Abstract
Interferons (IFNs) are induced as an initial response to viral infection after recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs). Here, we report that different PAMPs induce type I and III IFN expression at different ratios after mucosal administration in the vaginas of mice and that Toll-like receptor 9 (TLR9) stimulation evokes a particularly strong IFN-λ response, which is essential for optimal antiviral protection. Depletion of CD11c+ cells in vivo revealed that dendritic cells (DCs) in the vaginal epithelium are a key source of type I and III IFNs during herpes simplex virus infection and after specific stimulation of TLR9. A comparison of the signaling pathways activated by TLR9 and cytoplasmic PRRs, which induced lower levels of IFN-λ, revealed that high-level induction of IFN-λ correlated with strong activation of NF-κB p65. Inhibition of the NF-κB and interferon regulatory factor 3 (IRF-3) pathways with the NEMO-binding domain peptide and small interfering RNA (siRNA), respectively, revealed that transcription of the type III IFN genes was more dependent on the NF-κB pathway than that of the type I IFN genes, which relied more on the IRF system. Thus, the type I and III IFN genes are not induced through entirely identical pathways, which indicates differential expression of these two types of IFNs under certain conditions.
Viral infections elicit strong innate immune responses, characterized by production of interferons (IFNs) (17), which exert direct antiviral activity and also activate various cellular activities in the immune system, including cytotoxicity of natural killer cells and maturation of dendritic cells (DCs). Viral recognition by the innate immune system is mediated by pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs) and induce IFN expression (17). Viral nucleotides are potent IFN-inducting PAMPs.
Type I IFNs are well characterized and known to play essential roles for clearance of many viruses in murine models and in humans (1, 6, 11, 14). The more recently discovered type III IFNs (also known as interleukin 28A/B [IL-28A/B] and IL-29) share most of their functions with type I IFNs, most notably antiviral activity, but seem to be redundant for defense against many viral infections (1). This suggests that IFN-λs have a more specialized role in antiviral defense, being essential only under a subset of conditions. For instance, it has been reported that type III IFNs play an important role in optimal defense against influenza virus infections in mice (18). In humans, decreased expression of IFN-λ is associated with rhinovirus-induced asthma exacerbation (5), and genetic variation in IL-28B predicts spontaneous and treatment-induced hepatitis C virus clearance (8, 27).
Since the identification of type III IFNs (13, 24), it has been debated why higher organisms are in possession of two seemingly very similar systems for combating virus infections. However, important differences exist between the type I and type III IFN systems. It is now well established that one important difference between type I and III IFNs is the patterns of expression of their receptors, with the type I IFN receptor chains being abundantly expressed and the IFN-λ-specific IL-28RA chain being expressed in a very narrow subset of cells, particularly epithelial cells (1, 7, 18). With respect to the mechanisms that govern expression of the IFNs, the initial reports demonstrated that type I and III IFNs were induced by a panel of viruses and double-stranded RNA in identical patterns (13, 24). This was confirmed by subsequent studies where knockout cells and overexpression approaches were used (19, 20). However, a recent study showed that the human IFN-λ1 gene promoter is regulated by a distal cluster of NF-κB sites, which was suggested to act independently of the proximal IFN regulatory factor 3/7 (IRF-3/7) binding sites, hence implicating different mechanisms of regulation between the IFN-β and IFN-λ1 genes (28). In this work, we have investigated the mechanisms governing expression of type I versus type III IFNs in the vaginal epithelium in mice. We report that DCs constitute a key source of IFN-λ at epithelial surfaces in the female genital tract and that expression of type III IFN displays a higher level of dependence on the NF-κB pathway than expression of type I IFNs. Thus, the expressions of the two types of IFNs are not regulated by identical mechanisms, which may allow the infected host to establish innate antiviral activities more resistant to viral evasion strategies targeting the IRF pathway.
MATERIALS AND METHODS
Mice.
The mice used for this study were 8- to 12-week-old, age-matched, female, inbred, specific-pathogen-free C57BL/6, 129Sv (Taconic M&B), IFNAR−/− (B&K Universal), TLR9−/− (Oriental Yeast Co.), IL-28RA−/− (1), and CD11c-DTR-tg (The Jackson Laboratory) mice. All animal experiments described have been reviewed and approved by Danish Government authorities and hence comply with Danish law.
Cells.
