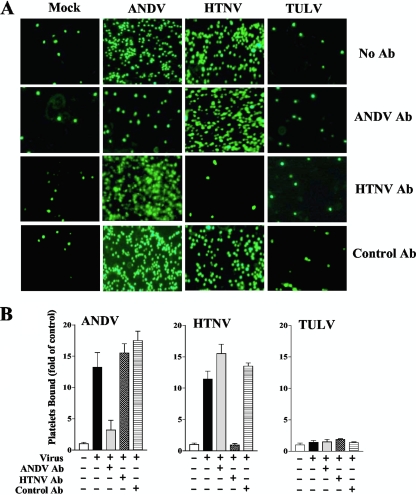
Hantavirus envelope glycoproteins are trafficked to the cis-Golgi and are not present on the cell surface when recombinantly expressed (33, 46, 54). Hantaviruses mature by budding into the lumen of the cis-Golgi, exiting cells through an incompletely understood vesicular process (54). In order to determine if platelet adherence to hantavirus-infected endothelial cells is dependent on the presence of cell surface-displayed hantavirus, we evaluated whether neutralizing antibody to ANDV or HTNV blocked platelet adherence to infected cells. Endothelial cells were infected with ANDV, HTNV, or TULV or mock infected, and at 3 days postinfection, endothelial cells were pretreated with neutralizing antibody to ANDV (62), HTNV, or control serum for 1 h on ice. Subsequently, calcein-labeled platelets (107/well) were added to monolayers, and platelet adherence was evaluated as described above (Fig. 4A and B). Neutralizing anti-ANDV or anti-HTNV sera decreased platelet adherence to only cognate ANDV- or HTNV-infected endothelial cells, respectively (>5-fold; P < 0.005), and had no effect on platelet binding to endothelial cells infected with a different hantavirus (Fig. 4A and B). Platelet adherence to ANDV- or HTNV-infected cells was unaltered by a control antibody, and there was no change in platelet adherence to TULV- or mock-infected cells following pretreatment with control, anti-ANDV, or anti-HTNV sera (Fig. 4A and B). This finding indicates that platelet binding to ANDV- and HTNV-infected endothelial cells is directed by virus present on the endothelial cell surface. These findings are consistent with electron microscopy findings indicating that pathogenic SNV coats the surfaces of infected cells days after infection (28) and suggest that pathogenic hantaviruses fundamentally alter the normal adherence properties of endothelial cells.
FIG. 4.
Neutralizing antibodies to ANDV or HTNV specifically inhibit platelet binding. Endothelial cells were grown in 96-well plates and infected with ANDV, HTNV, or TULV (MOI of 0.5) or mock infected as described in the legend to Fig. 2. At 3 days postinfection, antibodies (Ab) to the ANDV surface glycoproteins (neutralizing titer, 1:640; gift from J. W. Hooper, USAMRIID) (62), neutralizing antibody to HTNV (WHO Reference Center, Seoul, South Korea), or control rabbit serum was added (10 ng/ml) to infected or mock-infected cells for 1 h at 4°C. Subsequently, calcein-labeled platelets (107 platelets/well) were incubated with cells for 30 min at 37°C, and cells were washed three times with HBMT buffer. (A) Platelet adherence to endothelial cells was visualized using an Olympus IX51 fluorescence microscope. (B) Bound platelets were quantified, and results are expressed as fold changes for platelets bound versus the level of the control ± standard deviations for each group. A two-tailed Student t test was used to analyze the statistical differences between the control and treated groups.
β3 integrin antibodies inhibit platelet adherence to hantavirus-infected endothelial cells.
