Abstract
Sexual transmission is the major route of HIV-1 infection worldwide. Dendritic cells (DCs) from the mucosal layers are considered to be the initial targets of HIV-1 and probably play a crucial role in HIV-1 transmission. We investigated the role of cell-to-cell contact between HIV-1-exposed immature DCs and various lymphocyte subsets in the stimulation of HIV-1 replication. We found that HIV-1 replication and production in DCs were substantially enhanced by the coculture of DCs with primary CD4 T or nonpermissive B lymphocytes but not with primary activated CD8 T lymphocytes or human transformed CD4 T lymphocytes. Most of the new virions released by cocultures of HIV-1-exposed immature DCs and primary B lymphocytes expressed the DC-specific marker CD1a and were infectious for both immature DCs and peripheral blood mononuclear cells (PBMCs). Cocultured DCs thus produced large numbers of infectious viral particles under these experimental conditions. The soluble factors present in the supernatants of the cocultures were not sufficient to enhance HIV-1 replication in DCs, for which cell-to-cell contact was required. The neutralizing monoclonal antibody IgG1b12 and polyclonal anti-HIV-1 sera efficiently blocked HIV-1 transfer to CD4 T lymphocytes but did not prevent the increase in viral replication in DCs. Neutralizing antibodies thus proved to be more efficient at blocking HIV-1 transfer than previously thought. Our findings show that HIV-1 exploits DC-lymphocyte cross talk to upregulate replication within the DC reservoir. We provide evidence for a novel mechanism that may facilitate HIV-1 replication and transmission. This mechanism may favor HIV-1 pathogenesis, immune evasion, and persistence.
Most infectious agents of sexually transmitted diseases, including HIV-1, initiate infection via the mucosal epithelial surfaces of the genital tract. Immature dendritic cells (DCs) in the underlying mucosa are among the first antigen-presenting cells (APCs) encountered by HIV-1 after sexual transmission (7, 19, 22, 28, 53, 60, 61). These specialized APCs efficiently capture viruses through their specific uptake receptors for the processing and presentation of viral antigens to T or B lymphocytes (4). They establish stable or transient cell-to-cell contacts with various naive or memory T or B lymphocytes to generate and orchestrate adaptive virus-specific immune responses. HIV-1 replication in DCs, both in vivo and in vitro, has been firmly demonstrated, although HIV-1 infection of DCs in vitro is less efficient than the infection of primary CD4 T lymphocytes. The mechanisms responsible for the limited replication of HIV-1 in DCs have not been elucidated, but it has been suggested that intracellular restriction factors present in these cells, such as members of the APOBEC family or other, as yet unknown restriction factors, may interfere with the early steps of HIV-1 infection (15, 41, 54). The low availability of active transcription factors, such as NF-κB, in immature DCs is also known to limit HIV-1 replication. HIV-1 replication in DCs is poor, but many studies have reported the efficient transmission of infectious HIV-1 particles in trans from DCs to nearby permissive CD4 T lymphocytes, via several different pathways (7, 8, 18, 33, 36, 52, 57, 59, 60, 62). HIV-1 transfer from DCs to CD4 target cells probably increases the efficiency of HIV-1 trans-infection of CD4 T lymphocytes. It has been suggested that naive or memory CD4 T lymphocytes interacting with HIV-1-infected DCs form an immunological synapse (IS) mediating a T-cell activation program, leading to transmission of the virus from DCs to activated CD4 T lymphocytes, in which it replicates. This process of HIV-1 transmission has been proposed as an effective mode of cell-to-cell propagation of the initial infection and of latent infection generation in HIV-specific memory CD4 T lymphocytes. It has also been suggested that the mode of HIV-1 transfer from DCs to CD4 T target cells through an infectious synapse is resistant to antibody neutralization. However, conflicting data concerning the role of neutralizing antibodies in the HIV-1 trans-infection of CD4 T target cells have been reported (7-9, 13, 14).
An understanding of the way in which HIV-1 interacts with and replicates in the various types of primary target cells in the mucosal layers is essential for the design of therapeutic agents for preventing the early steps of HIV-1 infection at these sites of entry. Several studies have focused on the amplification of HIV-1 replication in primary CD4 T lymphocytes or human CD4 T-cell lines in the presence of mature DCs, but to our knowledge, the ability of HIV-1 to replicate in immature DCs during cell-to-cell interactions with primary T- or B-lymphocyte subsets has yet to be investigated. The development of a future HIV-1 vaccine candidate inducing protective mucosal immunity will depend on elucidation of the early events of HIV-1 replication in infected immature DCs interacting with various lymphocyte subsets. It will also be important to determine whether HIV-1-neutralizing antibodies can inhibit HIV-1 transfer from immature DCs to primary activated CD4 T lymphocytes.
We provide several lines of evidence that HIV-1 replication is efficiently stimulated in DCs during cross talk with primary CD4 T or B lymphocytes. We found that this enhancement of HIV-1 replication in DCs required early and specific cell-to-cell interaction between infected DCs and primary lymphocytes. We also showed that neutralizing polyclonal and monoclonal IgGs robustly inhibited productive HIV-1 trans-infection of primary CD4 T lymphocytes cocultured with infected DCs.
MATERIALS AND METHODS
Ethics statement.
Large volumes of sera from HIV-1-infected individuals and healthy HIV-1-seronegative donors were obtained by apheresis with the approval of the appropriate institutional review board (the Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale [CCPPRB]).
Cell preparation.
Immature monocyte-derived DCs (MoDCs) were generated by inducing the differentiation of human monocytes as described previously (21). Briefly, monocytes were isolated from human peripheral blood leukocytes from healthy HIV-1-seronegative donors by the selection of CD14-positive cells by isolation on magnetic beads (AutoMacs; Miltenyi Biotec, Cologne, Germany) and cultured for 6 days in RPMI 1640 with 5% heat-inactivated fetal bovine serum (FBS) in the presence of 20 ng/ml interleukin-4 (IL-4) and 10 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; both from R&D Systems, Minneapolis, MN). After the monocytes had been collected, residual CD19 B lymphocytes and CD4 and CD8 T lymphocytes were successively purified (up to 98% purity) by positive selection. Langerhans cells (LCs) were isolated from purified human cord blood CD34 progenitor cells by one-step immunomagnetic positive selection with materials from Miltenyi Biotec after Ficoll-Hypaque sedimentation. Purified CD34 cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 μg/ml), streptomycin (100 μg/ml), 50 ng/ml Flt3 ligand, 50 ng/ml GM-CSF, 25 ng/ml stem cell factor (SCF), and 10 ng/ml thrombopoietin (TPO; all from R&D Systems) for 7 days at 37°C under an atmosphere containing 5% CO2 to allow multiplication. The differentiation of these cells into LCs and interstitial DCs (intDCs) was then triggered by incubation with a mixture of 50 ng/ml GM-CSF, 6 ng/ml transforming growth factor β1 (TGF-β1), and 2 ng/ml tumor necrosis factor alpha (TNF-α) for a further 7 days. Autologous CD4 T lymphocytes from human cord blood samples were purified by positive selection after the collection of CD34 stem cells. For some experiments, peripheral blood mononuclear cells (PBMCs) or purified lymphocytes were frozen in liquid nitrogen until use (they were thawed for 18 h before addition to infected DCs). Human CD4 T-lymphocyte cell lines (Jurkat, MT4, CEMSS, and MT2 high CCR5) were cultured in RPMI 1640 containing 10% FBS with penicillin (100 μg/ml) and streptomycin (100 μg/ml).
Virus preparation.
