Abstract
The emergence in 1997 and continuance today of a highly lethal H5N1 avian influenza virus (AIV) causing human disease has raised concern about an impending pandemic and the need for a vaccine to prepare for such an occurrence. We previously generated an efficacious vesicular stomatitis virus (VSV)-based AIV vaccine expressing H5 hemagglutinin (HA) from the fifth genomic position of VSV (J. A. Schwartz et al., Virology 366:166-173, 2007). Here we have generated and characterized VSV-based vaccines that express the A/Hong Kong/156/1997 (clade 0) H5 HA from the first position of the VSV genome. These vectors induce broadly cross-neutralizing antibodies against homologous and heterologous H5N1 viruses of different clades in mice. The vaccines provide complete protection against morbidity and mortality after heterologous challenge with clade 0 and clade 1 strains in animals even 1 year after vaccination. Postchallenge pulmonary virus loads show that these vectors provide sterilizing immunity. Therefore, VSV-based AIV vaccines are potent, broadly cross-protective pandemic vaccine candidates.
Influenza viruses are negative-stranded, segmented RNA viruses of great public health importance. Not only do they cause epidemics affecting hundreds of thousands of people worldwide every year, but on rare occasions, they cause pandemics that can kill millions of people. The 20th century saw three such pandemics, and in the three pandemics combined, up to 100 million people worldwide have been estimated to have died. Pandemics occur when novel influenza virus subtypes infect humans and cause disease (42). The current H1N1 influenza pandemic appears to have arisen from a swine influenza reservoir, while others may have come from avian reservoirs (42). Influenza virus subtypes are based on the antigenicity of two envelope proteins, hemagglutinin (HA) and neuraminidase (NA). Currently, there are 16 known HA types and 9 NA types (8, 42).
In 1997, avian influenza viruses (AIVs) of the H5N1 subtype emerged and caused disease in humans. This was the first known instance of human disease by this subtype. The H5N1 viruses reemerged in 2003 and continue to cause disease in humans up to the present day. They also continue to circulate in poultry and migratory birds throughout Asia, Europe, and Africa (13). Viruses of this subtype are of great concern because of the high fatality rate (∼60%) in humans. These factors caused the Centers for Disease Control and Prevention and the World Health Organization to issue warnings of an impending pandemic resulting from H5N1 viruses (http://www.who.int/csr/disease/avian_influenza/en/).
To prepare for such a pandemic, vaccines are being developed using traditional and novel methodologies (reviewed in references 13 and 20). Currently, the annual seasonal trivalent influenza vaccine is directed against two influenza virus A subtypes (H1 and H3), and one influenza virus B type (36). Unfortunately, the antibodies induced by these vaccines do not cross-react well with AIV H5 strains. An additional concern with vaccine development is the ability of influenza viruses to undergo dramatic antigenic drift. AIVs within a subtype often undergo mutations, particularly within the HA, generating antigenically distinct sublineages (or clades) of HA (5, 41). Neutralizing antibodies against a strain in one clade are not always effective against strains in other clades, even within a subtype. Therefore, an important aspect to any pandemic influenza vaccine is that it elicit broadly cross-reactive immunity across clades.
In addition to the problems caused by the intrinsic biological properties of AIVs, difficulties exist with practical issues regarding traditional vaccine production strategies. One issue is the highly pathogenic nature of many AIVs. This raises biosafety and biocontainment risks associated with manufacture of AIV vaccines, which are typically inactivated, live attenuated, or subvirion vaccines. It also presents difficulty in generating high-titer vaccine stocks, since many of the highly pathogenic avian influenza (HPAI) viruses are pathogenic to chicken eggs in which traditional influenza vaccines are grown. Additionally and probably the most important in containing worldwide spread of a highly lethal infection is the 6- to 9-month period it takes for manufacture of traditional vaccines.
The ability of influenza viruses to undergo antigenic shift and drift makes it difficult to predict which subtype or clade will lead to a pandemic. The strains included in the annual seasonal influenza vaccine are based on predictions made by a group of international scientists several months before the influenza season begins. These predictions are based on reports of the previous year's circulating strains. This prediction is not always accurate, as exemplified by the year 2008 in which the strain causing the greatest incidence of disease was not included in the annual trivalent vaccine and therefore was not significantly effective. Thus, novel vaccine strategies that reduce the biosafety risks and increase the cross-reactivity against different clades and/or subtypes, thereby reducing the need to predict the future pandemic strain, would be beneficial.
One such strategy is the use of a virus-based vaccine vector system, such as vesicular stomatitis virus (VSV). Recombinant VSV (rVSV) vaccines have been efficacious against a wide number of viral diseases, including the respiratory diseases caused by respiratory syncytial virus (RSV) (23), severe acute respiratory syndrome (SARS) coronavirus (24, 25), and a mouse-adapted human influenza virus (32, 33). Additionally, we previously reported on rVSVs as effective vaccines against an AIV (38). These vectors expressed the H5 HA from a clade 0 H5N1 virus, A/Hong Kong/156/1997 (HK/156), from the fifth genomic position of the rVSV vectors. This vaccine induced broadly cross-neutralizing antibodies against the homologous virus as well as another clade 0 strain (A/Hong Kong/483/1997 [HK/483]) and two heterologous strains from clades 1 and 2.1.3. These fifth position vectors also completely protected mice against challenge with the antigenically distinct HK/483 virus in mice (38).