Pathogenic hantaviruses bind basal forms of β3 integrins (49, 67), and as a result, β3 integrins are likely to mediate the cell surface display of pathogenic hantaviruses and the concomitant adherence of platelets to infected cells. We hypothesized that pretreatment of infected monolayers with monovalent β3 integrin Fab fragments might displace pathogenic hantaviruses from the endothelial cell surface and thus abolish the adherence of platelets to infected endothelial cells. To address this, endothelial cells were infected with ANDV, HTNV, or TULV (MOI of 0.5) or mock infected, and at 3 days postinfection, cells were pretreated with an anti-β3 c7E3(ReoPro) Fab fragment (100 ng/ml) (10) or control serum for 1 h on ice. Complete β3 antibodies were not used, in order to prevent the direct cross-linking of endothelial cell and platelet β3 integrins. After antibody removal and monolayer washing, calcein-labeled platelets (107/well) were added to endothelial cells and platelet adherence was evaluated as described above (Fig. 5). Pretreatment of ANDV- and HTNV-infected endothelial cells with c7E3 decreased platelet adherence to cells >5-fold (P < 0.005) in comparison to infection alone or pretreatment of infected cells with control antibodies (Fig. 5). These findings suggest a primary role for β3 integrins in the cell association of pathogenic hantaviruses and further indicate that β3 integrin antibodies are capable of inhibiting hantavirus recruitment of platelets to the surface of infected endothelial cells.
FIG. 5.
β3 integrin antibodies inhibit platelet adherence to hantavirus-infected endothelial cells. Endothelial cells were infected with ANDV, HTNV, or TULV (MOI of 0.5) or mock infected as described in the legend to Fig. 2. At 3 days postinfection, cells were pretreated with c7E3(ReoPro) antibody (100 ng/ml) (10) or control serum (anti-β5 integrin [product no. 1926; Chemicon]) for 1 h at 4°C prior to platelet adherence. Calcein-labeled platelets (107/well) were added to monolayers as described in the legend to Fig. 4, and platelet adherence was evaluated. Bound platelets were quantified, and the results from two independent experiments are expressed as fold changes for platelets bound versus the level for the control ± standard deviations for each group. A two-tailed Student t test was used to analyze the statistical differences between the control and treated groups.
The normal function of endothelial cells and platelets is determined by receptor interactions, and β3 integrins play prominent roles in platelet activation, endothelial cell adherence, and regulation of vascular integrity (6, 8, 10, 12, 29, 30, 32, 38, 53, 56, 63). Approximately 80,000 copies of β3 integrin receptors are present on each platelet, making β3 integrins the most abundantly expressed platelet receptor and providing for rapid platelet activation (12). β3 integrins on endothelial cells perform discrete functions that direct vascular repair, angiogenesis, and endothelial cell migration cued by VEGF (6, 7, 18, 19, 30, 41, 50, 53, 56, 63). These functions in platelets and endothelial cells control vascular integrity through platelet activation and adherence to injured endothelium and by regulating permeabilizing responses of VEGF (6, 12, 18, 20-22, 30, 53). However, pathogenic hantaviruses bind inactive basal conformations of αvβ3 integrins on endothelial cells (49), and the findings presented here demonstrate that pathogenic ANDV and HTNV bind β3 integrins present on the surface of platelets. This finding provides a new role for platelet integrins during hantavirus infection and suggests that platelets may disseminate pathogenic hantaviruses within patients. Hantavirus binding to inactive β3 integrins also suggests a means by which hantaviruses could prevent normal platelet activation and is consistent with data indicating that platelets from HFRS patients are defective in platelet activation (14).
The binding of activated platelets to endothelial cells is of central importance to the regulation of vascular permeability and hemorrhagic disease, yet platelets normally do not bind endothelial cells and are maintained in a quiescent state by factors (ADPase and PGE1) secreted by endothelial cells (34, 44). This becomes interesting when findings on pathogenic-hantavirus use of β3 integrins are combined with the obscure observation that pathogenic SNV is cell associated and displayed on the surfaces of infected VeroE6 cells (28), which also express αvβ3. These observations suggested that cell surface-displayed hantaviruses could potentially interact with platelet β3 integrins and thereby recruit platelets to the surface of endothelial cells. Data presented here indicate that pathogenic-hantavirus-infected endothelial cells are able to bind quiescent platelets and that binding increased from 1 to 3 days postinfection (Fig. 3). Figure 4 demonstrates that neutralizing antibodies to ANDV or HTNV selectively blocked the binding of platelets to infected endothelial cells, and this indicates that cell surface-associated virus mediates platelet binding. These findings suggest that at late times postinfection, hantaviruses that are displayed on the surfaces of infected endothelial cells recruit platelets to the endothelial cell surface and thereby form a platelet covering on the surface of the endothelium. This could dramatically alter the appearance of infected endothelial cells to immune cells and enhance viral evasion of immune responses. Since endothelial cell factors prevent platelet activation (29, 34, 42, 44), recruiting inactive platelets to infected endothelial cells may further contribute to maintenance of platelet quiescence.
