Abstract
Divalent metal ions are components of numerous icosahedral virus capsids. Flock House virus (FHV), a small RNA virus of the family Nodaviridae, was utilized as an accessible model system with which to address the effects of metal ions on capsid structure and on the biology of virus-host interactions. Mutations at the calcium-binding sites affected FHV capsid stability and drastically reduced virus infectivity, without altering the overall architecture of the capsid. The mutations also altered the conformation of gamma, a membrane-disrupting, virus-encoded peptide usually sequestered inside the capsid, by increasing its exposure under neutral pH conditions. Our data demonstrate that calcium binding is essential for maintaining a pH-based control on gamma exposure and host membrane disruption, and they reveal a novel rationale for the metal ion requirement during virus entry and infectivity. In the light of the phenotypes displayed by a calcium site mutant of FHV, we suggest that this mutant corresponds to an early entry intermediate formed in the endosomal pathway.
During cellular entry, the capsids of nonenveloped viruses undergo conformational changes triggered by various host factors. The transitions include the exposure of membrane-active, hydrophobic viral polypeptides and the destabilization and disassembly of the capsid, culminating in the delivery of the viral genome to the cytoplasm. A detailed, stepwise pathway of disassembly is unavailable for most viruses and requires molecular characterization of the intermediates formed during this process. Replication of disassembly intermediates in vitro necessitates careful treatment of native virions to simulate conditions likely to be encountered inside host cells. Receptor binding induces conformational changes in poliovirus, and the poliovirus entry intermediates, the 135S and 80S particles, can be generated in vitro by treating the native virion with the purified receptor, or by heating (6, 10, 19). Reovirus, which requires cathepsin-mediated proteolysis in the late endosomes in order to undergo entry-related changes, produces infectious subvirion particles (ISVP) and core particles as disassembly intermediates upon treatment with proteases in vitro (11, 14).
Apart from receptors and cellular proteases, other host determinants affecting viruses include low pH and Ca2+ depletion in specialized cellular compartments. The removal of Ca2+ from capsids could be particularly crucial for viruses that depend on metal ions for assembly and stability, such as rotavirus, mouse polyomavirus, and dragon grouper nervous necrosis virus (DGNNV) (1, 39, 43). A few studies have reported that mutations at the metal-binding sites decrease virus infectivity by impairing the early stages of virus-host interaction and genome release (23, 24). When Ca2+ is removed from the capsids of plant viruses such as cowpea chlorotic mottle virus (CCMV), tomato bushy stunt virus (TBSV), and turnip crinkle virus (TCV) by metal chelators (2, 33, 35), the virion swells by almost 10% and becomes susceptible to RNase. The nature of these transitions indicates that loss of metal ions could induce instability in viral capsids, and the resulting conformational changes might represent a prelude to disassembly.
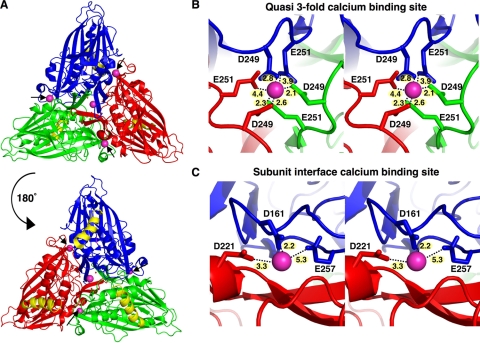
Flock House Virus (FHV), an insect nodavirus, incorporates 240 Ca2+ ions per capsid during assembly (17). FHV infects Drosophila cells in culture (32) and is a valuable model system for understanding nonenveloped virus entry, due to its small size and genetic tractability (4). The FHV capsid is a T=3 icosahedron composed of 180 copies of capsid protein α. The calcium ions are coordinated by electrostatic interaction with the carboxyl side chains of acidic amino acids in protein α (17). Each icosahedral asymmetric unit (iASU)—consisting of three copies of α, designated subunits A, B, and C—binds four calcium ions (Fig. 1A). The calcium ion at the quasi-3-fold axes of symmetry is coordinated by the side chains of aspartate-249 and glutamate-251 from each quasi-equivalent subunit (Fig. 1B). The other three calcium ions are bound at the interface between pairs of subunits and are coordinated by aspartate-221 from one subunit and aspartate-161 and glutamate-257 from the second subunit (Fig. 1C). The capsids of several other members of the family Nodaviridae also incorporate calcium ions. Black beetle virus (BBV) contains conserved acidic residues at identical positions and incorporates 5 calcium ions in each iASU (42). The capsid of Pariacoto virus (PaV) contains one calcium ion per iASU, which is coordinated at the quasi-3-fold axes of symmetry by aspartate-249 and glutamate-251 (36). The presence of conserved residues for metal chelation in the capsid protein indicates that calcium binding is essential for nodaviruses.