Bone marrow-derived DCs (BM-DCs) were obtained as follows. Femurs and tibia were surgically removed, freed of muscles and tendons, and briefly suspended in 70% ethanol. Ends were cut, the marrow was flushed with 10% RPMI 1640, and a cell suspension was filtrated over a 70-μm cell strainer (BD Falcon) and centrifuged for 5 min at 1,330 rpm. After 2 washes, cells were resuspended at 2 ×105 cells/ml in RPMI 1640 with 10% fetal calf serum (FCS) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (40 ng/ml) and seeded in bacteriological petri dishes for incubation at 37°C with 5% CO2. At day 3, fresh media were added, and at days 5 and 7, all media were replaced with fresh media. At day 10 of culture, nonadherent cells were harvested and centrifuged, and viable cells were counted in trypan blue, resuspended in RPMI 1640-10% FCS and GM-CSF (20 ng/ml), and seeded at 1 × 106 cells/ml in cell culture dishes. Cells were examined by flow cytometry, showing a myeloid DC phenotype (>95% were CD11c+ CD11b+) (data not shown).
Virus.
The virus used was the 333 strain of herpes simplex virus type 2 (HSV-2). Virus was grown on a Vero cell monolayer to complete cytopathic effect and prepared by one cycle of freezing and thawing, followed by centrifugation for 30 min at 5,000 × g for removal of cellular debris.
Reagents.
ODN1826, poly(I:C):LyoVec, and poly(dA:dT):LyoVec were all obtained from InvivoGen, and recombinant IFN-λ2 was from R&D Systems. The NF-κB pathway was inhibited using the NEMO-binding domain (NBD) peptide (Imgenex), and CD11c+ cells were depleted in CD11c-DTR-tg mice by use of diphtheria toxin (DTX; Sigma-Aldrich).
Depletion of CD11c+ cells in vivo.
To deplete CD11c+ cells, CD11c-DTR-tg mice were given a single dose of 100 ng DTX intraperitoneally (i.p.) 1 day before initiation of the experiments. Groups of wild-type (WT) mice were injected with the same dose of DTX as the control. In agreement with previous reports (12), this led to more than 85% reduction in the CD11chigh population (data not shown).
Vaginal viral infection and treatment with PAMPs and IFN-λ.
For vaginal HSV-2 infection, mice were pretreated by subcutaneous (s.c.) injection of 2 mg Depo-Provera (Pfizer). Five days later, mice were anesthetized with Isofluran (Baxter) and inoculated intravaginally with 20 μl of HSV-2 strain 333 (6.7 × 104 PFU), 5 μg recombinant carrier-free IFN-λ, 25 μg ODN1826, 100 μg poly(I:C):LyoVec, or 100 μg poly(dA:dT):LyoVec suspended in Iscove's modified medium. The mice were placed on their backs and maintained under anesthetics for at least 10 min. Vaginal fluids were collected at the indicated time points postinfection (p.i.) by pipetting 2 × 40 μl of Iscove's modified medium into and out of the vagina 12 to 15 times. The two wash samples were pooled. Genitally infected mice were examined daily and scored for vaginal inflammation, neurological illness, and death. The severity of disease was graded using the following scores: 0, healthy; 1, genital erythema; 2, moderate genital inflammation; 3, purulent genital lesion and/or generally bad condition; and 4, hind limb paralysis (at which level mice were sacrificed).
Viral plaque assay.
Vaginal wash samples from HSV-2-infected mice were titrated for viral content on Vero cells, which were seeded in minimal essential medium (MEM) supplemented with 5% FCS at a density of 1.2 × 106 in 5-cm-diameter plates and left overnight to settle. The cells were infected by incubation for 1 h at 37°C with 100 μl of serial dilutions of the vaginal wash samples and 400 μl of medium, during which time the tissue culture plates were rocked every 15 min to ensure even distribution of the virus. Subsequently, 8 ml of MEM supplemented with 2.5% FCS, and 0.2% human Ig was added to the plates. The cells were incubated at 37°C for 2 days and stained with 0.03% methylene blue, and finally, plaques were counted.
siRNA.
IRF-3-specific small interfering RNA (siRNA) was purchased from Dharmacon as a proprietary reagent. siRNA transfections targeting endogenous IRF-3 and scrambled controls were carried out in 24-well plates, using Lipofectamine 2000 as a transfection agent, by following the recommendations of the manufacturer (Invitrogen). The transfection medium was replaced at 5 h posttransfection and replaced with fresh medium. Cells were used for experiments 48 h later.
Measurement of type I and III IFNs.