R5 HIV-1 primary isolates were produced in human blood leukocytes as described previously (6). Virus stocks were collected at peak virus production and were concentrated by a factor of 80 with a 100-kDa-cutoff polyethersulfone filter (Centricon 80 Plus Biomax filter; Millipore, Molsheim, France). HIV-1Bx08 was provided by H. Fleury, and the HIV-1BaL isolates (subtype B R5 strains) were provided by S. Gartner, M. Popovic, and R. Gallo from the National Institutes of Health. HIV-1CN54 and HIV-1TV-1 were obtained from the National Institute for Biological Standards and Control (NIBSC) and S. Engelbrecht, respectively. Before their addition to cultures of immature DCs, concentrated viruses were purified by gel filtration with a Sephadex exclusion column used to remove fetal calf serum (FCS) proteins and free gp120 as described previously (6, 35). HIV-1 pseudo-virus-like particles (pcDNA3.1D/V5-His-TOPO-envSF162, backbone vector pSG3ΔEnv, and a vpr-green fluorescent protein [GFP] plasmid) were prepared as described previously (32).
Coculture of HIV-1-infected DCs with lymphocytes.
MoDCs were infected and cocultured with various types of primary lymphocytes under conditions similar to those described previously for HIV-1 transfer experiments (8, 18, 36, 59, 62). Briefly, immature MoDCs were infected with primary R5 HIV-1 isolates (500 ng/ml viral p24 antigen) by being incubated with the virus for 2 h. MoDCs were then washed thoroughly to remove unbound virus and resuspended at a density of 8 × 106 cells per ml in RPMI 1640 containing 5% FCS and supplemented with GM-CSF and IL-4. Aliquots (25 μl) of HIV-1-exposed immature MoDCs were added to 25 μl of a cell suspension containing 8 × 105 autologous or allogeneic phytohemagglutinin (PHA)-activated or nonactivated T or B lymphocytes, thawed 1 day before the infection of MoDCs with HIV-1, or 4 × 105 human CD4 T-cell lines in a flat-bottomed 96-well plate. In parallel, HIV-1-exposed MoDCs were cultured alone. Unless otherwise stated, we assessed productive infection after 48 h of culture by using flow cytometry to detect intracellular viral p24 antigen (21, 56). Virus release into the supernatant was assessed in the p24 viral antigen enzyme-linked immunosorbent assay (ELISA) with an Innotest assay kit (Innogenetics, Ghent, Belgium).
Microscopy.
Immature MoDCs were exposed to HIV-1 pSG3EnvSF162 vpr-GFP pseudoparticles (32) for 2 h and then washed and stained with Alexa Fluor 647-conjugated anti-human DC-SIGN monoclonal antibody (BD Biosciences [BD]). Labeled cells were washed, fixed with BD Cytofix solution, and transferred out of the biosafety level 3 (BSL3) laboratory. After cytospin centrifugation at 200 rpm for 5 min, MoDCs were covered with Aqua-Poly/Mount solution (Polysciences Europe GmbH, Eppelheim, Germany) and incubated under coverslips overnight at room temperature in the dark. Stained MoDCs were viewed with an Axio Observer Z1 microscope (Carl Zeiss Vision International GmbH, Oberkochen, Germany), and images were analyzed with Imaris software (Bitplane, Saint Paul, MN) after deconvolution with AutoDeblur software (AutoQuant X; Media Cybernetics, Bethesda, MD).
Fluorescence-activated cell sorter analysis.
Mouse monoclonal antibodies against human CD11b, DC-SIGN, CD1a, and CD3 were purchased from BD Biosciences (San Diego, CA); mouse anti-HIV-1 p24 monoclonal antibody (clone KC57) and monoclonal antibody against human CD207 (langerin) were obtained from Coulter Beckman (Fullerton, CA). Cells were labeled with antibodies directed against human cell surface molecules, washed, fixed, and permeabilized in Cytofix and Perm/Wash kit solutions (used according to the manufacturer's instructions). Cells were then stained for intracellular viral p24, washed again, and analyzed by flow cytometry. Multicolor data were acquired with a cytometer (LSRII SORP; BD Biosciences, San Jose, CA), as recommended elsewhere (39). Cytometer setup and tracking (CST) calibration particles (BD) were used to ensure the consistency of fluorescence intensity measurements throughout all experiments. Compensation was achieved with a flow cytometry CompBeads kit (BD). Gating on forward and side light scatter was used to exclude dead cells and debris from the analysis. Forward width and forward area were used to exclude doublet cells. The final analysis was performed and the graphical output was generated with FACSDiva software (BD).
Real-time RT-PCR.
Total RNA from cells subjected to various treatments was extracted with Trizol reagent used according to the instructions of the manufacturer (Invitrogen), and the RNA samples were treated with DNase I (Qiagen). TaqMan quantitative real-time reverse transcription (RT)-PCR analysis was performed with reagents from Applied Biosystems used according to the manufacturer's instructions in an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories, Inc.). Primers, the TaqMan probe long terminal repeat (LTR), and the external standard were used as described previously (46).
Transwell cell culture insert experiments.
Millicell-24 cell culture assembly insert plates with a pore size of 0.4 μm (Millipore, Bedford, MA) were used to prevent cell-to-cell contact. The outer wells contained HIV-1-exposed MoDCs, and the inner wells contained primary lymphocyte subsets. In a control plate, CD4 T lymphocytes were cultured in the inner wells together with uninfected or HIV-1BaL-infected MoDCs whereas the outer wells contained medium alone. In all cases, the final volume of medium was 250 μl, and triplicates were set up for each condition. Cells were harvested on days 2 and 5 (data not shown) for surface CD3, DC-SIGN, and intracellular p24 antigen staining analyses, followed by flow cytometry.
Characterization of the virus particles released.
Virus-containing culture supernatants were centrifuged to remove cellular debris before analysis for the presence of host cell-derived surface molecules incorporated into the envelope during budding. This analysis involved the capture of viral particles with magnetic microbeads coupled to specific antibodies directed against markers of different cell types (59). Briefly, cleared cell-free supernatants were incubated with magnetic beads coated with mouse anti-human CD1a or CD3 antibodies (Miltenyi Biotec) at a 1:2 bead-to-supernatant volume ratio for 15 min at 4°C. Magnetic bead-bound fractions were separated from the supernatant by selective retention on equilibrated μMACS magnetic columns (Miltenyi Biotec). Columns were washed four times with MACS running buffer (1× phosphate-buffered saline, 0.5% bovine serum albumin [BSA], and 2 mM EDTA), and the retained fractions were eluted with MACS rinse buffer (MACS running buffer without BSA). We determined the number of HIV-1 particles in each fraction bearing a given cell antigen by p24 ELISA. Supernatants from cell cultures maintained in the presence of azidothymidine (AZT), an inhibitor of HIV-1 replication, were used to determine p24 background levels (i.e., those from residual input virus particles or free or HIV-1-associated exosomes released from MoDCs). We evaluated the infectivity of viral particles produced under various MoDC-lymphocyte coculture conditions by measurement of the 50% tissue culture infective dose (TCID50).
Virus infectivity.
The capacities of viruses to infect PBMCs or immature MoDCs and to produce new viral particles were determined as reported previously (6). Cells were infected with serial dilutions of cell-free virus supernatant from HIV-1-exposed immature MoDCs cultured for 5 days alone or together with activated CD4 T lymphocytes or nonactivated B lymphocytes. After 4 days of infection, cells were washed twice and cultured for an additional 7 days in fresh culture medium. Virus-producing cultures were identified by subjecting culture supernatants to p24 ELISA. The TCID50 was calculated by the Reed-Muench method.
Neutralizing monoclonal antibodies.
The monoclonal neutralizing antibody IgG1b12, directed against the CD4 binding site of gp120, was a gift from Dennis Burton (Scripps, La Jolla, CA). Polyclonal neutralizing and non-HIV-1 IgG antibodies were purified from the sera of asymptomatic HIV-1-positive individuals and seronegative healthy blood donors, respectively (6, 21).
Inhibition of HIV transfer from immature DCs to T lymphocytes.