FIG. 1.
Calcium-binding sites in Flock House virus. (A) Locations of calcium-binding sites in one icosahedral asymmetric unit (iASU) of the FHV capsid. The A, B, and C subunits in the iASU are colored blue, red, and green, respectively, with the gamma peptides shown in yellow and bound calcium ions colored magenta. The view on the top is from the outside of the virus, while that on the bottom is from the interior of the virus. The positions of the metal ions at the interfaces of subunits are indicated by black arrows. (B) Close-up stereoscopic views of the residues involved in calcium chelation at the quasi-3-fold axes of symmetry (B) and the interface of A and B subunits (C). The distance between each residue and calcium ion is given in angstroms.
FHV enters host Drosophila cells through receptor-mediated endocytosis (26). Disruption of the endosomal membrane, essential for genome release, is carried out by the gamma (γ) peptide. Gamma is a 44-residue, amphipathic, membrane-active peptide usually sequestered inside the FHV capsid (17). It is generated by autoproteolytic cleavage of α during virus maturation (31), and maturation-defective mutants of FHV (FHVMD) are noninfectious (31), demonstrating the requirement for a covalently independent gamma peptide during entry. Interestingly, gamma supplied from noninfectious virus-like particles (VLPs) of FHV can rescue the infectivity of FHVMD, demonstrating that this peptide is the primary component required for membrane disruption during cellular entry of FHV (40). Gamma demonstrates a higher membrane disruption capacity at low pHs in vitro (26), indicating that it is probably externalized in early endosomes. However, not much is known about the structural changes occurring in the capsid prior to disassembly and the release of the genome across the endosomal barrier.
The lowering of pH in the early endosomal compartments is accompanied by an efflux of Ca2+, thus depleting the endosomes of the divalent cations. Both processes are inhibited by vacuolar proton ATPase blockers such as bafilomycin, indicating that they are coupled (20). We envisioned that a combination of low Ca2+ concentration and protonation of acidic side chains in endosomal compartments might predispose the FHV capsid for Ca2+ release, and we designed mutants that would not bind calcium, in order to replicate an entry intermediate in vitro. The calcium-coordinating residues in FHV capsid protein α were mutated, and the altered constructs were expressed as both authentic virus particles and VLPs. Changes in the biological and biochemical behaviors of the altered particles were studied in comparison to those of wild-type FHV. We show that mutations at calcium-binding sites, although not affecting capsid assembly or maturation, specifically affect virus entry and severely compromise infectivity. The data presented here demonstrate that the gamma peptide regions are externalized in the mutant, as is expected in an entry intermediate. Indeed, FHV particles lacking calcium, due either to mutagenesis or to chelation, are less stable than wild-type capsids. Thus, our data show that the inclusion of metal ions provides an essential temporal control for entry-related conformational alterations in the capsid and the initiation of host membrane disruption.
MATERIALS AND METHODS
Cells.
Drosophila melanogaster (Schneider's line 1 or DL-1) was maintained at 27°C in Schneider's insect medium (Sigma) supplemented with 15% fetal bovine serum and antibiotics. Spodoptera frugiperda (line IPLB-Sf21) cells were maintained at 27°C in TC100 medium (Sigma) supplemented with 10% fetal bovine serum and antibiotics.
Mutation in the capsid protein sequence.
cDNA for FHV capsid protein α in plasmid p2BS(+) (31) was used as a template for the generation of mutated sequences. The QuikChange site-directed mutagenesis strategy (Stratagene) was utilized to convert aspartates to asparagines and glutamates to glutamines at the calcium-binding sites. All constructs were sequenced to confirm the incorporation of proper mutations.
Expression of virus.
Wild-type, calcium site mutant, or maturation-defective FHV was produced by transfection of FHV RNA1 and mutated RNA2 into Drosophila DL-1 cells by using Transfectene (Bio-Rad) as described in reference 31.
Expression of VLPs.