In order to generate a greater and potentially more robust cross-protective response against distantly related AIV strains, we constructed rVSVs that express the HK/156 H5 HA from the first genomic position of VSV, near the 3′ end of the negative-strand RNA genome (30). Because VSV transcription is sequentially attenuated along the genome (22), this position provides optimal expression of the HA transgene. We show here that these first position rVSV vectors expressing the HK/156 H5 HA induce broadly cross-neutralizing antibodies against clade 0, 1, and 2.1.3 viruses. The rVSV vaccines induce sterilizing immunity and are highly efficacious against infection with heterologous AIVs. Furthermore, this protective response is maintained in mice for at least a year postvaccination.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were propagated in Dulbecco's modified essential medium (DMEM) (Invitrogen, Carlsbad, CA) containing penicillin and streptomycin (1× Pen-Strep; Invitrogen) and 5% fetal bovine serum (FBS) (Gemini Bio Products, West Sacramento, CA). Madin-Darby canine kidney (MDCK) cells were grown in minimum essential medium (MEM) (Invitrogen) containing 1× Pen-Strep and 10% FBS.
Recombinant VSVs were grown and titrated on BHK-21 cells as described previously (29, 37). The following AIVs, used in the neutralization assays, were provided by N. Cox and A. Klimov (Influenza Branch, Centers for Disease Control and Prevention [CDC], Atlanta, GA): A/Hong Kong/156/1997 (HK/156; clade 0), A/Hong Kong/483/1997 (HK/483; clade 0), A/Vietnam/1203/2004 (VN/1203; clade 1), and A/Indonesia/5/2005 (INA/5; clade 2.1.3). For challenge virus stocks, MDCK cells were infected at a low multiplicity of infection (MOI) in MEM plus 0.3% bovine serum albumin (BSA), 1× Pen-Strep, and 1 μg/ml trypsin that had been treated with tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK). AIV stocks were titrated on MDCK cells in media supplemented with 1 μg/ml trypsin that had been treated with TPCK.
Construction and recovery of recombinant VSVs.
The H5 HA gene from HK/156 (provided by Y. Kawaoka, University of Wisconsin, Madison, WI; GenBank accession number AF046088), amplified by PCR from pBS-H5 as previously described (38), was cloned using XhoI and NheI into the recombinant wild-type VSV vector (Indiana serotype), pVSV1XN (30), which incorporates the H5 HA gene into the first genomic position of VSV to generate the pVSV-H5(1) plasmid. The serotype switch vector, pVSV-NJG-H5(1), expressing the H5 HA gene from the first position and the G gene from the VSV New Jersey (NJ) serotype was made by switching the ApaI-XbaI fragment containing the portion of the rVSV genome with the NJ G gene from pVSV-NJG-XN (35) with that from pVSV-H5(1).
The rVSVs, VSV-H5(1) and VSV-NJG-H5(1), were recovered from the above plasmid DNAs as previously described (27, 38). Briefly, BHK-21 cells infected with a vaccinia virus expressing T7 polymerase, vTF7-3 (9), at an MOI of 10 for 1 h were transfected with 50 μg of the appropriate H5 HA plasmid plus the support plasmids, pBS-N (15 μg), pBS-P (25 μg), pBS-G (20 μg), and pBS-L (5 μg). At 48 h posttransfection, the cell culture medium was collected, filtered (0.2 μm), and passaged onto BHK-21 cells. Once a cytopathic effect (CPE) was observed, the culture medium was filtered (0.1 μm), plaque purified, and used to grow virus stocks.
Western blot analysis.
Western blot analysis was performed as described previously (38). Briefly, whole-cell extracts from BHK-21 cells infected at an MOI of ∼20 of the indicated viruses were collected at 4.5 hours postinfection (hpi). After the monolayers were washed twice with ice-cold phosphate-buffered saline (PBS), the cells were incubated for 5 min on ice with lysis buffer. The lysates were subjected to SDS-PAGE on a 4 to 12% bis-Tris NuPAGE gel (Invitrogen), and the proteins were transferred to nitrocellulose using a semidry transfer apparatus (Bio-Rad Laboratories, Inc., Hercules, CA). The blot was blocked overnight in PBS containing 0.5% Tween 20 (PBST) and 4% nonfat milk (PBST-milk) and then incubated in PBST-milk containing the NR-665 polyclonal anti-influenza virus H5 HA A/Hong Kong/156(483)/97 sheep antiserum (NIH Biodefense and Emerging Infectious Research Repository, NIAID, NIH) at a 1:1,000 dilution for 1 h at room temperature. After three 5-min washes in PBST, the blot was incubated as before in a 1:10,000 dilution of donkey anti-sheep IgG conjugated to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA) in PBST-milk. The blot was washed 3 more times and subjected to chemiluminescence analysis using a LAS-3000 imaging system (Fujifilm).