Prevention of platelet activation is entirely different from platelet aggregation directed by dengue virus infection and other viral infections associated with thrombocytopenia (36, 48, 61). Activation of platelets results in aggregation and granule deposition as well as immune cell recruitment and activation of damaged endothelium (1, 3, 60, 66). Consistent with dengue virus infection, platelet deposition and aggregation may result in thrombocytopenia by reducing the effective concentration of platelets in circulation (36). In contrast, the absence of platelet activation following hantavirus infection (14) could result in thrombocytopenia due to the transduction of platelet-rich serum into tissues. Recruitment of quiescent platelets to infected endothelial cells suggests a second mechanism by which hantaviruses may cause thrombocytopenia. The endothelium is estimated to contain 1011 cells and to cover an area of >1,000 m2 (1, 60), and thus, recruitment of quiescent platelets to hantavirus-infected endothelial cells could be a significant means of reducing the level of circulating platelets following infection.
The presence of pathogenic hantaviruses on the surfaces of endothelial cells also suggests that hantavirus β3 integrin receptor interactions may alter endothelial cell functions. Interestingly, increased platelet adherence to ANDV- and HTNV-infected cells occurs synchronously with the inhibition of β3-directed endothelial cell migration (23) and the enhanced permeability of ANDV- and HTNV-infected endothelial cells in response to VEGF (25). This mimics the enhanced permeability of β3 integrin knockout endothelial cells in response to VEGF (53) and functionally suggests that cell-associated hantaviruses might contribute to this response. Based on platelet adherence, Fab fragments directed at β3 appear to remove hantavirus from the cell surface. Although there is no information on whether this prevents hantavirus-induced endothelial cell permeability, β3 Fab fragments have the potential to normalize β3 integrin regulation of VEGF responses (10-12) and prevent endothelial cell permeability.
A layer of platelets on the surfaces of endothelial cells may also alter capillary permeability by affecting gas exchange within vast pulmonary capillary beds and thereby contribute to a hypoxic state (4, 16, 57). Hantavirus patients are hypoxic, and high-altitude-induced pulmonary edema results from hypoxic conditions inducing VEGF (4, 16, 31, 47). Since hypoxia-induced factor 1α (HIF1α) induces VEGF and VEGF induces transcription of HIF1α, an autocrine loop which amplifies endothelial cell permeability in response to hypoxia is formed (16, 47, 57, 59). The importance of these interactions during hantavirus infection is fostered by understanding that hantaviruses inactivate β3 functions that normally regulate VEGF responses.
Collectively, our findings indicate that hantavirus binding to inactive β3 integrins affects both platelet and endothelial cell interactions which dynamically regulate vascular integrity. Our data suggest a new paradigm in which pathogenic hantaviruses alter the endothelial cell surface as well as endothelial cell functions through the recruitment and display of quiescent platelets and further suggest a potential means for therapeutic intervention by the targeting of these receptors.
Acknowledgments
We thank Dmitry Gnatenko at Stony Brook University for help with platelet studies and Jean Wainer for excellent technical support. We thank Brian Hjelle at the University of New Mexico for generously providing ANDV for these studies and J. W. Hooper at USAMRIID for generously providing anti-ANDV antisera.