HIV-1 trans-infection of CD4 T lymphocytes was inhibited with antibodies as described previously (24). Briefly, immature MoDCs were loaded with HIV-1 for 2 h and then washed. Neutralizing antibodies were then added to the cells, together with autologous primary activated CD4 T lymphocytes. After 48 h, the inhibitory effect of neutralizing antibodies was determined by comparing the percentages of the two cell types positively stained for intracellular p24 antigen in the presence and absence of neutralizing antibodies.
Statistical analysis.
A nonparametric two-tailed Mann-Whitney U test was used to assess differences among groups. Values of P of <0.05 were considered to be statistically significant for the two-tailed Mann-Whitney U test, and values of P of <0.017 were considered to be statistically significant for the two-tailed Mann-Whitney U test with Bonferroni's correction. Statistical calculations were performed using R software (Department of Statistics and Mathematics, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
HIV-1 replication and production in immature MoDCs, LCs, and intDCs are strongly enhanced in the presence of primary human CD4 T lymphocytes.
DCs infected in vivo at mucosal sites may be a source of viral particles for other responding CD4 target cells, but little is known about their HIV-1 replication capacity during cross talk with various lymphocyte populations. We studied the effect of DC-lymphocyte interactions on HIV-1 replication in DCs by incubating immature MoDCs or CD34-derived LCs and intDCs with R5 HIV-1 primary isolates for 2 h before adding purified primary blood lymphocytes or human transformed CD4 T lymphocytes.
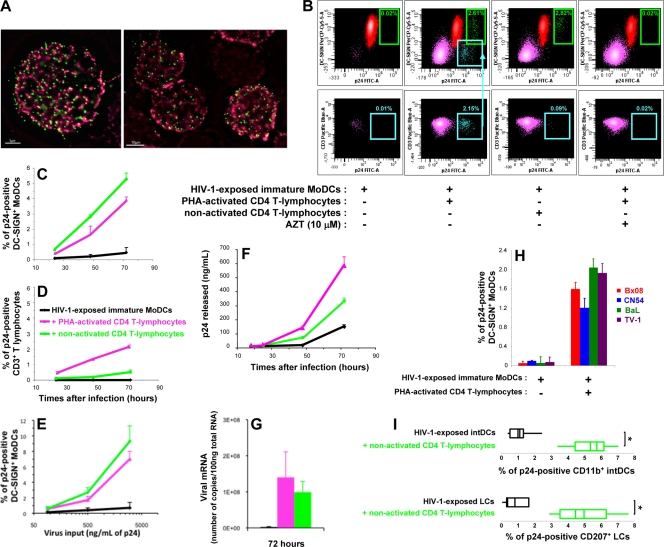
We used flow cytometry to detect intracellular p24 viral antigen—a reliable early indicator of productive infection (56)—and cell-specific markers for the phenotypic characterization of infected cells. Immature DC-SIGN-positive (DC-SIGN+) MoDCs exposed to HIV-1 for 2 h efficiently captured viral particles, as shown by fluorescence microscopy (Fig. 1A), but only a few MoDCs were able to replicate HIV-1 (0.02% of DC-SIGN+ MoDCs were p24 positive) (Fig. 1B, first column). However, in the presence of autologous PHA-activated CD4 T lymphocytes, the HIV-1 infection of immature MoDCs was markedly enhanced, as shown by intracellular p24 antigen levels (2.61% of DC-SIGN+ MoDCs were p24 positive) (Fig. 1B, second column). Indeed, viral p24 antigen was detectable in MoDCs by 24 h (Fig. 1C). At 48 h, the percentage of infected MoDCs in the coculture was markedly higher than the percentage of infected MoDCs among HIV-1-exposed MoDCs cultured alone (Fig. 1C). The percentage of infected MoDCs in the coculture continued to increase from the 48-h time point to day 3 (Fig. 1C) and day 5 (data not shown). HIV-1 replication in PHA-activated CD4 T lymphocytes in the coculture was also observed (Fig. 1B, bottom row, and D), confirming previous observations that infected DCs can efficiently transmit infectious viral particles (8, 18, 33, 36, 52, 57). As a control, AZT, a reverse transcriptase inhibitor, was added 2 h after the infection of MoDCs, at the same time that CD4 T lymphocytes were added. AZT prevented HIV-1 replication in both MoDCs and CD4 T lymphocytes, indicating that the viral p24 antigen detected in the absence of AZT resulted from de novo synthesis (Fig. 1B). The relationship between virus input and the percentage of p24-positive DC-SIGN+ MoDCs was also investigated. In experiments with serial dilutions of virus inoculum, a dose-dependent response curve was obtained when the amount of virus loaded and the percentage of infected MoDCs after 2 days of coculture with CD4 T lymphocytes were plotted (Fig. 1E). Thus, a plateau was not reached with this virus input (500 ng/ml viral p24 antigen used in our experiments). The number of virions released, assessed by determining extracellular p24 antigen levels, was greater in the MoDC-T-lymphocyte coculture than in cultures of HIV-1-exposed MoDCs alone (Fig. 1F). Furthermore, the quantification of viral mRNA in the cells demonstrated viral transcription levels to be higher in cocultured cells, regardless of the activation status of the primary CD4 T lymphocytes (Fig. 1G). The increase in viral p24 production in MoDCs did not require HIV-1 multiplication in CD4 T lymphocytes, as this effect was also observed in the presence of nonactivated CD4 T lymphocytes unable to support viral replication (Fig. 1B and C). The increase in viral replication in intracellular p24-positive DC-SIGN+ MoDCs was actually greater in the presence of nonactivated CD4 T lymphocytes than in the presence of activated ones. The exposure of immature MoDCs to a panel of different R5 HIV-1 clade B primary isolates led to similar increases in HIV-1 replication in MoDCs (Fig. 1H). The percentages of CD34-derived LCs and intDCs infected with clade B R5 HIV-1BaL isolates (Fig. 1I) and clade C R5 HIV-1TV-1 isolates (data not shown) were also higher in the presence of autologous primary CD4 T lymphocytes than in the absence of such cells. This phenomenon, therefore, occurs with various R5 isolates and DC subsets and is not restricted to DC-SIGN+ MoDCs. In contrast, we detected no stimulation of HIV-1 production in monocyte-derived macrophages cocultured with autologous nonactivated primary CD4 T lymphocytes (data not shown), demonstrating that the stimulatory process is specific to DCs. Our findings indicate that coculture of DCs and CD4 T lymphocytes promotes efficient HIV-1 replication in immature DCs, even if the primary CD4 T lymphocytes are not infected.
FIG. 1.
Increase in HIV-1 p24 antigen production in immature MoDCs in the presence of cocultured primary CD4 T lymphocytes. (A) Immunofluorescence analysis of the binding of HIV-1 pSG3EnvSF162 vpr-GFP pseudoparticles (green) to immature MoDCs 2 h postinfection. Membranes were stained with anti-human DC-SIGN IgG (red). Bars, 3 μm (left) and 10 μm (right). (B) Dot plots of intracellular p24-positive DC-SIGN+ MoDCs (green) and CD3+ CD4 T lymphocytes (purple) after 48 h of culture. The teal arrowhead indicates the percentage of infected CD4 T lymphocytes in the CD3-positive gate. PerCP Cy5.5; peridinin-chlorophyll cyanine 5.5; FITC, fluorescein isothiocyanate. (C and D) Immature MoDCs were exposed to HIV-1BaL for 2 h before the addition of uninfected autologous PHA-activated or nonactivated CD4 T lymphocytes. Percentages of MoDCs (C) and CD4 T lymphocytes (D) producing intracellular p24 antigen were determined by flow cytometry. Data are the means ± standard deviations (SD) of triplicate results. (E) Dose-dependent response curve obtained by plotting the amount of input virus loaded into immature DCs against the percentage of p24-positive DC-SIGN+ MoDCs detected after 48 h of coculture with autologous CD4 T lymphocytes. Data are the means ± SD of triplicate results. Data from one representative experiment of four are shown. (F) Cell-free supernatants were collected after various amounts of time, and extracellular viral p24 antigen levels were determined by ELISA. Data are the means ± SD of triplicate results. (G) Quantification of viral mRNA in cocultures of HIV-1-exposed MoDCs and activated or nonactivated CD4 T lymphocytes (n = 3) by RT-PCR. (H) Immature MoDCs were infected with various R5 HIV-1 primary isolates and cultured with or without activated CD4 T lymphocytes for 48 h (n = 3). (I) Box plot analyses of intracellular viral p24 antigen in cocultures (n = 5) of HIV-exposed LCs or intDCs and uninfected autologous nonactivated CD4 T lymphocytes after 3 days. Data are the means ± standard errors of the means (SEM) of results from n independent experiments. A two-tailed Mann-Whitney U test with Bonferroni's correction was used to assess differences between groups. A value of P of <0.025 was considered significant. *, P < 0.025 versus the control group.