Vaccinations.
All vaccination procedures were approved by the Institutional Animal Care and Use Committee of Yale University. Six- to 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were primed by intramuscular (i.m.) injection with 50 μl of 107 PFU of VSV-H5(1) or VSV-SIVGag(1), an rVSV (30) expressing the unrelated simian immunodeficiency virus (SIV) Gag gene from the first genomic position (L. Buonocore and J. Rose, unpublished data). At 2 or 2.5 months postprime, all of the mice were boosted i.m. with 50 μl of 107 PFU of VSV-NJG-H5(1) or VSV-NJG-SIVGag(1), a NJ serotype switch vector expressing the unrelated SIV Gag gene from the first genomic position (L. Buonocore and J. Rose, unpublished data).
Microneutralization assays.
Neutralizing antibody titers against the H5 HA were determined under enhanced biosafety level 3 (BSL-3) containment procedures in laboratories approved for use by the U.S. Department of Agriculture and CDC as previously described (38, 39). All AIVs used in this assay were grown in the allantoic cavities of specific-pathogen-free eggs as previously described (39). In brief, heat-inactivated, serially diluted mouse serum was combined with an equal volume of ∼100 50% tissue culture infective dose (TCID50) of the indicated virus, incubated at room temperature for 1 h, and then added to MDCK cells in quadruplicate. Neutralizing antibody titers were determined by the reciprocal dilution at which infectivity was neutralized, which is defined as the absence of CPE at 4 days postinfection (dpi). An arbitrary titer of 10 was given to any sample that did not display neutralizing activity.
AIV challenge and viral load.
Challenge infections were performed as previously described (38) under an approved animal use protocol according to the guidelines of the Canadian Council on Animal Care using BSL-4 containment procedures. At various times postvaccination, mice were anesthetized with isoflurane and challenged intranasally (i.n.) with 100 50% lethal doses (LD50s) of either HK/483 or VN/1203 in 50 μl MEM containing 0.3% BSA. The LD50s were determined as described previously (31). Mice were weighed daily and analyzed for disease. Mice displaying severe morbidity were humanely euthanized.
On day 3 postchallenge, the lungs were harvested from mice challenged with the indicated virus. The tissues were homogenized, and viral load was determined by plaque assay on MDCK cells.
RESULTS
Construction and characterization of vectors containing an AIV H5 HA gene in the first position of the VSV genome.
The five genes in VSV (N, P, M, G, and L) are transcribed sequentially starting from the 3′ end of the genome with transcription attenuation of about 30% occurring at each gene junction (22). Because of this attenuation, foreign genes expressed from the first position of the VSV genome are typically expressed about 4-fold better than genes expressed from the fifth position (J. Rose, unpublished results).
Previously, we described a VSV-based H5N1 vaccine vector in which the HK/156 H5 HA gene was expressed from the fifth position of a VSV recombinant (38). To generate vectors expressing higher levels of the H5 HA and to maximize the immune responses to HA, we incorporated the HK/156 (clade 0) H5 HA gene into the first position of the VSV (Indiana serotype) genome upstream of the N gene (Fig. 1A). We also prepared a vector [VSV-NJG-H5(1)] for booster vaccination in which the Indiana G gene from VSV-H5(1) was replaced with the G gene from the VSV New Jersey (NJ) serotype (Fig. 1A). Vaccination with VSV vectors generates high levels of G neutralizing antibodies, which precludes efficient boosting with the same vector (35). Therefore, the NJ serotype switch vector was used for boosting.
FIG. 1.
Construction and characterization of recombinant VSVs expressing an H5 HA gene from the first genomic position of VSV. (A) Schematic diagram of the rVSV genomes showing the insertion of the HK/156 H5 HA gene (H5) into the first genomic position upstream of the VSV N gene in a recombinant wild-type (rWT) background [Indiana strain; VSV-H5(1)] or a rWT background where the G gene was replaced with the G gene from another VSV serotype [New Jersey (NJ); VSV-NJG-H5(1)]. The numbers in parentheses indicate the VSV genomic position at which the H5 HA gene is inserted. (B) Western blot analysis of whole-cell extracts from cells mock infected or infected with virus using a polyclonal antibody to the HK/156 H5 HA. The cells were mock infected or infected with the following viruses: rVSV (Indiana) (WT); first position vectors VSV-H5(1) [H5(1)] and VSV-NJG-H5(1) [NJG-H5(1)]; and fifth position vectors VSV-H5 HA [H5(5)] and VSV-NJG-H5 HA [NJG-H5(5)] (38). The full-length HA (HA0) and cleaved isoforms of HA (HA1 and HA2) are indicated by arrows to the right of the gel. The positions (molecular mass [in kilodaltons]) of molecular size markers are indicated to the left of the gel.