Five hundred nanograms of the purified pBacPAK9 vector, containing the mutated α sequence, was used to transfect Spodoptera frugiperda (Sf21) cells, along with baculovirus DNA, using the transfection agent bacfectin (Clontech). Single recombinant baculovirus particles were isolated and identified by plaque picking and Western blotting with an anti-FHV polyclonal antibody. The baculovirus particles were amplified twice, and mutated VLPs were produced by infecting 4.5 × 107 Sf21 cells with recombinant baculovirus at a multiplicity of infection (MOI) of 5, as described elsewhere (18, 29).
Purification of virus and VLPs.
Wild-type or mutated FHV or virus-like particles were purified by pelleting the particles from disrupted cells on a 30% sucrose cushion and subsequently layering them on a 10 to 40% sucrose gradient as described previously (40). Purified virus or VLPs were dialyzed in 50 mM HEPES (pH 7.0), concentrated, and stored at 4°C in the same buffer. Virus and virus-like particles were quantified by using the optical density at 260 nm (OD260) (an OD260 of 4.15, with an OD260-to-OD280 ratio [OD260/280] of between 1.6 and 1.7, represents a virus concentration of 1 mg/ml).
Negative staining of virus particles.
Two microliters of VLPs, in 50 mM HEPES (pH 7.0) and at a concentration of ∼10 mg/ml, was applied to a glow-discharged, carbon-coated copper grid (300 mesh) and allowed to adsorb onto the surface. After removal of excess solution, the grids were washed three times in 50 mM HEPES (pH 7.0) and were then stained with a 2% Nano-W solution (pH 6.8) (Nanoprobes). Grids were air dried and were then viewed with a CM100 transmission electron microscope (EM) (Philips) at 80 keV.
Differential scanning calorimetry.
For calorimetry, virus or virus-like particles at a concentration ranging from 0.2 to 0.4 mg/ml, in 50 mM HEPES (pH 7.0), were used. EGTA-treated FHV was prepared by dialyzing wild-type FHV in a 50 mM HEPES (pH 7.0)-5 mM EGTA solution overnight at 4°C. Calorimetry was carried out in a MicroCal VP-DSC (differential scanning calorimetry) calorimeter containing a 0.6-ml cell and operating at a scan rate of 1.5°/min. The reference cell contained 50 mM HEPES buffer with or without 5 mM EGTA, as appropriate. The reference scans (buffer versus buffer) were subtracted from the sample scans (sample versus buffer) for each set. A temperature scan from 10°C to 110°C was carried out for each sample to determine the temperatures of endothermic transition.
Plaque assay.
The infectivity titers of wild-type FHV and calcium site mutants were detected by counting plaques formed by the particles on monolayers of Drosophila DL-1 cells, as described elsewhere (31). The assay was repeated three times, in triplicate, with similar results, and the values shown in Table 1 each represent the average of triplicates from one assay.
TABLE 1.
Infectivity titers of wild-type and calcium-site-mutated FHV
| FHV variant | Infectivity (PFU/mg)a |
|---|---|
| Wild type | 3.1 × 1010 ± 0.41 × 1010 |
| FHVCM1 | 1.9 × 108 ± 0.28 × 108* |
| FHVCM2 | 1.6 × 106 ± 0.25 × 106* |
| FHVCM3 | 0.56 × 106 ± 0.04 × 106* |
An asterisk indicates a plaque size smaller than that of the wild type.
Flow cytometry.
A total of 2 × 106 Drosophila DL-1 cells were collected, centrifuged at 500 × g for 5 min, and resuspended in ice-cold serum- and antibiotic-free medium (SFM). Wild-type or calcium-site-mutated VLPs (1 × 105/cell) were allowed to bind to the cells at 4°C for 1 h. Cells were washed three times in SFM before being fixed with 2% paraformaldehyde in SFM. Then cells were sequentially incubated with a solution of 5% bovine serum albumin (BSA) in SFM, a primary polyclonal antibody against FHV, and a secondary antibody conjugated with Alexa 488 (Molecular Probes). Control cells were incubated with BSA only, followed by primary and secondary antibody incubations. All incubations were carried out at 4°C, and cells were washed three times with ice-cold SFM between incubations. Fluorescence-activated cell sorter (FACS) analysis was carried out on a FACSCalibur flow cytometer (Becton Dickinson) with cells gated against forward and side scatter. Data analysis was carried out with CellQuestPro software.
Gamma trans-complementation assay.