IFN-α/β bioactivity was measured by using a L929-cell based bioassay. L929 cells (2 × 104 cells/well in 100 μl) in MEM with 5% FCS were incubated overnight at 37°C in successive 2-fold dilutions of samples (UV inactivated for 6 min) or murine IFN-α as a standard. Twenty-four hours later, vesicular stomatitis virus (VSV/V10) was added to the wells, and the cells were incubated for 3 days. The dilution mediating 50% protection was defined as 1 U of IFN-αβ/ml. High levels of recombinant IFN-λ or IFN-γ did not interfere with the assay. IFN-λ was detected by an enzyme-linked immunosorbent assay (ELISA). For detection, MaxiSorp plates (Nunc) were coated overnight at room temperature with 1 μg/ml rat anti-mouse IL-28 (R&D Systems) diluted in phosphate-buffered saline (PBS). After being blocked for 1 h at room temperature with PBS containing 1% (wt/vol) of bovine serum albumin (BSA) (Sigma-Aldrich), samples and standard dilutions were added to the wells, and the plates were incubated for 2 h at room temperature. Subsequently, the wells were incubated for 2 h at room temperature with biotinylated rat anti-mouse IL-28 detection antibody (R&D Systems) at a concentration of 250 ng/ml in PBS with 1% (wt/vol) of BSA. For development, horseradish peroxidase (HRP)-conjugated streptavidin, diluted 1:200 in PBS with 1% (wt/vol) of BSA, was added and incubated for 20 min at room temperature, after which 100 μl of substrate buffer (R&D Systems) was added to the wells. The color reaction was stopped with 5% (vol/vol) H2SO4. Between each step, the plates were washed three times with PBS containing 0.05% (vol/vol) Tween 20. The detection limit of the ELISA was 15 pg/ml.
DNA-binding assays for NF-κB p65 and IRF-3.
Cells were stimulated as indicated in the text. At the appropriate times, the cells were harvested, washed, and lysed in a buffer containing 10 mM HEPES, 400 mM KCl, 10% glycerol, 2 mM EDTA, 1 mM EGTA, 0.01% Triton X-100, 0.5 mM dithiothreitol, 1 mM NaVO4, and protease inhibitors (Complete; Roche). Cleared cell lysates were incubated with streptavidin-agarose beads (Neutravidin; Pierce) coupled to 5′-biotinylated, 6-bp-5′-extended oligonucleotides (DNA Technology). The oligonucleotides used were IFN-β PRDI-III (IRF-3) (5′-GGA TCC GAA AAC TGA AAG GGA GAA GTG AAA GTG-3′ and 5′-GGA TCC CAC TTT CAC TTC TCC CTT CTT TCA GTT TTC-3′) and IFN-β PRDII (NF-κB) (5′-GG ATC CGG AAT TTC CCG GAA TTT CCC-3′ and 5′-GGA TCC GGG AAA TTC CGG GAA ATT CC-3′). The binding reactions were performed for 2 h at 4°C in binding buffer containing 10 mM HEPES, 133 mM KCl, 10% glycerol, 2 mM EDTA, 1 mM EGTA, 0.01% Triton X-100, 0.5 mM dithiothreitol, 1 mM NaVO4, and protease inhibitors. After being washed, the oligonucleotide-bound proteins were released in SDS sample buffer, separated by 10% SDS-polyacrylamide gel electrophoresis, and transferred onto membranes (Bio-Rad). Membranes were blocked with 5% skim milk in Tris-buffered saline (TBS) containing 0.05% Tween 20. Rabbit anti-IRF-3 antibody or goat anti-p65 antibody (both from Santa Cruz Biotechnology), diluted 1:2,000 in TBS containing 0.05% Tween 20, were allowed to bind overnight at 4°C. Peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:2,000 dilution) or anti-goat IgG (both from Dako) was allowed to bind for 1 h at room temperature. The signal was detected using the FijiFilm LAS 3000 imaging system and quantified using ImageJ.
Statistical analysis.
The data are presented as means ± standard deviations (SD). Statistical significance was estimated with the Wilcoxon rank sum test (P values of <0.05 were considered to be statistically significant).
RESULTS
Vaginal infection with HSV and treatment with nucleotide PAMPs induce expression of type I and III IFN.
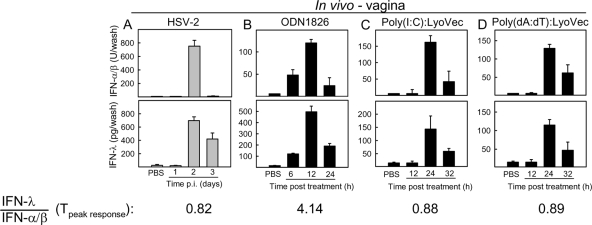
We have previously shown that HSV-2 infection in the female genital tract induces expression of IFNs (1). As shown in Fig. 1A, both IFN-α/β and IFN-λ were detectable on day 2 p.i., and IFN-λ showed a more prolonged kinetics with high levels also on day 3 p.i. HSV infection induces expression of IFNs via recognition by Toll-like receptor 9 (TLR9), RIG-like receptors (RLRs), and cytosolic DNA receptors (DNAR) (4, 21, 22, 25), so we were interested in knowing how the different PRRs triggered by HSV infection modulated IFN expression in the vagina. Mice were treated with the TLR9 agonist ODN1826, the RLR agonist poly(I:C) (in complex with the transfection agent LyoVec), and the DNAR agonist poly(dA:dT) (in complex with LyoVec). As shown in Fig. 1B to D, all PAMPs induced expression of type I and III IFNs. However, we noted that the TLR9 agonist ODN1826 induced more IFN-λ than the two PAMPs targeting cytoplasmic PRRs but that all stimuli led to comparable peak levels of type I IFN.