To determine how well the H5 HAs were expressed from the first position vectors (Fig. 1A), we performed indirect immunofluorescence (data not shown) and Western blot analyses (Fig. 1B). H5 HA accumulates to high levels in cells infected with either VSV-H5(1) or VSV-NJG-H5(1) (Fig. 1B). Cells infected with the first position vectors accumulated more H5 HA than cells infected with the fifth position vectors (Fig. 1B).
Induction of neutralizing antibodies against heterologous H5 HAs in mice after vaccination with VSV-based vectors.
To determine the immunogenicity of the first position vectors, 24 mice were inoculated intramuscularly (i.m.) with 107 PFU of VSV-H5(1) and 2.5 months later were boosted with the serotype switch vector, VSV-NJG-H5(1). Another group of 24 mice (controls) were primed and boosted with first position vectors expressing an unrelated foreign antigen. Initial dose-response studies (data not shown) showed that this dose was sufficient to generate maximal neutralizing antibody (nAb) titers against the homologous AIV. Doses above 107 PFU did not yield higher levels of nAb titers to the homologous AIV.
Using a stringent microneutralization assay (100% inhibition of CPE), we evaluated the ability of the individual animals to mount a serum nAb response against a homologous clade 0 AIV, HK/483 (Fig. 2A), and a heterologous clade 1 AIV, VN/1203 (Fig. 2B). All vaccinated animals (n = 24) generated high nAb titers against HK/483 after priming alone, with a significant increase after boosting. The nAb response against HK/483 for the 8 mice that were subsequently challenged with HK/483 is shown in Fig. 2A.
FIG. 2.
Induction of heterologous cross-protective immune responses in mice after vaccination with rVSVs expressing H5 HA from the optimal first genomic position. (A and B) Mice (n = 8) were intramuscularly primed and boosted at 2.5 months postprime with 107 PFU of VSV-H5(1) and VSV-NJG-H5(1), respectively. Sera collected from vaccinated mice at 2.5 months postprime (preboost; white bars) or 1 month postboost (3.5 months postprime; gray bars) were subjected to a microneutralization assay (39) against the challenge virus (HK/483[A] and VN/1203 [B]). All mice vaccinated with the negative-control vectors (n = 8) had undetectable neutralizing activity that corresponds to a titer of 10 in this assay (data not shown). Asterisks indicate sera from H5 HA-vaccinated animals with undetectable nAb. (C to F) At 2 months postboost (4.5 months postprime), vaccinated mice were intranasally challenged with either HK/483 (clade 0; n = 8) (C and E) or VN/1203 (clade 1; n = 8) (D and F). (C and D) Pathogenesis was assessed by weight loss and is shown as the average percentage of original prechallenge weight. (E and F) Kaplan-Meier plots depict the percent survival of negative-control mice (controls) or H5 HA-vaccinated mice (vaccinees).
We also determined the nAb titers in individual animals against the clade 1 VN/1203 strain. Unlike the response against the highly homologous HK/483, only 50% of the mice had a detectable nAb titer against VN/1203 after prime only, while 87.5% had nAb titers after boosting. Out of 24 boosted animals, only 3 (12.5%) had nAb titers that were below the level of detection in the assay. The nAb titers against VN/1203 for the group of 8 mice subsequently challenged with VN/1203 are shown (Fig. 2B). The difference in nAb titers to the two viruses is consistent with the degree of homology to the HA (HK/156) expressed by the vaccine virus. The clade 0 strain, HK/156, isolated in 1997 has 98.6% amino acid identity with HK/483, also a clade 0 virus from 1997, compared to 96% identity with VN/1203, a clade 1 strain from 2004.
Vaccination provides protection against a heterologous AIV challenge.
The majority of vaccinated mice had sufficient nAb titers to indicate that they would be protected from challenge with strain VN/1203 (Fig. 2B). To determine the degree of protection against heterologous AIV challenge, mice primed and boosted with the first position H5 HA vectors were challenged i.n. with either the highly homologous HK/483, a clade 0 virus (Fig. 2C and E), or the heterologous VN/1203, a clade 1 virus (Fig. 2D and F). All vaccinees were protected against HK/483 challenge (Fig. 2E) and did not display any signs or symptoms of disease, including weight loss (Fig. 2C). All vaccinated mice were also protected against challenge with VN/1203 (Fig. 2F) despite significantly lower nAb titers against this strain (Fig. 2B). Furthermore, none of the animals showed significant weight loss following challenge (Fig. 2D). Vaccination reduced the severity of disease, since none of the vaccinated animals challenged with either HK/483 (Fig. 2C) or VN/1203 (Fig. 2D) displayed significant weight loss.
Challenge virus does not replicate in vaccinated animals.