This work was supported by National Institutes of Health grants R01AI47873, PO1AI055621, R21AI1080984, and U54AI57158 (Northeast Biodefense Center [director, W. I. Lipkin]) and by a Veterans Affairs Merit Award to E.R.M.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Aird, W. C. 2004. Endothelium as an organ system. Crit. Care Med. 32:S271-S279. [DOI] [PubMed] [Google Scholar]
- 2.Banno, A., and M. H. Ginsberg. 2008. Integrin activation. Biochem. Soc. Trans. 36:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner-Parzer, S. M., and W. K. Waldhausl. 2001. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp. Clin. Endocrinol. Diabetes 109(Suppl. 2):S166-S179. [DOI] [PubMed] [Google Scholar]
- 4.Berger, M. M., C. Hesse, C. Dehnert, H. Siedler, P. Kleinbongard, H. J. Bardenheuer, M. Kelm, P. Bartsch, and W. E. Haefeli. 2005. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 172:763-767. [DOI] [PubMed] [Google Scholar]
- 5.Bombeli, T., B. R. Schwartz, and J. M. Harlan. 1998. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J. Exp. Med. 187:329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges, E., Y. Jan, and E. Ruoslahti. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867-39873. [DOI] [PubMed] [Google Scholar]
- 7.Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. [PubMed] [Google Scholar]
- 8.Calvete, J. J. 1999. Platelet integrin GPIIb/IIIa: structure-function correlations. An update and lessons from other integrins. Proc. Soc. Exp. Biol. Med. 222:29-38. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. P., and T. M. Cosgriff. 2000. Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul. Fibrinolysis 11:461-483. [DOI] [PubMed] [Google Scholar]
- 10.Coller, B. 1997. GPIIb/IIIa antagonists: pathophysiologic and therapeutic insights from studies of c7E3 Fab. Thromb. Haemost. 78:730-735. [PubMed] [Google Scholar]
- 11.Coller, B. 1997. Platelet GPIIb/IIIb antagonists: the first anti-integrin receptor therapeutics. J. Clin. Invest. 99:1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coller, B. S., and S. J. Shattil. 2008. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood 112:3011-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgriff, T. M. 1991. Mechanisms of disease in Hantavirus infection: pathophysiology of hemorrhagic fever with renal syndrome. Rev. Infect. Dis. 13:97-107. [DOI] [PubMed] [Google Scholar]
- 14.Cosgriff, T. M., H. W. Lee, A. F. See, D. B. Parrish, J. S. Moon, D. J. Kim, and R. M. Lewis. 1991. Platelet dysfunction contributes to the haemostatic defect in haemorrhagic fever with renal syndrome. Trans. R. Soc. Trop. Med. Hyg. 85:660-663. [DOI] [PubMed] [Google Scholar]
- 15.Cosgriff, T. M., and R. M. Lewis. 1991. Mechanisms of disease in hemorrhagic fever with renal syndrome. Kidney Int. Suppl. 35:S72-S79. [PubMed] [Google Scholar]
- 16.Dehler, M., E. Zessin, P. Bartsch, and H. Mairbaurl. 2006. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur. Respir. J. 27:600-606. [DOI] [PubMed] [Google Scholar]
- 17.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, R. L. Moolenaar, S. E. Reef, K. B. Nolte, M. M. Gallaher, J. C. Butler, R. F. Breiman, and H. S. Group. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N. Engl. J. Med. 330:949-955. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak, H. F. 2006. Discovery of vascular permeability factor (VPF). Exp. Cell Res. 312:522-526. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak, H. F., L. F. Brown, M. Detmar, and A. M. Dvorak. 1995. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146:1029-1039. [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia, J. G., F. Liu, A. D. Verin, A. Birukova, M. A. Dechert, W. T. Gerthoffer, J. R. Bamberg, and D. English. 2001. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108:689-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavard, J., and J. S. Gutkind. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223-1234. [DOI] [PubMed] [Google Scholar]
- 22.Gavard, J., V. Patel, and J. S. Gutkind. 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14:25-36. [DOI] [PubMed] [Google Scholar]