Stimulation of HIV-1 replication in MoDCs is also detected in the presence of primary B lymphocytes but not in the presence of PHA-activated CD8 T lymphocytes or human transformed CD4 T cells.
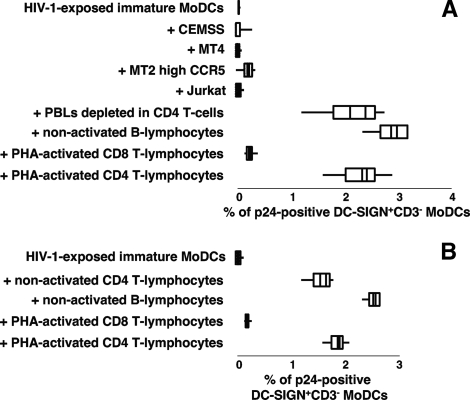
CD4 T lymphocytes were not the only leukocyte population able to increase the proportion of MoDCs producing viral p24 antigen. A similar effect was observed following the addition of peripheral blood lymphocytes (PBLs) depleted of CD4 target cells (Fig. 2A). Indeed, the presence of purified primary B lymphocytes in the coculture strongly stimulated HIV-1 replication in MoDCs. In contrast, the addition of PHA-activated CD8 T lymphocytes to infected MoDCs had no significant effect on the percentage of p24-positive DC-SIGN+ MoDCs (Fig. 2A). In parallel, we determined the levels of viral mRNA in these various cocultures by real-time RT-PCR. The number of HIV-1 mRNA copies in the coculture of HIV-1-exposed MoDCs and primary B lymphocytes was 70 times higher than that in the culture of infected MoDCs alone. In contrast, the quantity of HIV mRNA in the coculture with activated CD8 T lymphocytes was not significantly different from that in the culture of infected MoDCs alone. The lack of increase in the number of HIV-1-infected MoDCs and in viral mRNA in the coculture with PHA-activated CD8 T lymphocytes may be due to the apoptosis of HIV-infected MoDCs induced by these activated T lymphocytes. Indeed, we observed an increase, by a factor of five, in the number of hypodiploid DNA particles in MoDCs (data not shown). Transformed human CD4 T lymphocytes, which are commonly used as HIV-1 target cells in studies of HIV-1 transfer, had no significant effect on the rate of MoDC infection (Fig. 2A). In contrast, coculture with allogeneic primary CD4 T or B lymphocytes at a DC/lymphocyte ratio of 1:4 resulted in an increase in HIV-1 replication in MoDCs. Thus, autologous or allogeneic primary lymphocytes stimulate HIV-1 replication in MoDCs (Fig. 2B).
FIG. 2.
Stimulation of HIV-1 replication in infected MoDCs cocultured with primary B lymphocytes but not with human CD4 T-cell lines. (A) Box plot analysis of the percentage of p24-positive DC-SIGN+ CD3− MoDCs following the coculture of HIV-1-exposed MoDCs and autologous CD4 T lymphocyte-depleted PBLs (n = 4), nonactivated B lymphocytes (n = 5), PHA-activated CD8 T lymphocytes (n = 7), PHA-activated CD4 T lymphocytes (n = 10), or various human CD4 T-cell lines (n = 4) for 48 h. (B) Box plot analysis of the percentage of infected DC-SIGN+ CD3− MoDCs in the presence of allogeneic T- or B-lymphocyte populations (n = 3) after 48 h of coculture. Data are the means ± SEM of results from n independent experiments.
The infectious virions released in coculture are produced by infected MoDCs.
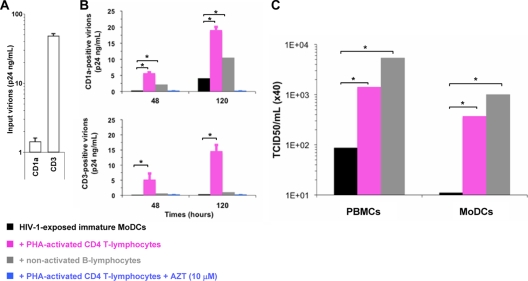
We investigated the cellular origin of the virions released into the supernatant of the coculture. We checked for host cell-derived molecules (37, 59), such as CD1a and CD3, which are incorporated at the surfaces of virus particles budding from MoDCs and CD4 T lymphocytes, respectively. The input virus produced in PBLs expressed mostly the CD3 marker (Fig. 3A), whereas a significant proportion of virus particles released in the DC-CD4 T-lymphocyte coculture carried the DC-specific markers CD1a (Fig. 3B) and CD11b (data not shown). In addition, the virions produced in the MoDC-B-lymphocyte coculture were mostly CD1a positive (i.e., produced by DCs). The number of CD1a-positive virus particles increased with the time of infection (Fig. 3B). Moreover, the resulting virus suspensions were infectious, as they productively infected MoDCs and PBMCs (Fig. 3C). No virus particles were detected in the supernatants of cocultures containing AZT (Fig. 3B) or cocultures of HIV-1-exposed MoDCs and PHA-activated CD8 T lymphocytes (data not shown). Thus, MoDCs efficiently produced infectious HIV-1 particles when cocultured with primary CD4 T lymphocytes or nonpermissive B lymphocytes.
FIG. 3.
The infectious HIV-1 particles released into the supernatant of the coculture are produced principally by infected MoDCs. (A and B) Virus particles positive for CD1a or CD3 were subjected to immunomagnetic separation and quantification by p24 ELISA (n = 4). Levels of these host cell surface markers detected on the envelopes of virions from input virus (A) or cell-free supernatants from cocultures (B) are shown. (B) Amounts of CD1a (top)- or CD3 (bottom)-positive virus particles collected after 2 and 5 days of culture (n = 4). The values plotted on all graphs are the means ± SEM of results from n independent experiments. (C) Infectivities (TCID50s) of the cell-free virus supernatants for PBMCs and immature MoDCs. Two-tailed Mann-Whitney U tests with Bonferroni's correction were used to assess differences between groups. A value of P of <0.017 was considered statistically significant. *, P < 0.017 versus controls.
Early cell-to-cell contacts between infected DCs and primary lymphocytes are required for the stimulation of HIV-1 production in MoDCs.