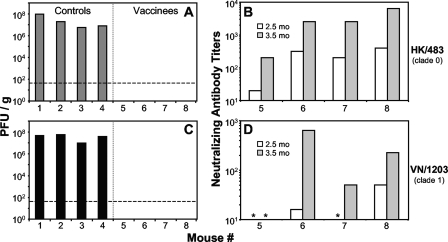
To further analyze the extent of protection provided by the VSV-based vectors, pulmonary loads of challenge virus were examined in four vaccinated mice and four control mice 3 days after i.n. challenge. Mice that received the control vectors showed robust AIV pulmonary replication after challenge as indicated by virus titers in lung homogenates (Fig. 3A and C). However, no virus was found in the lungs of vaccinated mice after challenge with either HK/483 (Fig. 3A) or VN/1203 (Fig. 3C). Because there was no evidence of challenge virus infection in animals vaccinated with the VSV vectors expressing H5 HA, it appears that sterilizing immunity was achieved.
FIG. 3.
VSV-based vaccines provide sterilizing immunity against heterologous AIVs. Mice (n = 4) were vaccinated with the first position H5 HA vectors (vaccinees) (A and C) or negative-control vectors (controls) (A and C) and challenged with either HK/483 (A and B) or VN/1203 (C and D) as in Fig. 2. (C to F) At 3 days postchallenge, the lungs were harvested, and the virus titer was determined by plaque assay. No virus was detected in any vaccinee homogenate. The limit of detection for this assay was ≤50 PFU/g (dashed line) where the average lung mass was 100 mg. (B and D) Sera collected from vaccinated mice (mouse 5 to mouse 8) at 2.5 months postprime (preboost; white bars) or 1 month postboost (3.5 months postprime; gray bars) were subjected to a microneutralization assay (39) against the challenge virus (HK/483 [B] and VN/1203 [D]). All mice vaccinated with the negative-control vectors (mouse 1 to mouse 4) had undetectable nAb activity, which corresponds to a titer of 10 in this assay (data not shown). Asterisks indicate sera from H5 HA-vaccinated animals with no detectable nAb.
To examine the range of nAb responses in these animals, sera collected before and after boosting were analyzed in a microneutralization assay. As expected, the mice challenged with HK/483 had high nAb titers against this highly homologous virus (Fig. 3B) prior to challenge. Consistent with what was seen in sera from other vaccinated animals (Fig. 2B), only half the animals had a nAb response prior to boosting (Fig. 3D), and one animal did not have any detectable nAbs against VN/1203 after boosting (Fig. 3D). However, despite the lack of detectable nAbs against VN/1203 prior to challenge, this animal (Fig. 3C and D, mouse 5) showed no evidence of challenge virus replication in the lungs (Fig. 3C).
Induction of a broadly cross-neutralizing antibody response.
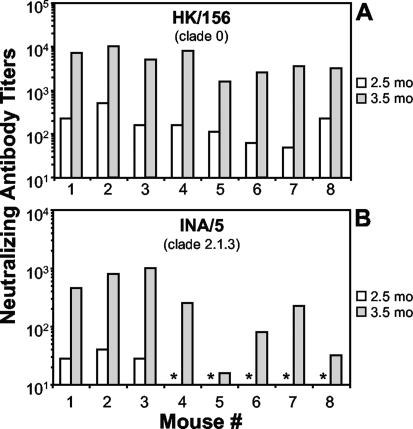
Previously, we showed that VSV-based vectors expressing the HK/156 H5 HA protein from the fifth position generated a cross-neutralizing antibody response against clade 0, 1, and 2.1.3 H5N1 viruses (38). To determine whether broadly cross-neutralizing antibodies were also generated by the first position vectors, we measured the nAb response against the more highly diverged clade 2.1.3 virus, A/Indonesia/5/2005 (INA/5). The nAb titers against HK/156 (Fig. 4A) and INA/5 (Fig. 4B) are shown for the individual serum samples previously examined in Fig. 2B against VN/1203. All H5 HA-vaccinated animals (n = 24) had high nAbs against HK/156 before and after boosting. The titers against HK/156 for 8 representative mice are shown (Fig. 4A). Similar to what was found for VN/1203 nAbs, only 50% of the mice (n = 24) had detectable nAbs against INA/5 after priming. However, all animals had detectable nAbs against INA/5 after boosting. The nAbs titers tested against INA/5 for 8 representative mice are shown (Fig. 4A).
FIG. 4.
Vaccination induces a broad cross-neutralizing antibody response. Sera collected at 2.5 months postprime (preboost; white bars) or 1 month postboost (3.5 months postprime; gray bars) from vaccinated mice (n = 8) that were challenged with VN/1203 (described in the legend to Fig. 2B, D, and F) were subjected to microneutralization assays (39) against the indicated viruses (HK/156 [A] and INA/5 [B]). All mice vaccinated with the negative-control vectors (n = 8) had undetectable neutralizing activity, which corresponds to a titer of 10 in this assay (data not shown). Asterisks indicate sera from H5 HA-vaccinated animals with undetectable nAb.
Vaccination confers long-term immunity to and protection against a heterologous AIV.