- 23.Gavrilovskaya, I., T. Peresleni, E. Geimonen, and E. Mackow. 2002. Pathogenic hantaviruses selectively inhibit beta-3 integrin directed endothelial cell migration. Arch. Virol. 147:1913-1931. [DOI] [PubMed] [Google Scholar]
- 24.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 73:3951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrilovskaya, I. N., E. E. Gorbunova, N. A. Mackow, and E. R. Mackow. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82:5797-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. beta3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnatenko, D. V., J. J. Dunn, S. R. McCorkle, D. Weissmann, P. L. Perrotta, and W. F. Bahou. 2003. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood 101:2285-2293. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith, C. S., L. H. Elliott, C. J. Peters, and S. R. Zaki. 1995. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch. Virol. 140:2107-2122. [DOI] [PubMed] [Google Scholar]
- 29.Hawiger, J. 1995. Mechanisms involved in platelet vessel wall interaction. Thromb. Haemost. 74:369-372. [PubMed] [Google Scholar]
- 30.Hodivala-Dilke, K. M., K. P. McHugh, D. A. Tsakiris, H. Rayburn, D. Crowley, M. Ullman-Cullere, F. P. Ross, B. S. Coller, S. Teitelbaum, and R. O. Hynes. 1999. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopkins, S. R., J. Garg, D. S. Bolar, J. Balouch, and D. L. Levin. 2005. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 171:83-87. [DOI] [PubMed] [Google Scholar]
- 32.Hynes, R. O., and K. M. Hodivala-Dilke. 1999. Insights and questions arising from studies of a mouse model of Glanzmann thrombasthenia. Thromb. Haemost. 82:481-485. [PubMed] [Google Scholar]
- 33.Kamrud, K. I., and C. S. Schmaljohn. 1994. Expression strategy of the M genome segment of Hantaan virus. Virus Res. 31:109-121. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima, Y., T. Nagasawa, and H. Ninomiya. 2000. Contribution of ecto-5′-nucleotidase to the inhibition of platelet aggregation by human endothelial cells. Blood 96:2157-2162. [PubMed] [Google Scholar]
- 35.Koster, F., K. Foucar, B. Hjelle, A. Scott, Y. Y. Chong, R. Larson, and M. McCabe. 2001. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am. J. Clin. Pathol. 116:665-672. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamurti, C., R. A. Peat, M. A. Cutting, and S. W. Rothwell. 2002. Platelet adhesion to dengue-2 virus-infected endothelial cells. Am. J. Trop. Med. Hyg. 66:435-441. [DOI] [PubMed] [Google Scholar]
- 37.Lahdevirta, J. 1982. Clinical features of HFRS in Scandinavia as compared with East Asia. Scand. J. Infect. Dis. Suppl. 36:93-95. [PubMed] [Google Scholar]
- 38.Lampugnani, M. G., and E. Dejana. 2007. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb. Res. 120(Suppl. 2):S1-S6. [DOI] [PubMed] [Google Scholar]
- 39.Luo, B. H., J. Karanicolas, L. D. Harmacek, D. Baker, and T. A. Springer. 2009. Rationally designed integrin beta3 mutants stabilized in the high affinity conformation. J. Biol. Chem. 284:3917-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackow, E. R., and I. N. Gavrilovskaya. 2001. Cellular receptors and hantavirus pathogenesis. Curr. Top. Microbiol. Immunol. 256:91-115. [DOI] [PubMed] [Google Scholar]
- 41.Mahabeleshwar, G. H., W. Feng, D. R. Phillips, and T. V. Byzova. 2006. Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 203:2495-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McVerry, B. J., and J. G. Garcia. 2004. Endothelial cell barrier regulation by sphingosine 1-phosphate. J. Cell. Biochem. 92:1075-1085. [DOI] [PubMed] [Google Scholar]
- 43.Montrucchio, G., E. Lupia, E. Battaglia, L. Del Sorbo, M. Boccellino, L. Biancone, G. Emanuelli, and G. Camussi. 2000. Platelet-activating factor enhances vascular endothelial growth factor-induced endothelial cell motility and neoangiogenesis in a murine matrigel model. Arterioscler. Thromb. Vasc. Biol. 20:80-88. [DOI] [PubMed] [Google Scholar]
- 44.Nalbandian, R. M., and R. L. Henry. 1978. Platelet-endothelial cell interactions. Metabolic maps of structures and actions of prostaglandins, prostacyclin, thromboxane and cyclic AMP. Semin. Thromb. Hemost. 5:87-111. [DOI] [PubMed] [Google Scholar]
- 45.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110-120. [DOI] [PubMed] [Google Scholar]