Drosophila DL-1 cells (1 × 108) were coinfected with FHVMD (1.5 × 103 particles/cell) and VLPs (9 × 103 particles/cell), and the progeny virus was labeled with 35S, purified, and quantified as described elsewhere (40).
Liposome disruption assay.
Dioleoyl phosphatidylcholine (DOPC) liposomes containing sulforhodamine B (Molecular Probes) were prepared as described previously (26). The quantity of liposomes used in disruption assays was determined empirically for each assay; the amount that produced the maximum signal-to-noise ratio within the range of the instrument (usually 1 to 2 μl) was used. Assays under different pH conditions were carried out with liposomes in different pH buffers. Fluorescence measurements were carried out at excitation/emission maxima of 535/585 nm for sulforhodamine B on a Cary Eclipse fluorescence spectrophotometer (Varian). One hundred percent rupture of dye-containing liposomes was obtained by adding Triton X-100 to the liposomes at a final concentration of 0.1%.
Partial protease digestion and mass spectrometry (MS).
Proteolysis reactions were conducted at 25°C with FHV particles at 0.5 mg/ml in 50 mM HEPES, pH 7.3. Aliquots from reaction products were diluted 5-fold and were spotted directly onto the matrix-assisted laser desorption ionization (MALDI) analysis plate. Protease digestion was stopped by the addition of either alpha-cyano-4-hydroxycinnamic acid or 3,5-dimethoxy-4-hydroxycinnamic acid in a solution of 50% acetonitrile with 0.05% trifluoroacetic acid. Spots were allowed to air dry before analysis. Calibration was performed using bradykinin and insulin peptide standards. Spectra were collected on a Biflex III MALDI-time-of-flight (TOF) mass spectrophotometer (Bruker Daltonics, Billerica, MA). Reactions with the proteases trypsin and Glu-C were conducted with purified virus particles at enzyme-to-virus ratios from 1:100 to 1:4,000 (wt/wt). The result from Glu-C confirmed that wild-type FHV protein capsids are less dynamic than the mutant forms.
Crystallization, data collection, and structure determination.
Purified FHVCM3 VLPs (Fig. 2A) were concentrated to 10 mg/ml in an Amicon filter with a 100,000-Da cutoff (Millipore). Crystals were obtained by the sitting drop vapor diffusion method at room temperature (20°C) in a 1:1 solution (1 μl each) of protein to mother liquor. The mother liquor consisted of 100 mM sodium citrate (pH 4.0), 4% polyethylene glycol 8000 (PEG 8000), and 100 mM lithium sulfate. Crystals typically grew over a 2- to 3-week time frame. The crystals were cryoprotected sequentially in mother liquor containing 10%, 20%, and 30% glycerol and were flash frozen in liquid nitrogen. Diffraction data extending to 3.2 Å resolution were collected from one frozen crystal at the Advanced Photon Source (APS) synchrotron at Argonne National Laboratories, beamline 14-BMC. Data from 150 frames were indexed, integrated, and scaled using the HKL2000 package (27). The crystals belong to space group C2, with four particles per unit cell and one particle in each crystallographic asymmetric unit, defining a 60-fold noncrystallographic symmetry (NCS). The 5-fold self-rotation function was calculated using the GLRF program (37, 38), and the orientation of particles in the unit cell was further refined using a 1-dimensional (1D) locked rotation function search. All four particles in the unit cell have identical orientations (90°, 90°, 40.2°). The positions of particles in the unit cell were determined (0.2450, 0, 0.2500) using a combined translation rotation script, with wild-type FHV as the search model.
FIG. 2.
Calcium site mutations do not affect the assembly and maturation of FHV. (A) Amino acid changes in three calcium site mutants (expressed as virus or virus-like particles) and the maturation-defective mutant (expressed as virus) of FHV. (B) SDS-PAGE of purified virus particles produced in Drosophila DL-1 cells transfected with FHV RNA1 (which codes for FHV polymerase) along with either wild-type RNA2 (which codes for capsid protein α) or altered RNA2 corresponding to FHVCM1, FHVCM2, FHVCM3, or FHVMD. Each lane represents an equivalent volume of purified particles, and the positions of α and the maturation cleavage product β are indicated. Virus particles were purified from cells 36 h posttransfection; thus, they were produced from multiple cycles of replication. (C) SDS-PAGE of purified virus-like particles (VLPs) containing either the wild-type or a calcium-site-mutated capsid protein sequence. Each lane represents an equivalent volume of purified protein, and the positions of α and β are indicated. (D) Electron micrographs of wild-type and FHVCM3 VLPs, stained with 2% Nano-W.