Previously, we showed that mice vaccinated with fifth position H5 HA VSV-based vectors retained nAb responses at 5.5 months postvaccination and complete protection from homologous challenge at 7.5 months postvaccination (38). Here we found that even at 11 months after vaccination, animals maintained levels of nAbs (Fig. 5A) similar to those seen a few months after vaccination (Fig. 2A and B and 4). The nAbs 11 months postvaccination had the same distribution and broad cross-reactivity to all four antigenically distinct strains (compare Fig. 5A to Fig. 2A and B and 4), with the highest neutralizing activity against the homologous clade 0 strains and ∼1-log-unit-lower, but relatively similar, activity against the heterologous clade 1 and 2.1.3 strains (Fig. 5A).
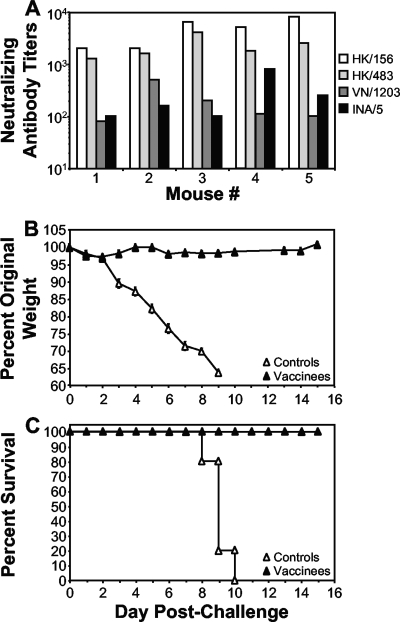
FIG. 5.
Long-term protective heterologous immunity in vaccinated mice. Mice (n = 5) were vaccinated and challenged at 1 year postprime with VN/1203, as described in the legend to Fig. 2. (A) Sera collected from vaccinated mice at 11 months postprime (9 months postboost) were subjected to microneutralization assays (39) against the indicated viruses (HK/156, HK/483, VN/1203, and INA/5). A titer of 10 in this assay is negative. (B and C) At 1 year postprime (10 months postboost), vaccinated mice were challenged intranasally with VN/1203 (clade 1). (B) Morbidity was assessed by weight loss and is shown as the average original prechallenge weight. (C) A Kaplan-Meier plot depicts the percent survival of negative-control or H5 HA-vaccinated mice. The control animals shown in panels B and C are the same animals as those in Fig. 2 and are shown here for comparison.
Because the animals were able to retain significant levels of nAbs long-term, we determined whether these animals were still protected against a heterologous challenge. At 1 year postvaccination, the mice were i.n. challenged with the heterologous virus, VN/1203. The vaccinated mice showed little or no postchallenge weight loss or clinical symptoms of disease (Fig. 5B). All vaccinees survived the challenge with VN/1203, while all of the control animals died by 10 days postchallenge (Fig. 5C).
DISCUSSION
Previously, we showed that VSV-based vectors expressing the H5 HA from HK/156 could protect mice against lethal challenge with a highly homologous AIV (38). Here we show that VSV-based vectors can protect mice from lethal challenge with a heterologous AIV. Additionally, our data show that not only do our vaccines impart a 100% survival rate, they also provide apparent sterilizing immunity in that challenge virus was not detectable in the lungs 3 days after challenge.
The concern about an impeding pandemic created by an H5N1 AIV has led to the need for a vaccine. It is difficult to predict exactly which AIV clade might create a pandemic. Therefore, development of vaccines that protect across a broad range of clades is important. Although inactivated H5N1 vaccines are now available, their immunogenicity is not optimal. The split virion vaccines without adjuvant induce antibody titers predictive of protection in only ∼40% of vaccinated individuals (40). The use of a whole virion vaccine (7) or the inclusion of oil-in-water adjuvants (1, 4, 28) clearly improve the immunogenicity of inactivated H5N1 virus vaccines. In any case, due to numerous issues with traditional vaccine development for pandemic strains, it is likely that novel vaccine therapies will be needed in the event of a pandemic.
The broad cross-reactivity of our VSV-based vaccines is a distinct advantage in developing a pandemic AIV vaccine. It provides heterologous protection against and prevents infection by clade 0 and 1 viruses. Additionally, nAb titers suggest that it would also be protective against another highly divergent strain, INA/5, a clade 2.1.3 virus. Other vaccines containing the 1997 HK/156 HA have shown protection against newer, more recent strains of AIV after vaccination with a cold-adapted influenza vaccine (39), a DNA vaccine (26), and an adenovirus vector (18). The adenovirus vector was able to protect against a lethal AIV challenge, but not against replication of the challenge virus.
The major correlate of protection against influenza viruses is antibody. Our vaccine vectors may have an advantage over other systems because they induce such high levels of homologous nAbs, a fraction of which are heterologous cross-reactive nAbs. We also noted that protection against pathogenesis and AIV replication occurred even in the absence of a detectable nAb response in some mice. Protection from challenge in the absence of detectable nAbs has been seen with other H5N1 vaccines (2, 14, 17-19, 21). To date, no other study has shown protection from virus pulmonary replication in the absence of detectable nAbs in individual animals. Hoelscher et al. (19) showed heterologous protection with little to no virus pulmonary replication after vaccination with adenovirus vectors. However, their data are presented for the vaccination group only as a whole and do not show data for individual animals. In our study, it is possible that all of the mice were at least primed and able to generate nAb rapidly after challenge. Alternatively, due to the limits of detection in the nAb assay, the assay may not have been sensitive enough to detect low but protective levels of nAb present in the mice in this study. VSV-based vectors also generate potent cellular immune responses (15, 16), and these responses could also play a role in those animals that were fully protected in the absence of detectable neutralizing antibody.