FIG. 1.
Vaginal infection with HSV and treatment with nucleotide PAMPs induce expression of type I and III IFN. C57BL/6 mice were infected intravaginally with 6.7 × 104 PFU of HSV-2 (A) or treated with 25 μg of ODN1826, 100 μg of poly(I:C):LyoVec, or 100 μg of poly(dA:dT):LyoVec (B to D). At the indicated time points posttreatment, vaginal wash samples were collected and levels of IFN-α/β bioactivity (top row) and total IFN-λ levels (bottom row) were measured. The data are shown as means ± SD (n = 4 to 7). T, time point.
Depletion of CD11c+ cells reduces expression of type I and III IFNs in the vagina.
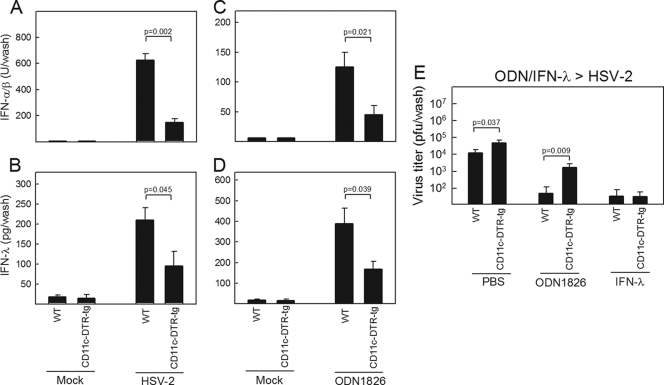
We have previously shown that DC populations are the most potent producers of type I and III IFNs after HSV-2 infection in vitro (1). In order to identify the cellular sources of IFN-λ in the vaginal epithelium in vivo, we took advantage of the transgenic CD11c-DTR-tg mouse strain. CD11c+ cells were depleted in vivo, and the mice were either infected with HSV-2 or treated with ODN1826 in the vagina. Deletion of DCs led to a significant reduction in the levels of both type I and type III IFNs in the vaginal wash samples collected from mice receiving either treatment (Fig. 2A to D). Moreover, the ability of ODN1826 to stimulate antiviral protection against vaginal HSV-2 infection was dependent on the presence of CD11c+ cells, whereas IFN-λ treatment still gave full protection in mice depleted for DCs (Fig. 2E). Thus, DCs in the vaginal mucosa represent a major source of IFN-λ, which exerts its antiviral activity through other cell types, most likely epithelial cells.
FIG. 2.
Depletion of CD11c+ cells reduces expression of type I and III IFNs in the vagina. C57BL/6 and CD11c-DTR-tg mice were treated with 100 ng diphtheria toxin 1 day prior to experiments. The mice were infected intravaginally with 6.7 × 104 PFU of HSV-2 (A, B), treated with 25 μg of ODN1826 (C to E), or treated with PBS or 5 μg of IFN-λ2 (E). Vagina wash samples were collected at 2 days (A, B) and 12 h (C, D) posttreatment for measurement of IFN-α/β (A, C) and IFN-λ (B, D). (E) The mice were infected intravaginally with 6.7 × 104 PFU of HSV-2 6 h after PBS/ODN1826/IFN-λ treatment. Twenty-four hours after infection, vaginal wash samples were collected, and viral load was measured by a plaque assay. All data are shown as means ± SD (n = 5 [A to D] and n = 7 to 10 [E]).