- 46.Pensiero, M. N., G. B. Jennings, C. S. Schmaljohn, and J. Hay. 1988. Expression of the Hantaan virus M genome segment by using a vaccinia virus recombinant. J. Virol. 62:696-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pham, I., T. Uchida, C. Planes, L. B. Ware, R. Kaner, M. A. Matthay, and C. Clerici. 2002. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1133-L1142. [DOI] [PubMed] [Google Scholar]
- 48.Rahbar, A., and C. Soderberg-Naucler. 2005. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J. Virol. 79:2211-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond, T., E. Gorbunova, I. N. Gavrilovskaya, and E. R. Mackow. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. U. S. A. 102:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds, A. R., L. E. Reynolds, T. E. Nagel, J. C. Lively, S. D. Robinson, D. J. Hicklin, S. C. Bodary, and K. M. Hodivala-Dilke. 2004. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 64:8643-8650. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds, L. E., L. Wyder, J. C. Lively, D. Taverna, S. D. Robinson, X. Huang, D. Sheppard, R. O. Hynes, and K. M. Hodivala-Dilke. 2002. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8:27-34. [DOI] [PubMed] [Google Scholar]
- 52.Rhee, J. S., M. Black, U. Schubert, S. Fischer, E. Morgenstern, H. P. Hammes, and K. T. Preissner. 2004. The functional role of blood platelet components in angiogenesis. Thromb. Haemost. 92:394-402. [DOI] [PubMed] [Google Scholar]
- 53.Robinson, S. D., L. E. Reynolds, L. Wyder, D. J. Hicklin, and K. M. Hodivala-Dilke. 2004. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler. Thromb. Vasc. Biol. 24:2108-2114. [DOI] [PubMed] [Google Scholar]
- 54.Schmaljohn, C. S., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, p. 1581-1602. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 55.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soldi, R., S. Mitola, M. Strasly, P. Defilippi, G. Tarone, and F. Bussolino. 1999. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stenmark, K. R., K. A. Fagan, and M. G. Frid. 2006. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ. Res. 99:675-691. [DOI] [PubMed] [Google Scholar]
- 58.Takagi, J., B. M. Petre, T. Walz, and T. A. Springer. 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110:599-611. [DOI] [PubMed] [Google Scholar]
- 59.Tang, N., L. Wang, J. Esko, F. J. Giordano, Y. Huang, H. P. Gerber, N. Ferrara, and R. S. Johnson. 2004. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6:485-495. [DOI] [PubMed] [Google Scholar]
- 60.Valbuena, G., and D. H. Walker. 2006. The endothelium as a target for infections. Annu. Rev. Pathol. 1:171-198. [DOI] [PubMed] [Google Scholar]
- 61.Visser, M. R., P. B. Tracy, G. M. Vercellotti, J. L. Goodman, J. G. White, and H. S. Jacob. 1988. Enhanced thrombin generation and platelet binding on herpes simplex virus-infected endothelium. Proc. Natl. Acad. Sci. U. S. A. 85:8227-8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wahl-Jensen, V., J. Chapman, L. Asher, R. Fisher, M. Zimmerman, T. Larsen, and J. W. Hooper. 2007. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J. Virol. 81:7449-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weis, S. M., J. N. Lindquist, L. A. Barnes, K. M. Lutu-Fuga, J. Cui, M. R. Wood, and D. A. Cheresh. 2007. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood 109:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yanagihara, R., and D. J. Silverman. 1990. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch. Virol. 111:281-286. [DOI] [PubMed] [Google Scholar]
- 65.Zaki, S., P. Greer, L. Coffield, C. Goldsmith, K. Nolte, K. Foucar, R. Feddersen, R. Zumwalt, G. Miller, P. Rollin, T. Ksiazek, S. Nichol, and C. Peters. 1995. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]
- 66.Zarbock, A., and K. Ley. 2009. The role of platelets in acute lung injury (ALI). Front. Biosci. 14:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu, J., B. H. Luo, T. Xiao, C. Zhang, N. Nishida, and T. A. Springer. 2008. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 32:849-861. [DOI] [PMC free article] [PubMed] [Google Scholar]