VSV vectors show promise as human vaccines. Recently, a VSV-based vaccine vector was used successfully in a person in an emergency situation with little to no apparent adverse reaction. This live-attenuated VSV-based Ebola vaccine was administered to a German researcher following a needle stick injury with a syringe that had been used to inject mice with a highly lethal Ebola virus strain (http://blogs.sciencemag.org/scienceinsider/2009/03/researchers-aro.html). Furthermore, attenuated VSV-based human immunodeficiency virus type 1 (HIV-1) vaccine vectors (6) are expected to receive final FDA approval and be moved into clinical trials as HIV-1 vaccines in 2010. Single-cycle VSV-based vectors lacking the VSV glycoprotein gene have also been tested and are highly effective (25).
VSV-based vectors have significant advantages over other virus-based vaccine platforms (34). These advantages include the fact that there is no significant preexisting immunity to VSV in humans and the availability of multiple serotypes that would allow multiple vaccine applications. VSV can also be delivered by mucosal routes, potentially providing improved protection against mucosal challenges (34). They have been shown to provide protection against exposure to Ebola and Marburg viruses in nonhuman primates (10-12). In addition, a VSV-based influenza vaccine has recently been shown to provide postexposure protection from influenza virus challenge in a mouse model (3).
VSV can be grown rapidly with only BSL-2 containment in cell lines approved for human vaccine production. The long lag time required to produce an influenza vaccine by traditional methods has caused a critical delay in the current H1N1 swine influenza pandemic. In a pandemic caused by a highly lethal influenza virus, such as H5N1 AIV, cutting the vaccine production time could be even more critical to public safety.
Acknowledgments
This work was funded in part by the following: NIH grants RO1 AI080781 and U54 AI057158; the Intramural Research Program of the NIH, NIAID; the Public Health Agency of Canada; and grants 166339 and 310641 from the Canadian Institute for Health Research.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Banzhoff, A., R. Gasparini, F. Laghi-Pasini, T. Staniscia, P. Durando, E. Montomoli, P. L. Capecchi, P. di Giovanni, L. Sticchi, C. Gentile, A. Hilbert, V. Brauer, S. Tilman, and A. Podda. 2009. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baras, B., K. J. Stittelaar, J. H. Simon, R. J. Thoolen, S. P. Mossman, F. H. Pistoor, G. van Amerongen, M. A. Wettendorff, E. Hanon, and A. D. Osterhaus. 2008. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One 3:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barefoot, B. E., K. Athearn, C. J. Sample, and E. A. Ramsburg. 2009. Intramuscular immunization with a vesicular stomatitis virus recombinant expressing the influenza hemagglutinin provides post-exposure protection against lethal influenza challenge. Vaccine 28:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, N. J., and G. L. Plosker. 2008. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs 22:279-292. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H., G. J. Smith, K. S. Li, J. Wang, X. H. Fan, J. M. Rayner, D. Vijaykrishna, J. X. Zhang, L. J. Zhang, C. T. Guo, C. L. Cheung, K. M. Xu, L. Duan, K. Huang, K. Qin, Y. H. Leung, W. L. Wu, H. R. Lu, Y. Chen, N. S. Xia, T. S. Naipospos, K. Y. Yuen, S. S. Hassan, S. Bahri, T. D. Nguyen, R. G. Webster, J. S. Peiris, and Y. Guan. 2006. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc. Natl. Acad. Sci. U. S. A. 103:2845-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, D., K. J. Wright, P. C. Calderon, M. Guo, F. Nasar, J. E. Johnson, J. W. Coleman, M. Lee, C. Kotash, I. Yurgelonis, R. J. Natuk, R. M. Hendry, S. A. Udem, and D. K. Clarke. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and G gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich, H. J., M. Muller, H. M. Oh, P. A. Tambyah, C. Joukhadar, E. Montomoli, D. Fisher, G. Berezuk, S. Fritsch, A. Low-Baselli, N. Vartian, R. Bobrovsky, B. G. Pavlova, E. M. Pollabauer, O. Kistner, and P. N. Barrett. 2008. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N. Engl. J. Med. 358:2573-2584. [DOI] [PubMed] [Google Scholar]
- 8.Fouchier, R. A., V. Munster, A. Wallensten, T. M. Bestebroer, S. Herfst, D. Smith, G. F. Rimmelzwaan, B. Olsen, and A. D. Osterhaus. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79:2814-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisbert, T. W., K. M. Daddario-Dicaprio, J. B. Geisbert, D. S. Reed, F. Feldmann, A. Grolla, U. Stroher, E. A. Fritz, L. E. Hensley, S. M. Jones, and H. Feldmann. 2008. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 26:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert, T. W., K. M. Daddario-Dicaprio, M. G. Lewis, J. B. Geisbert, A. Grolla, A. Leung, J. Paragas, L. Matthias, M. A. Smith, S. M. Jones, L. E. Hensley, H. Feldmann, and P. B. Jahrling. 2008. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 4:e1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisbert, T. W., K. M. Daddario-DiCaprio, K. J. Williams, J. B. Geisbert, A. Leung, F. Feldmann, L. E. Hensley, H. Feldmann, and S. M. Jones. 2008. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J. Virol. 82:5664-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillim-Ross, L., and K. Subbarao. 2006. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19:614-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govorkova, E. A., R. J. Webby, J. Humberd, J. P. Seiler, and R. G. Webster. 2006. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 194:159-167. [DOI] [PubMed] [Google Scholar]