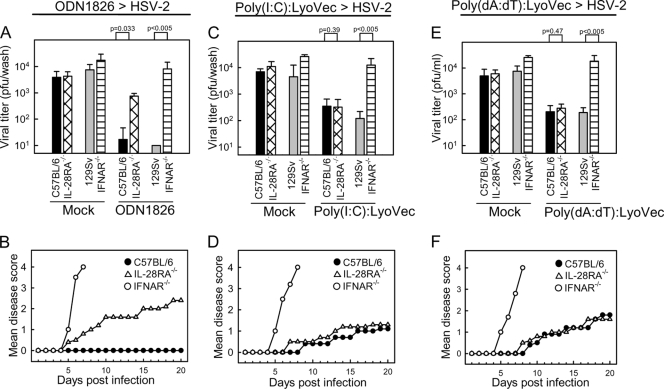
Important role for type III IFN in antiviral protection stimulated through TLR9 but not cytosolic nucleotide sensors.
We and others have previously reported that TLR9 agonists potently stimulate antiviral protection against genital HSV-2 infections (2, 10). We therefore examined whether cytoplasmic delivery of poly(I:C) or poly(dA:dT) also protected mice from subsequent HSV-2 infection. As seen in Fig. 3, vaginal administration of poly(I:C) or poly(dA:dT) in complex with LyoVec partly protected the mice from HSV-2 infection as well as development of disease, whereas ODN1826 led to total protection. For all three PAMPs, the ability to evoke antiviral protection was highly dependent on the type I IFN system. However, only in the case of ODN1826 was the antiviral response also dependent on a functional IFN-λ system, which correlated with the higher level of induction of IFN-λ by the TLR9 agonist than by the PAMPs stimulating cytoplasmic PRRs. Thus, activation of optimal antiviral protection by TLR9 agonists in the female genital tract relies on the type III IFN system.
FIG. 3.
Important role for type III IFN in antiviral protection stimulated through TLR9 but not cytosolic nucleotide sensors. IL-28RA−/−, IFNAR−/−, and the respective WT littermates were treated with 25 μg of ODN1826 (A, B), 100 μg of poly(I:C):LyoVec (C, D), or 100 μg of poly(dA:dT):LyoVec (E, F) 6 h before intravaginal infection with HSV-2 (6.7 × 104 PFU). (A, C, E) Vaginal wash samples were collected on day 1 p.i., and viral load was determined by a plaque assay. The virus titers are shown as mean values ± SD. (B, D, F) The mice were followed for 20 days and scored clinically. Data are shown as mean disease scores (n = 8 [A, B], n = 5 to 8 [C, D], and n = 6 to 8 [E, F]).
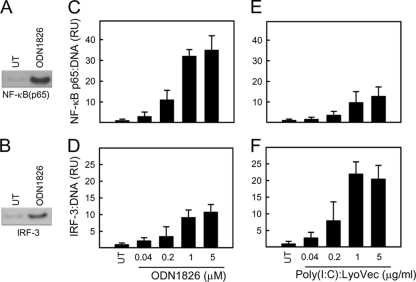
Strong induction of IFN-λ correlates with high-level activation of the NF-κB pathway.
Given the above-mentioned findings that the TLR9 pathway induced more IFN-λ than the RLR pathway and that IFN-λ contributed to TLR-mediated antiviral defense in vivo, we were interested in comparing the intracellular signaling pathways activated by TLR9 versus those activated by RLR agonists in DCs. We looked at the activation of NF-κB (p65) and IRF-3 DNA binding in extracts from cells stimulated with ODN1826 and poly(I:C):LyoVec. The transcription factor bound to DNA in the in vitro assay was detected by Western blotting (Fig. 4A and B) and quantified using ImageJ. Stimulation of the DCs with ODN1826 led to a very strong induction of NF-κB p65 DNA-binding activity (Fig. 4C) and a more modest activation of IRF-3 (Fig. 4D). This was in contrast to what we observed in cells transfected with poly(I:C), where IRF-3 activation was stronger than NF-κB p65 activation (Fig. 4E and F). On the basis of these data, we conclude that strong induction of IFN-λ correlates with high-level activation of the NF-κB pathway.
FIG. 4.
Strong induction of IFN-λ correlates with high-level activation of the NF-κB pathway. (A, B) Cell lysates from DCs treated for 5 h with 1 μM ODN1826 were precipitated with bead-coupled consensus sequences for NF-κB and IRF-3 binding sites. The presences of NF-κB p65 and IRF-3 were detected by Western blotting. UT, untreated control. (C to F) Lysates from DCs treated for 5 h with ODN1826 or poly(I:C):LyoVec in the indicated doses were analyzed as in panels A and B. The data were quantified and are presented as mean numbers of relative units (RU) ± SD for triplicates.
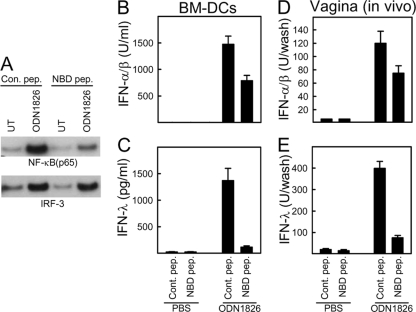
Inhibition of the NF-κB pathway abrogates expression of type III but not type I IFN in vitro and in vivo.