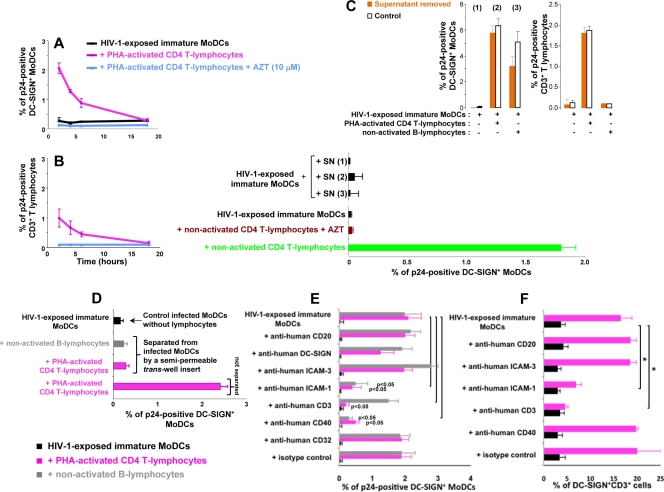
We then added primary lymphocytes to HIV-1-exposed MoDCs at various time points. Kinetic analysis showed that the stimulation of HIV-1 replication in MoDCs was less efficient if the addition of PHA-activated CD4 T lymphocytes to HIV-1-exposed MoDCs was delayed. The percentage of p24-positive MoDCs detected after 2 days (Fig. 4A) or 3 days (data not shown) decreased as a function of time between HIV-1 exposure and the addition of CD4 T lymphocytes, suggesting that the addition of lymphocytes may stimulate an early step of HIV-1 replication in MoDCs. Moreover, when MoDCs were infected for a longer period of time before the addition of primary CD4 T lymphocytes, the percentage of p24-positive CD4 T lymphocytes also decreased (Fig. 4B). Thus, the HIV-1 trans-infection of CD4 T lymphocytes was efficient only for a limited period of time after the loading of MoDCs with virus; after this period, HIV-1 transfer in cis may occur, as suggested in previous studies (13, 18, 57). Moreover, kinetic analysis after the addition of AZT suggested that the reverse transcription of viral RNA was more rapid after the addition of lymphocytes (data not shown), consistent with the higher viral mRNA levels in the presence of lymphocytes as detected by RT-PCR (Fig. 1G).
FIG. 4.
Early cell-to-cell contact is required for the increase in HIV-1 replication in infected DCs. (A and B) CD4 T lymphocytes were added at various time points after the infection of immature MoDCs. The percentage of DC-SIGN+ DCs (A) or CD3+ CD4 T lymphocytes (B) expressing p24 was determined after 48 h. (C) Supernatants (SN) from HIV-1-exposed MoDCs, cultured alone [SN (1)] or cocultured with CD4 T lymphocytes [SN (2)] or B lymphocytes [SN (3)], were collected at 6 h and added to infected immature MoDCs. (Bottom) After 48 h, intracellular p24 levels were determined. (Top) In parallel, we used flow cytometry to measure intracellular p24 production at 48 h in cells from which supernatants (1, 2, and 3) had been removed (orange bars) or not removed (white bars). (D) HIV-1-exposed MoDCs and uninfected lymphocytes were left in contact or separated with a Transwell insert, and intracellular p24 levels were determined after 48 h. (E) PHA-activated CD4 T or B lymphocytes were pulsed with 20 μg/ml mouse monoclonal IgG2a directed against human CD3 (clone OKT3 [azide free]; Miltenyi Biotec) before being added to HIV-1-exposed immature MoDCs. We added 20 μg/ml mouse monoclonal IgG1 directed against CD40 (clone B-B20 [azide free]; Abcam), ICAM-3 (clone B-R1 [azide free]; Abcam), ICAM-1 (clone 1H4 [azide free]; Abcam), CD20 (clone MEM-97 [azide free]; Abcam), DC-SIGN (clone DCN46; BD Pharmingen), CD32 (clone 3D3; BD Pharmingen), or a mouse monoclonal IgG1 isotype (clone NCM1; Abcam) to the coculture at the same time as CD4 T lymphocytes. After 48 h of coculture, intracellular p24 levels were determined by flow cytometry. (F) In parallel, MoDC-CD4 T-cell conjugates were evaluated by determining the percentage of DC-SIGN+ CD3+ cells by flow cytometry. The values plotted on all graphs are means ± SD of triplicate results from a single representative experiment. A value of P of <0.05 was considered significant. *, P < 0.05 versus the corresponding control group.
We investigated whether the release of infectious viral particles (e.g., those associated with exosomes) or soluble factors was involved in the stimulation of HIV-1 replication in cocultured MoDCs by removing the supernatants from MoDCs infected in the presence of CD4 T or B lymphocytes after 6 h of coculture and adding them to HIV-1-exposed immature MoDCs. The addition of these supernatants to HIV-1-exposed MoDCs did not result in the stimulation of HIV-1 replication (Fig. 4C, bottom). Moreover, HIV-1 replication in MoDCs in the coculture from which the supernatant was removed was not significantly affected (Fig. 4C, top).
In contrast, when cell-to-cell contact was prevented by a cell culture membrane insert, coculture with CD4 T or B lymphocytes no longer affected MoDCs (Fig. 4D). The increase in HIV-1 replication in cocultured MoDCs was also substantially decreased when PHA-activated CD4 T lymphocytes, but not B lymphocytes, were first pulsed or continuously incubated with 20 μg/ml monoclonal anti-human CD3 antibody (Fig. 4E). Moreover, the addition of 20 μg/ml monoclonal antibody directed against human CD40 receptor or ICAM-1 to HIV-1-exposed immature MoDCs at the same time as PHA-activated CD4 T lymphocytes (Fig. 4E) or nonactivated B and CD4 T lymphocytes (data not shown) significantly inhibited the stimulation of HIV replication in MoDCs. In the presence of anti-human CD40 antibody, the decrease in HIV replication in MoDCs was associated with the induction of DC maturation markers (more than 85% of DC-SIGN+ MoDCs were CD83 positive). This DC maturation may contribute to the decrease in R5 HIV-1 replication in infected MoDCs (as mature MoDCs do not support R5 HIV-1 replication [41]). In contrast, in the presence of anti-human CD3 or ICAM-1 antibody, no DC maturation was recorded (data not shown). In parallel, the percentage of cells positive for both DC-SIGN and CD3 was determined by flow cytometry. Blocking ICAM-1-LFA-1 interaction between MoDCs and activated CD4 T lymphocytes markedly decreased the percentage of DC-SIGN+ CD3+ cells, whereas inhibiting the CD40-CD40L interaction had no significant effect on this percentage (Fig. 4F). The purified mouse monoclonal IgG directed against CD3 masked the CD3 binding site, hindering the detection of cell conjugates by flow cytometry. We were therefore unable to draw any firm conclusions on the inhibition of DC-SIGN+ CD3+ cell conjugate formation by anti-human CD3 antibody under these conditions.
The addition of monoclonal antibody directed against DC-SIGN, CD32, CD20, or ICAM-3 did not significantly affect the percentage of DC-SIGN+ CD3+ cells or the proportion of infected MoDCs in cocultures with activated CD4 T lymphocytes (Fig. 4E and F and data not shown). In addition, no doubly positive cells were detected by flow cytometry when HIV-exposed MoDCs were cocultured with nonactivated B or CD4 T lymphocytes (data not shown), although anti-ICAM-1 and anti-CD40 antibodies inhibit HIV stimulation in MoDCs. Thus, overall, our data demonstrate that efficient HIV-1 replication in MoDCs is dependent on cellular interactions between MoDCs and lymphocytes in primary cultures but not on viral particles or soluble factors released during early cell-to-cell contact.
Neutralizing IgGs inhibit HIV-1 trans-infection.