Initial phases obtained from a polyalanine model of the wild-type FHV particle were combined with 60-fold NCS averaging and solvent flattening routines with the CCP4 and RAVE packages (12, 22) to generate an averaged electron density map for FHVCM3 VLPs. Most residues fit well in the density, except for the gamma helices (residues 364 to 381) in the A and B subunits and the N arms in the A subunit. No density corresponding to calcium was detected, although nearby sulfate ions were still present.
Crystallographic refinement.
The residues corresponding to areas of disordered density were removed from the model, and the residues mutated in FHVCM3 VLPs were altered in the model in order to achieve a better fit in the density. Real space refinement was carried out manually using the Coot graphics program (15), followed by positional B-factor refinement in the Crystallography & NMR System (CNS) (9). Cross-validation is not reported, since the test reflections are related to working reflections by the 60-fold noncrystallographic symmetry (16).
Protein structure accession numbers.
The coordinates for the FHVCM3 VLP crystal structure have been submitted to the Protein Data Bank (PDB ID 3LOB, RCSB ID RCSB057525).
RESULTS
Calcium site mutations do not affect the assembly or maturation of FHV.
Three separate sets of mutations were introduced into FHV capsid protein α (Fig. 2A). In the first set, aspartate-249 and glutamate-251, which coordinate calcium at the quasi-3-fold axes of symmetry (Fig. 1B), were changed to asparagine and glutamine (construct designated FHVCM1) (Fig. 2A). In the second construct, residues chelating calcium ions at the subunit interfaces (aspartate-161, aspartate-221, and glutamate-257) (Fig. 1C) were changed to corresponding asparagines and glutamines (FHVCM2) (Fig. 2A). A third construct, containing both sets of mutations, was produced (FHVCM3) (Fig. 2A).
The cDNA for the three FHV constructs was utilized for in vitro synthesis of mutated positive-sense RNA2 corresponding to FHV capsid protein α, and these RNA2 mutants were transfected into Drosophila DL-1 cells along with the viral RNA1, as described previously (31). Transfection with positive-sense RNA imposes one round of translation resulting in the production of virus capsid protein and assembled particles, and the newly synthesized particles, if infectious, can subsequently infect more cells to produce progeny virus. When DL-1 cells were transfected as described, and authentic virus particles were purified 36 h posttransfection, FHVCM1 was produced in substantial amounts, whereas only small quantities of FHVCM2 and FHVCM3 could be obtained (Fig. 2B). The low yields of FHVCM2 and FHVCM3 particles could be due to an assembly defect or to the reduced infectivity of progeny virions. The latter occurs with maturation-defective FHV (FHVMD) and results in low yields (Fig. 2B). Maturation cleavage was normal in all of the calcium-site-mutated particles, since the capsid protein migrated as both α and β on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Fig. 2B).
All three constructs were also produced as virus-like particles (VLPs) in Sf21 cells, by expressing the mutated capsid proteins from a baculovirus vector (29). VLPs encapsidate insect cell RNA instead of viral genomic RNA and are therefore noninfectious; however, their structures are very similar to those of authentic virions. FHVCM1, FHVCM2, and FHVCM3 VLPs could be purified in quantities equivalent to those of wild-type VLPs, indicating that they were stable (Fig. 2C). They also underwent normal maturation cleavage (Fig. 2C) and displayed no differences from wild-type particles upon negative-stain EM (an example is shown in Fig. 2D). The yield of mutated VLPs in the mature form demonstrated that metal ion binding is not absolutely essential for assembly or maturation.
Calcium site mutations affect the thermal stability of VLPs.