The differential induction of type III IFN by TLR and RLR agonists correlated with activation of NF-κB. We therefore hypothesized that type III IFNs are regulated more by NF-κB than type I IFNs. To test this in primary DCs and in vivo, we took advantage of the cell-permeable inhibitory NBD peptide, which interferes with the interaction between NEMO and IκB kinases (IKKs) (15) and hence blocks the NF-κB pathway. Treatment of DCs with NBD peptide reduced TLR9-mediated NF-κB activation by over 60% without affecting IRF-3 activation (Fig. 5A). Importantly, while type I IFN induction by ODN1826 was inhibited between 30 and 40% by the NBD peptide, the type III IFN response was reduced over 90% by this treatment (Fig. 5B and C). Treatment of whole mice with the NBD peptide followed by vaginal treatment with ODN1826 and collection of vaginal wash samples for measurement of IFNs gave very similar results, i.e., modest reduction in the type I IFN response and strong inhibition of the type III IFN response.
FIG. 5.
Inhibition of the NF-κB pathway abrogates expression of type III but not type I IFN in vitro and in vivo. (A to C) BM-DCs were treated with 100 μM NBD peptide 12 h before stimulation with 1 μM ODN1826. Cell lysates and culture supernatants harvested at 5 h and 18 h poststimulation, respectively, were analyzed for DNA-binding activity (NF-κB and IRF-3) (A) and IFN-α/β and -λ levels (B, C). (E, F) Mice were treated with 1.25 mg/kg of body weight of NBD peptide through i.p. injection 16 h prior to stimulation in the vagina with 25 μg ODN1826. Vaginal wash samples were collected at 12 h poststimulation for measurement of IFN-α/β and -λ. The IFN data are presented as means ± SD of measurements from triplicate cultures (B, C) using 5 mice per group (D, E).
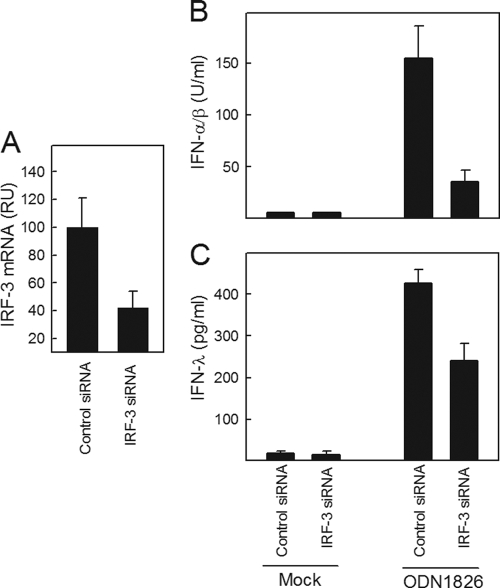
To corroborate these findings, we also performed siRNA-mediated knockdown of IRF-3 and looked for induction of the type I and III IFNs. The siRNA was able to reduce the IRF-3 mRNA levels by about 60% (Fig. 6A). Under these conditions, the ability of ODN1826 to induce expression of type I IFNs was reduced between 65 and 75%, whereas the type III IFN response was reduced about 40% (Fig. 6B and C). Finally, we found that both types of IFNs were dependent on some degree of signaling from both the NF-κB and IRF pathways, since no IFN induction in response to poly(I:C) was observed in murine embryonic fibroblasts (MEFs) lacking either NEMO or IKKɛ/TBK-1 (data not shown). Collectively, these data demonstrate that the genes encoding type I and III IFNs are regulated by NF-κB and IRF-3, but the type III IFN genes seem to be more dependent on the NF-κB pathway than the type I IFN genes, which rely more on the IRF system.
FIG. 6.
siRNA-mediated knockdown of IRF-3 strongly impairs type I IFN expression. BM-DCs were transfected with control and IRF-3 siRNA. Forty-eight hours after transfection, RNA was harvested for analysis of IRF-3 mRNA levels by quantitative reverse transcription-PCR (qRT-PCR) (A) or cells were stimulated with 1 μM ODN1826 (B, C). Culture supernatants were harvested at 18 h poststimulation, and levels of IFN-α/β (B) and IFN-λ (C) were measured. The data are presented as means ± SD of measurements from 4 cultures. RU, relative units.