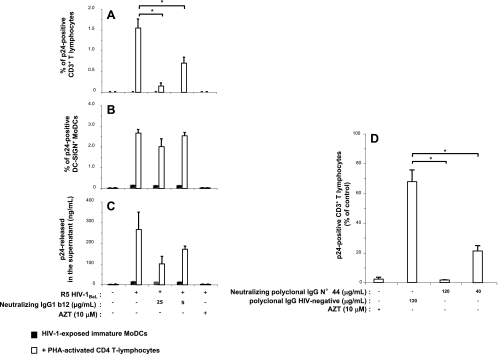
The greater efficiency of HIV-1 production in immature MoDCs cocultured with primary lymphocytes highlights the need to reconsider the potential inhibitory effects of antibodies on R5 HIV-1 transfer from DCs to CD4 T lymphocytes. Indeed, in previous studies based on a similar in vitro experimental design, the neutralizing activities of antibodies were recorded by analyzing virus p24 particles released into the supernatant but the increase in HIV-1 particle release from DCs was not taken into account. In this study, we determined the percentage of infected cells by flow cytometry and found that adding 25 μg/ml of neutralizing monoclonal IgG1b12 antibody (recognizing a conserved epitope overlapping the CD4 binding site of HIV-1 gp120) at the same time as PHA-activated CD4 T lymphocytes to HIV-1-exposed immature MoDCs abolished the HIV-1 infection of the CD4 T lymphocytes (Fig. 5A), despite the absence of a strong inhibitory effect on HIV-1 replication in MoDCs (Fig. 5B). As a consequence, only a limited decrease in p24 levels in the supernatant in the presence of neutralizing IgG1b12 antibodies occurred (Fig. 5C). Thus, IgG1b12, when added 2 h after the infection of immature DCs, efficiently blocked productive HIV-1 transfer from infected MoDCs to activated CD4 T lymphocytes, although this effect was not apparent from determinations of soluble p24 Gag levels in the supernatant. Similarly, CD4 T lymphocyte trans-infection was inhibited by a neutralizing polyclonal antibody preparation purified from the serum of an HIV-1 patient (polyclonal IgG no. 44) (Fig. 5D). These data clearly demonstrate that HIV-1 transfer from MoDCs to CD4 T lymphocytes in trans was not resistant to HIV-1 neutralization. Our findings therefore provide strong evidence that neutralizing monoclonal and polyclonal antibodies can prevent early HIV-1 cell-to-cell transfer to a much greater extent than was previously recognized.
FIG. 5.
Neutralizing activities of anti-HIV-1 monoclonal or polyclonaI IgGs toward the trans-infection of CD4 T lymphocytes. Monoclonal neutralizing IgG1b12 (A, B, and C) or polyclonal neutralizing IgG preparation no. 44 (D) was added, together with activated CD4 T lymphocytes, to HIV-1-exposed immature MoDCs. After 48 h of coculture, intracellular and extracellular p24 production levels were determined by flow cytometry and p24 ELISA, respectively. Shown are the percentages of p24-positive primary CD3+ CD4 T lymphocytes (A and D) and of p24-positive DC-SIGN+ MoDCs (B) and amounts of viral p24 released into the supernatant (C) when HIV-1-exposed immature MoDCs were cultured with or without activated CD4 T lymphocytes in the presence or absence of neutralizing IgGs or AZT. The values shown are the means ± SD of triplicate results. Purified neutralizing polyclonal IgGs were obtained from the serum of HIV-1-positive asymptomatic patient no. 44, and nonneutralizing polyclonal IgGs were obtained from a pool of sera from HIV-1-negative healthy donors. A value of P of <0.05 was considered significant. *, P < 0.05 versus the corresponding control group.
DISCUSSION
The importance of virus trafficking in DCs for transmission has been clearly demonstrated for HIV-1 and for other viruses, including human T-cell leukemia virus type 1 (HTLV-1) and Ebola virus (1, 24, 25). In the case of HIV-1 infection, it was initially suggested that DCs could not support efficient HIV-1 replication due to intracellular restriction factors and their capacity to capture and degrade viral pathogens for antigen presentation (26, 34, 36). However, the potential modulation of HIV-1 replication in DCs during their cross talk with various lymphocyte subsets was not taken into account when this conclusion was reached.
By directly measuring productive HIV-1 infection in each cell subset present in the cocultures of MoDCs and lymphocytes, we obtained evidence for an unexpected increase in HIV-1 replication in MoDCs. The addition of primary autologous or allogeneic CD4 T lymphocytes to HIV-1-exposed immature MoDCs effectively stimulated HIV-1 replication in the MoDCs. The increase in HIV-1 replication in MoDCs was also observed in the presence of nonactivated CD4 T lymphocytes and nonpermissive primary B lymphocytes, indicating that this phenomenon was not dependent on HIV-1 replication in lymphocytes. The virions produced in these cocultures resulted from efficient viral replication in MoDCs and were able to infect both PBMCs and immature MoDCs. Saïdi et al. recently reported that cross talk between activated NK cells and MoDCs induces the accumulation of HIV-1 DNA in MoDCs and significantly increases HIV-1 production in immature MoDCs (48). These data indicate that HIV-1 replication in immature MoDCs may be induced by interaction with different leukocyte populations. Interestingly, we detected no enhancement of HIV-1 replication in MoDCs cocultured with various human transformed CD4 T-lymphocyte cells, although infected MoDCs efficiently transfer infectious R5 or X4 HIV-1 particles to these CD4 T cells (data not shown) (2, 62), further indicating that HIV-1 transfer to CD4 T target cells and stimulation in DCs are not necessary associated. Moreover, we observed a similar increase in HIV-1 replication when LCs and intDCs were cocultured with primary CD4 T lymphocytes, whereas no such increase was observed when the cells were cocultured with human transformed CD4 T cells (unpublished results). As immature LCs and intDCs are considered to be among the first targets of HIV-1 in vivo after sexual transmission, this increase in HIV-1 replication may be of physiological relevance. De Witte et al. found epithelial LCs to be refractory to HIV infection and transmission and proposed a mechanism of HIV capture and degradation involving langerin (10). Their results contrast with our observation of efficient HIV replication and transfer in LCs and intDCs. This discrepancy may be attributed to differences in the experimental culture conditions used: the concentrations of input virus and the origins of the LCs used (LCs isolated from skin versus LCs differentiated from cord blood CD34 stem cells). Nonetheless, we and others have shown that LCs are infected in vitro and can transmit HIV particles to CD4 T cells (11, 27-29). In addition, LCs and memory T lymphocytes emigrating from human skin explants have been found to facilitate productive HIV-1 infection (43), consistent with our results.
In our kinetic experiments, in which primary CD4 T or B lymphocytes were added at different time points, the detection of an increased viral mRNA level after lymphocyte addition is consistent with the stimulation of an early step of HIV-1 RNA reverse transcription in MoDCs. Using cell culture inserts, we showed that cell-to-cell contact was required. DC-lymphocyte interactions are known to lead to the formation of an IS. This IS involves the local reorganization of an array of receptors (e.g., CD3, CD4, CD8, talin, ICAM-1, DC-SIGN, and CD40/CD40L) that may vary with the lymphocyte phenotype and the presence or absence of antigens (5, 45, 51). Such interactions are important for immunological cross talk between DCs and T or B lymphocytes (30, 31, 45, 51). We investigated whether these interactions triggered early stages of HIV-1 replication in immature MoDCs. The abolition of IS formation (T-cell receptor-CD3 or ICAM-1-LFA-1 cell interactions) mediated by antibodies directed against CD3 or ICAM-1 prevented the stimulation of HIV-1 replication in MoDCs cocultured with primary CD4 T lymphocytes. In contrast, the addition of monoclonal antibody against ICAM-3 to infected MoDCs cocultured with CD4 T lymphocytes had no effect on HIV replication in DCs. Others have shown that blocking ICAM-1-LFA-1 interactions on primary CD4 T lymphocytes significantly decreases HIV-1 transmission from immature DCs to lymphocytes but that blocking ICAM-2 and ICAM-3 does not inhibit DC-mediated HIV-1 transfer (49, 58). Furthermore, the insertion of ICAM-1 into HIV-1 particles enhances the infection of CD4 T lymphocytes expressing LFA-1 (12, 55). The importance of such interactions for HIV-1 transmission has been highlighted by the results of in vitro HIV transfer experiments using primary lymphocytes from leukocyte adhesion deficiency type 1 (LAD-1) patients harboring LFA-1 defects (17). The absence of LFA-1 results in the impairment of HIV-1 replication, cell-to-cell viral transfer, and the formation of virological synapses (47). In addition to its role in cell-to-cell adhesion during antigen presentation or cross talk, ICAM-1 may be involved in signal transduction across cell membranes, like gene transcription, as suggested previously (23), or in the modulation of restriction factor activity.