- 15.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76:7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman, D., M. J. Hossain, H. Song, Y. Araya, A. Solorzano, and D. R. Perez. 2008. An avian live attenuated master backbone for potential use in epidemic and pandemic influenza vaccines. J. Gen. Virol. 89:2682-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoelscher, M. A., S. Garg, D. S. Bangari, J. A. Belser, X. Lu, I. Stephenson, R. A. Bright, J. M. Katz, S. K. Mittal, and S. Sambhara. 2006. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 367:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelscher, M. A., N. Singh, S. Garg, L. Jayashankar, V. Veguilla, A. Pandey, Y. Matsuoka, J. M. Katz, R. Donis, S. K. Mittal, and S. Sambhara. 2008. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J. Infect. Dis. 197:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horimoto, T., and Y. Kawaoka. 2006. Strategies for developing vaccines against H5N1 influenza A viruses. Trends Mol. Med. 12:506-514. [DOI] [PubMed] [Google Scholar]
- 21.Horimoto, T., A. Takada, K. Fujii, H. Goto, M. Hatta, S. Watanabe, K. Iwatsuki-Horimoto, M. Ito, Y. Tagawa-Sakai, S. Yamada, H. Ito, T. Ito, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, W. Lim, Y. Guan, M. Peiris, and Y. Kawaoka. 2006. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine 24:3669-3676. [DOI] [PubMed] [Google Scholar]
- 22.Iverson, L. E., and J. K. Rose. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477-484. [DOI] [PubMed] [Google Scholar]
- 23.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapadia, S. U., I. D. Simon, and J. K. Rose. 2008. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology 376:165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodihalli, S., H. Goto, D. L. Kobasa, S. Krauss, Y. Kawaoka, and R. G. Webster. 1999. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J. Virol. 73:2094-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroux-Roels, I., M. Van der Wielen, F. Kafeja, C. Vandermeulen, R. Lazarus, M. D. Snape, T. John, C. Carre, N. Nougarede, S. Pepin, G. Leroux-Roels, K. Hoppenbrouwers, A. J. Pollard, and P. Van Damme. 2009. Humoral and cellular immune responses to split-virion H5N1 influenza vaccine in young and elderly adults. Vaccine 27:6918-6925. [DOI] [PubMed] [Google Scholar]
- 29.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsburg, E., J. Publicover, L. Buonocore, A. Poholek, M. Robek, A. Palin, and J. K. Rose. 2005. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J. Virol. 79:15043-15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed, L., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. (Lond.) 27:493-497. [Google Scholar]
- 32.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose, J., and M. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1240. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 35.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell, C. A., T. C. Jones, I. G. Barr, N. J. Cox, R. J. Garten, V. Gregory, I. D. Gust, A. W. Hampson, A. J. Hay, A. C. Hurt, J. C. de Jong, A. Kielso, A. I. Klimov, T. Kageyama, N. Komadina, A. S. Lapedes, Y. P. Lin, A. Mosterin, M. Obuchi, T. Odagiri, A. D. Osterhaus, G. F. Rimmelzwaan, M. W. Shaw, E. Skepner, K. Stohr, M. Tashiro, R. A. Fouchier, and D. J. Smith. 2008. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 26(Suppl. 4):D31-D34. [DOI] [PubMed] [Google Scholar]
- 37.Schnell, M. J., J. E. Johnson, L. Buonocore, and J. K. Rose. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849-857. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz, J. A., L. Buonocore, A. Roberts, A. Suguitan, Jr., D. Kobasa, G. Kobinger, H. Feldmann, K. Subbarao, and J. K. Rose. 2007. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology 366:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suguitan, A. L., Jr., J. McAuliffe, K. L. Mills, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treanor, J. J., J. D. Campbell, K. M. Zangwill, T. Rowe, and M. Wolff. 2006. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354:1343-1351. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization Global Influenza Program Surveillance Network. 2005. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Dis. 11:1515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, P., and R. Webster. 2001. Orthomyxoviruses, p. 1533-1579. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.