DISCUSSION
IFN-λ is a class of cytokines with potent antiviral activity in vitro and in vivo and has recently been described as having essential functions in host defense against important human pathogenic viruses (18, 27). While most cell types respond to type I IFNs, it seems that only a restricted subset of cell types, and in particular epithelial cells, express IFN-λ receptors and respond to the cytokine (1, 7, 18). Therefore, induction of IFN-λ expression at epithelial surfaces is important for activating the initial antiviral barrier at this major portal of viral entry. It has been reported that viruses and PRRs induce expression of type I and type III IFNs through identical mechanisms (19, 20). However, a study of hepatic expression of IFNs and IFN-stimulated genes during chronic hepatitis C virus infection has demonstrated that liver biopsy specimens from patients contain elevated levels of type III IFN but not type I IFN mRNA compared to specimens from healthy controls (16). Such findings suggest that the two classes of IFNs are not always concomitantly expressed and support the idea that differences exist between the mechanisms regulating the expression of type I and type III IFNs.
Here, we report that administration of a TLR9 agonist in the female genital tract induces more IFN-λ than treatment with nucleotide PAMPs targeting cytoplasmic PRRs and that the IFN-λ system was essential for TLR9-induced antiviral activity. DCs represent the main source of type III IFN during vaginal infection with HSV-2 or treatment with a TLR9 agonist, and the ability of DCs to induce IFN-λ expression was largely dependent on a functional NF-κB pathway. This was in contrast to type I IFN expression, which was primarily driven by the IFN pathway. Thus, although NF-κB and IRFs both contribute to induction of both type I and type III IFNs, there seem to be differential relative requirements for the two pathways in induction of IFN-α/β versus induction of IFN-λ.
One important finding of this work is that depletion of DCs from mice strongly reduces the expression of type III IFNs in the vagina after HSV-2 infection or treatment with ODN1826. Together with previous reports (1), this suggests that recognition of viruses in the vaginal tissue by DCs triggers IFN-λ expression, which exerts antiviral activity on the epithelial cells. This is very similar to a previous report on type I IFN expression in the female genital tract after stimulation through TLR9, in which plasmacytoid DCs were identified as the main source of type I IFN (23). Thus, although we did not distinguish between different subclasses of DCs in our work, these studies together suggest that type I and III IFNs are produced by the same cell types in response to infection in the vagina.
The type I IFN promoters have been extensively studied and are well understood. The IFN-β promoter is regulated through concerted action of IRFs, NF-κB, and ATF2/cJUN (26), whereas the IFN-α gene promoters are controlled almost exclusively by IRF-3 and -7 (9). Our finding that type I IFN expression displayed a major dependency on IRF-3 and a minor dependency on NF-κB is in agreement with this idea. The type III IFN promoters share some similarity with the IFN-β promoter by containing binding sites for both IRF-3 and NF-κB, and two reports have used knockout cells and ectopic expression of IRF-3 to show that the type I and III IFN systems are induced through common pathways (19, 20). Recently, Thomson and associates identified NF-κB sites in the distal region of the IFN-λ1 promoter, which they demonstrated to play a key role in activating gene transcription in response to lipopolysaccharide (LPS) (28). The authors proposed a model in which NF-κB and IRFs activate IFN-λ1 gene expression independently, which is in contrast to the current model for IFN-β induction with concerted action of IRFs, NF-κB, and ATF2/cJUN (26). Our present findings support the idea that NF-κB plays a key role in induction of IFN-λ expression and extents the previous work by suggesting that the NF-κB pathway may play the dominant role in driving the IFN-λ response on epithelial surfaces and in DCs.
One implication of our findings is that there could be infectious conditions where expression of type III IFN dominates that of type I IFN. Many viruses specifically inhibit the IRF signaling pathway and hence impair type I IFN expression (3). Our present finding suggests that under such conditions, the expression of IFN-λ will be less affected and hence allow the host to retain IFN expression and antiviral activity despite viral attempts to evade the innate antiviral response.
In summary, expressions of type I and type III IFNs are regulated through the same signaling pathways, but in contrast to the type I IFNs, which are induced mainly by IRFs, the NF-κB pathway plays a more dominant role in induction of IFN-λ in the vaginal mucosa, a phenomenon that may also apply to other epithelial surfaces.
Acknowledgments
This work was supported by The Lundbeck Foundation (grant number R17-A1526), the Kathrine og Vigo Skovgards Fond, and the Aarhus University Research Foundation.