The agonist monoclonal antibody against the human CD40 receptor inhibited HIV-1 replication in MoDCs in the presence of CD4 T or B lymphocytes. This antibody is known to mimic CD40-CD40L engagement, which upregulates the maturation markers CD86 and CD83 and increases APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G) expression (40). The resulting increase in the amount of APOBEC3G in the cell is correlated with an increase in resistance to HIV-1 infection (38). Thus, CD40 engagement on infected MoDCs triggers the production of APOBEC3G, which is involved in decreasing HIV-1 replication and infectivity (41, 50). Similarly, in our experiments, the addition of human anti-CD40 monoclonal antibody to HIV-1-exposed immature MoDCs induced MoDC maturation, potentially accounting for the inhibition of HIV-1 stimulation in MoDCs. Conversely, the productive infection of DCs with HIV-1 may be stimulated by unknown retrograde signals delivered to DCs by primary lymphocytes, which may downregulate or inhibit intracellular HIV-1 restriction factors, such as APOBEC3G (41, 54) or the unknown factor counteracted by Vpx (15). Other factors may also modify the life cycle of HIV-1 in DCs. The upregulation of transcription factors, such as NF-κB, in infected DCs may also account for efficient HIV replication in cocultured DCs, as shown following the formation of syncytia between infected DCs and memory CD4 T lymphocytes (16). Moreover, these heterologous syncytia contain high levels of active Rel and Sp1 factors, likely to induce strong stimulation of the HIV-1 promoter, leading to high levels of virion production (16). In contrast to findings for cells in the presence of PHA-activated CD4 T lymphocytes, no syncytium formation or DC-SIGN+ CD3+ or CD20+ cells in the presence of nonactivated CD4 T or B lymphocytes were observed by microscopy or flow cytometry 2 days after infection (data not shown), although HIV-1 replication was enhanced in MoDCs. This discrepancy between HIV-1 replication and cell conjugate formation demonstrates that HIV-1 stimulation in DCs may be mediated by different mechanisms involving an exchange of information during syncytium formation that cannot in itself account for the stimulation of HIV-1 replication. As the HIV-1 particles taken up by DCs are rapidly degraded in endolysosomal compartments (7, 62), the addition of primary lymphocytes may affect the fate of these incoming viruses, allowing them to evade the degradation process. Alternatively, a more efficient route of viral entry in the nonlysosomal compartment or higher integration efficiency may contribute to the increase in HIV-1 replication in DCs. In vivo, the enhancement of HIV-1 replication in immature DCs surrounded by lymphocytes may create a favorable environment for HIV-1 transmission, dissemination, and persistence. It may also be highly detrimental to the immune system, as HIV-1 replication in DCs may hijack immune functions of these cells, thus disrupting the early innate immune response and the initiation of adaptive immunity to HIV-1 (3, 42). Thus, an understanding of the precise mechanism responsible for the stimulation of HIV-1 replication would facilitate the development of inhibitors of HIV-1 replication in DCs.
As the HIV-1 trans-infection of CD4 T target cells plays an important role in the propagation of infection, we also evaluated virus transmission to primary CD4 T lymphocytes in coculture. Productive HIV-1 trans-infection of activated CD4 T lymphocytes was detected in the coculture on day 1 after infection. The percentage of infected CD4 T lymphocytes decreased with increasing duration of the period of infection of immature MoDCs. Consistent with this pattern, the number of new viral particles released into the supernatant of the coculture also decreased as a function of the duration of DC infection (data not shown). Pope et al. also showed that viral p24 antigen release into the supernatant decreased with increasing duration of the period of incubation of T lymphocytes with infected DCs (44). Cavrois et al. recently investigated this aspect further, using a virion-based HIV-1 fusion assay. They confirmed that levels of R5 or X4 HIV-1 transmission decreased sharply when virions were presented by immature or mature MoDCs incubated with HIV-1 for up to 120 min at 37°C before the addition of CD4 T cells (8).
We have shown previously that neutralizing monoclonal antibodies strongly inhibit the HIV-1 infection of immature MoDCs (20, 21). In this study, we assessed the capacity of neutralizing IgGs to inhibit HIV-1 transfer from immature MoDCs to primary CD4 T lymphocytes. By analyzing the percentages of infected DCs and CD4 T lymphocytes, we showed that neutralizing IgGs strongly inhibited the infection of primary CD4 T lymphocytes, with no marked effect on HIV-1 replication in MoDCs. Moreover, HIV-1 particles continued to be released into the supernatant in the presence of neutralizing antibodies. Thus, the determination of viral p24 antigen levels in the coculture medium underestimated the inhibition of HIV-1 cell-to-cell transfer by antibodies. Thus, quantification of the HIV-1 released into the supernatant is not a reliable method for assessing the inhibition of HIV-1 transfer by antibodies. Our findings may explain some of the discrepancies among previous reports concerning the capacity of antibodies to inhibit HIV-1 transfer (7, 9, 13, 14). The efficient inhibition of HIV-1 transfer by neutralizing antibodies described here opens up new perspectives in the search for an effective HIV-1 vaccine. Indeed, one of the primary goals of vaccination should be the induction of HIV-1-specific antibodies at mucosal sites, to block DC infection and viral transfer from DCs to CD4 T cells.
Acknowledgments
We thank Lisa Chakrabarti from the Pasteur Institute (Unité d'Immunogénétique Cellulaire) in Paris for fruitful discussions and for critically reading the manuscript. We thank J. Penichon at the Institute of Virology in Strasbourg for his excellent technical assistance. Special thanks go to D. Mirisky and I. Nisand (Gynecology and Maternity Department) at the University Hospital Center in Strasbourg-Hautepierre for supplying cord blood samples from healthy donors.
V.H., A.-M.A., and C.M. conceived and designed the experiments; K.X., V.H., M.P., A.L., M.E.B., M.D., S.S., T.D., and G.L. performed the experiments; M.P., K.X., V.H., and M.E.B. analyzed data; S.S., T.D., K.X., and G.L. contributed reagents/materials/analysis tools; and V.H., A.-M.A., and C.M. wrote the paper.
This work was supported by funds from EuroNeut 41 (grant no. FP7-HELTH-2007-A-201038), EuroPrise (grant no. LSHP-CT-2006-037611), ANRS, and Dormeur Investment Service Ltd. M.P. and K.X. were supported by scholarships from Sidaction and from EuroPrise, respectively.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkow, S., F. Krux, K. Loser, J. U. Becker, S. Grabbe, and U. Dittmer. 2007. Friend retrovirus infection of myeloid dendritic cells impairs maturation, prolongs contact to naive T cells, and favors expansion of regulatory T cells. Blood 110:3949-3958. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Brossard, C., V. Feuillet, A. Schmitt, C. Randriamampita, M. Romao, G. Raposo, and A. Trautmann. 2005. Multifocal structure of the T cell-dendritic cell synapse. Eur. J. Immunol. 35:1741-1753. [DOI] [PubMed] [Google Scholar]
- 6.Burrer, R., S. Haessig-Einius, A. M. Aubertin, and C. Moog. 2003. Polyclonal immunoglobulin G from patients neutralizes human immunodeficiency virus type 1 primary isolates by binding free virions, but without interfering with an initial CD4-independent attachment of the virus to primary blood mononuclear cells. J. Virol. 77:11385-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavrois, M., J. Neidleman, and W. C. Greene. 2008. The Achilles heel of the Trojan horse model of HIV-1 trans-infection. PLoS Pathog. 4:e1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 3:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, P., W. Hubner, M. A. Spinelli, and B. K. Chen. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81:12582-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Witte, L., A. Nabatov, M. Pion, D. Fluitsma, M. A. de Jong, T. de Gruijl, V. Piguet, Y. van Kooyk, and T. B. Geijtenbeek. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367-371. [DOI] [PubMed] [Google Scholar]
- 11.Fahrbach, K. M., S. M. Barry, S. Ayehunie, S. Lamore, M. Klausner, and T. J. Hope. 2007. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J. Virol. 81:6858-6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72:9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goujon, C., V. Arfi, T. Pertel, J. Luban, J. Lienard, D. Rigal, J. L. Darlix, and A. Cimarelli. 2008. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J. Virol. 82:12335-12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granelli-Piperno, A., M. Pope, K. Inaba, and R. M. Steinman. 1995. Co-expression of NF-κB/Rel and Sp1 transcription factors in human immunodeficiency virus 1-induced, dendritic cell-T-cell syncytia. Proc. Natl. Acad. Sci. U. S. A. 92:10944-10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groot, F., T. W. Kuijpers, B. Berkhout, and E. C. de Jong. 2006. Dendritic cell-mediated HIV-1 transmission to T cells of LAD-1 patients is impaired due to the defect in LFA-1. Retrovirology 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gummuluru, S., V. N. KewalRamani, and M. Emerman. 2002. Dendritic cell-mediated viral transfer to T cells is required for human immunodeficiency virus type 1 persistence in the face of rapid cell turnover. J. Virol. 76:10692-10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783-792. [DOI] [PubMed] [Google Scholar]
- 20.Holl, V., M. Peressin, T. Decoville, S. Schmidt, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holl, V., M. Peressin, S. Schmidt, T. Decoville, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard, A. K., and R. Rothlein. 2000. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 28:1379-1386. [DOI] [PubMed] [Google Scholar]
- 24.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 25.Jones, K. S., C. Petrow-Sadowski, Y. K. Huang, D. C. Bertolette, and F. W. Ruscetti. 2008. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4+ T cells. Nat. Med. 14:429-436. [DOI] [PubMed] [Google Scholar]
- 26.Jones, L., D. McDonald, and D. H. Canaday. 2007. Rapid MHC-II antigen presentation of HIV type 1 by human dendritic cells. AIDS Res. Hum. Retroviruses 23:812-816. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. U. S. A. 100:8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura, T., S. E. Kurtz, A. Blauvelt, and S. Shimada. 2005. The role of Langerhans cells in the sexual transmission of HIV. J. Dermatol. Sci. 40:147-155. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura, T., Y. Koyanagi, Y. Nakamura, Y. Ogawa, A. Yamashita, T. Iwamoto, M. Ito, A. Blauvelt, and S. Shimada. 2008. Significant virus replication in Langerhans cells following application of HIV to abraded skin: relevance to occupational transmission of HIV. J. Immunol. 180:3297-3304. [DOI] [PubMed] [Google Scholar]
- 30.Koopman, G., H. K. Parmentier, H. J. Schuurman, W. Newman, C. J. Meijer, and S. T. Pals. 1991. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J. Exp. Med. 173:1297-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushnir, N., L. Liu, and G. G. MacPherson. 1998. Dendritic cells and resting B cells form clusters in vitro and in vivo: T cell independence, partial LFA-1 dependence, and regulation by cross-linking surface molecules. J. Immunol. 160:1774-1781. [PubMed] [Google Scholar]
- 32.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 34.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 35.Moog, C., C. Spenlehauer, H. Fleury, F. Heshmati, S. Saragosti, F. Letourneur, A. Kirn, and A. M. Aubertin. 1997. Neutralization of primary human immunodeficiency virus type 1 isolates: a study of parameters implicated in neutralization in vitro. AIDS Res. Hum. Retroviruses 13:19-27. [DOI] [PubMed] [Google Scholar]
- 36.Moris, A., A. Pajot, F. Blanchet, F. Guivel-Benhassine, M. Salcedo, and O. Schwartz. 2006. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108:1643-1651. [DOI] [PubMed] [Google Scholar]
- 37.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retroviruses 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 38.Peng, G., T. Greenwell-Wild, S. Nares, W. Jin, K. J. Lei, Z. G. Rangel, P. J. Munson, and S. M. Wahl. 2007. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood 110:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perfetto, S. P., D. Ambrozak, R. Nguyen, P. Chattopadhyay, and M. Roederer. 2006. Quality assurance for polychromatic flow cytometry. Nat. Protoc. 1:1522-1530. [DOI] [PubMed] [Google Scholar]
- 40.Pido-Lopez, J., T. Whittall, Y. Wang, L. A. Bergmeier, K. Babaahmady, M. Singh, and T. Lehner. 2007. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4+ T cells and dendritic cells. J. Immunol. 178:1671-1679. [DOI] [PubMed] [Google Scholar]
- 41.Pion, M., A. Granelli-Piperno, B. Mangeat, R. Stalder, R. Correa, R. M. Steinman, and V. Piguet. 2006. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J. Exp. Med. 203:2887-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollara, G., A. Kwan, P. J. Newton, M. E. Handley, B. M. Chain, and D. R. Katz. 2005. Dendritic cells in viral pathogenesis: protective or defective? Int. J. Exp. Pathol. 86:187-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope, M., M. G. H. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 44.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revy, P., M. Sospedra, B. Barbour, and A. Trautmann. 2001. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2:925-931. [DOI] [PubMed] [Google Scholar]
- 46.Rouet, F., D. K. Ekouevi, M. L. Chaix, M. Burgard, A. Inwoley, T. D. Tony, C. Danel, X. Anglaret, V. Leroy, P. Msellati, F. Dabis, and C. Rouzioux. 2005. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J. Clin. Microbiol. 43:2709-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudnicka, D., J. Feldmann, F. Porrot, S. Wietgrefe, S. Guadagnini, M. C. Prévost, J. Estaquier, A. T. Haase, N. Sol-Foulon, and O. Schwartz. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83:6234-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saïdi, H., M. T. Melki, and M. L. Gougeon. 2008. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS One 3:e3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 51.Shishkova, Y., H. Harms, G. Krohne, E. Avota, and S. Schneider-Schaulies. 2007. Immune synapses formed with measles virus-infected dendritic cells are unstable and fail to sustain T cell activation. Cell. Microbiol. 9:1974-1986. [DOI] [PubMed] [Google Scholar]
- 52.Sowinski, S., C. Jolly, O. Berninghausen, M. A. Purbhoo, A. Chauveau, K. Kohler, S. Oddos, P. Eissmann, F. M. Brodsky, C. Hopkins, B. Onfelt, Q. Sattentau, and D. M. Davis. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10:211-219. [DOI] [PubMed] [Google Scholar]
- 53.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stopak, K. S., Y. L. Chiu, J. Kropp, R. M. Grant, and W. C. Greene. 2007. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J. Biol. Chem. 282:3539-3546. [DOI] [PubMed] [Google Scholar]
- 55.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turville, S. G., M. Aravantinou, H. Stossel, N. Romani, and M. Robbiani. 2008. Resolution of de novo HIV production and trafficking in immature dendritic cells. Nat. Methods 5:75-85. [DOI] [PubMed] [Google Scholar]
- 57.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 58.Wang, J. H., C. Kwas, and L. Wu. 2009. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J. Virol. 83:4195-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley, R. D., and S. Gummuluru. 2006. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. U. S. A. 103:738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, L. 2008. Biology of HIV mucosal transmission. Curr. Opin. HIV AIDS 3:534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L., and V. N. KewalRamani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, H. J., M. A. Reuter, and D. McDonald. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 4:e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]