The technical support provided by Kirsten Stadel Petersen is greatly appreciated.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Ank, N., M. B. Iversen, C. Bartholdy, P. Staeheli, R. Hartmann, U. B. Jensen, F. Dagnaes-Hansen, A. R. Thomsen, Z. Chen, H. Haugen, K. Klucher, and S. R. Paludan. 2008. An important role for type III interferon (IFN-lambda/IL-28) in Toll-like receptor-induced antiviral activity. J. Immunol. 180:2474-2485. [DOI] [PubMed] [Google Scholar]
- 2.Ank, N., H. West, C. Bartholdy, K. Eriksson, A. R. Thomsen, and S. R. Paludan. 2006. Lambda interferon (IFN-{lambda}), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowie, A. G., and L. Unterholzner. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu, Y. H., J. B. Macmillan, and Z. J. Chen. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contoli, M., S. D. Message, V. Laza-Stanca, M. R. Edwards, P. A. Wark, N. W. Bartlett, T. Kebadze, P. Mallia, L. A. Stanciu, H. L. Parker, L. Slater, A. Lewis-Antes, O. M. Kon, S. T. Holgate, D. E. Davies, S. V. Kotenko, A. Papi, and S. L. Johnston. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 12:1023-1026. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis, S., E. Jouanguy, S. Al Hajjar, C. Fieschi, I. Z. Al Mohsen, S. Al Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al Gazlan, H. Al Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald, K. A., E. M. Palsson-McDermott, A. G. Bowie, C. A. Jefferies, A. S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M. T. Harte, D. McMurray, D. E. Smith, J. E. Sims, T. A. Bird, and L. A. O'Neill. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78-83. [DOI] [PubMed] [Google Scholar]
- 8.Ge, D., J. Fellay, A. J. Thompson, J. S. Simon, K. V. Shianna, T. J. Urban, E. L. Heinzen, P. Qiu, A. H. Bertelsen, A. J. Muir, M. Sulkowski, J. G. McHutchison, and D. B. Goldstein. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399-401. [DOI] [PubMed] [Google Scholar]
- 9.Genin, P., A. Vaccaro, and A. Civas. 2009. The role of differential expression of human interferon-a genes in antiviral immunity. Cytokine Growth Factor Rev. 20:283-295. [DOI] [PubMed] [Google Scholar]
- 10.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson, C., J. D. Wetzel, J. He, C. Mikacenic, T. S. Dermody, and B. L. Kelsall. 2007. Type I interferons produced by hematopoietic cells protect mice against lethal infection by mammalian reovirus. J. Exp. Med. 204:1349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 14.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 16.Mihm, S., M. Frese, V. Meier, P. Wietzke-Braun, J. G. Scharf, R. Bartenschlager, and G. Ramadori. 2004. Interferon type I gene expression in chronic hepatitis C. Lab. Invest. 84:1148-1159. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen, T. H. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordstein, M., G. Kochs, L. Dumoutier, J. C. Renauld, S. R. Paludan, K. Klucher, and P. Staeheli. 2008. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4:e1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onoguchi, K., M. Yoneyama, A. Takemura, S. Akira, T. Taniguchi, H. Namiki, and T. Fujita. 2007. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 282:7576-7581. [DOI] [PubMed] [Google Scholar]
- 20.Osterlund, P. I., T. E. Pietila, V. Veckman, S. V. Kotenko, and I. Julkunen. 2007. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 179:3434-3442. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen, S. B., S. B. Jensen, C. Nielsen, C. Quartin, Z. J. Chen, R. H. Silverman, S. Akira, and S. R. Paludan. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and RIG-like receptors, which synergizes to induce type I interferon production. J. Gen. Virol. 90:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen, S. B., L. N. Sorensen, L. Malmgaard, N. Ank, J. D. Baines, Z. J. Chen, and S. R. Paludan. 2007. Type I IFN production during HSV infection is controlled by cell-type specific viral recognition by TLR9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81:13315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen, H., and A. Iwasaki. 2006. A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN-based vaginal microbicide. J. Clin. Invest. 116:2237-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 25.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 26.Thanos, D., and T. Maniatis. 1995. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell 83:1091-1100. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, D. L., C. L. Thio, M. P. Martin, Y. Qi, D. Ge, C. O'Huigin, J. Kidd, K. Kidd, S. I. Khakoo, G. Alexander, J. J. Goedert, G. D. Kirk, S. M. Donfield, H. R. Rosen, L. H. Tobler, M. P. Busch, J. G. McHutchison, D. B. Goldstein, and M. Carrington. 2009. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461:798-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson, S. J., F. G. Goh, H. Banks, T. Krausgruber, S. V. Kotenko, B. M. Foxwell, and I. A. Udalova. 2009. The role of transposable elements in the regulation of IFN-lambda1 gene expression. Proc. Natl. Acad. Sci. U. S. A. 106:11564-11569. [DOI] [PMC free article] [PubMed] [Google Scholar]