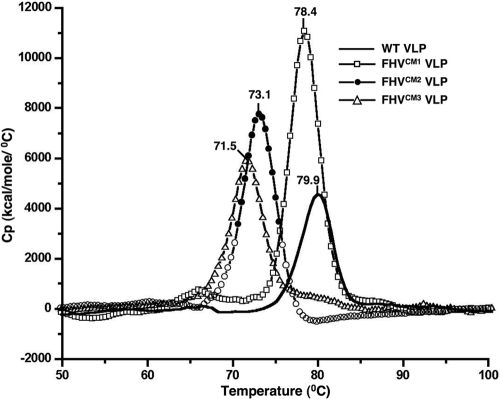
Differential scanning calorimetry (DSC) has been successfully utilized to monitor thermally induced changes in the capsid architecture of bacteriophages (13). This technique, which measures the heat capacity of a system as a function of temperature, was used to scrutinize the heat-induced structural changes in wild-type and mutated VLPs. Upon heating of wild-type VLPs in a scanning calorimeter, a single peak, indicating a major structural transition, was observed at 79.9°C (Fig. 3). The mutated VLPs demonstrated a similar degradation profile, with a single prominent thermal transition. However, while the structural transitions of wild-type and FHVCM1 VLPs occurred at similar temperature ranges, indicating comparable stability, the FHVCM2 and FHVCM3 VLPs showed a substantial loss in stability. From our analysis, mutations at the subunit interfaces decrease the thermal stability of VLPs by ∼8°C (Fig. 3).
FIG. 3.
Calcium site mutations affect the stability of VLPs. Results of differential scanning calorimetry of wild-type (solid line), FHVCM1 (open squares), FHVCM2 (filled circles), and FHVCM3 (open triangles) VLPs, with each VLP at a concentration between 0.2 and 0.4 mg/ml in 50 mM HEPES (pH 7.0), are shown. The Cp (kilocalories of heat absorbed per mole per °C) of the sample is plotted as a function of temperature. The curves for the buffer solution only were subtracted from the sample curves in each case. The temperatures for the major endothermal transitions are given. The traces are representative data from three separate experiments.
The stability of authentic FHV decreases upon Ca2+ removal.
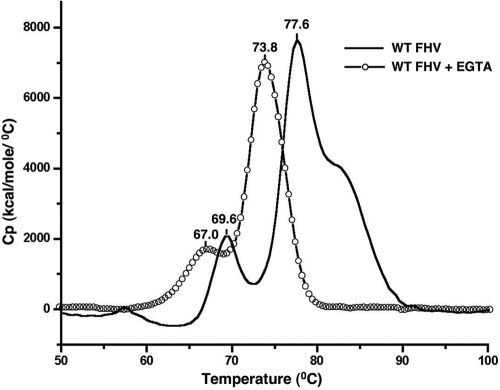
DSC of wild-type virions (Fig. 4) showed an obvious difference from that of wild-type VLPs (Fig. 3). Thermal treatment of virions produced two prominent peaks, at 69.6°C and 78°C (Fig. 4); the latter was very similar to the single structural transition observed in VLPs (Fig. 3). A third, partial transition in authentic particles was detected close to 83°C. The minor peak at 69.9°C and the partial peak at 83°C, reproducibly present in virion profiles, are absent in VLPs, suggesting a possible role for genomic RNA in these transitions. The decrease in stability induced in VLPs by the calcium site mutations could be replicated in authentic FHV virions by dialyzing them in a buffer containing the metal chelator EGTA at 5 mM. The major thermal transitions of EGTA-treated virions shifted to 67.0°C and 73.8°C, respectively, although the overall pattern of the transitions remained similar (Fig. 4). We confirmed, using atomic absorption spectroscopy, that the EGTA-treated FHV particles contained much less bound calcium (<10 ions/particle) than untreated FHV (∼240 ions/particle) and that the EGTA treatment of FHV does not affect particle integrity, since no major structural changes (41) were visible upon negative-stain electron microscopy (data not shown). This indicates that the change in the stability of the particles upon EGTA treatment is likely due to the loss of calcium, which, however, does not cause the capsid to disassemble.
FIG. 4.
Calcium removal decreases the stability of the FHV capsid. Results of differential scanning calorimetry of wild-type FHV in 50 mM HEPES (pH 7.0) (solid line) and wild-type FHV dialyzed against a 50 mM HEPES-5 mM EGTA solution (open circles), both at a concentration of 0.3 mg/ml, are shown. The traces are representative data from three separate experiments.
Calcium site mutations decrease the infectivity of FHV.
FHVCM1, FHVCM2, and FHVCM3, as authentic virions, were purified from Drosophila DL-1 cells (Fig. 2B), and their infectivities were quantified using a plaque assay (31). The PFU per milligram of FHVCM1 was 10−2 times that of the wild-type virions, while FHVCM2 and FHVCM3 produced 10−4 times the number of plaques that the wild type produced (Table 1). Plaques produced by the mutated virions were smaller in diameter than those produced by wild-type virions. This drastic decrease in infectivity cannot be attributed only to the decreased capsid stability of the mutants, since FHVCM1, which as a VLP showed stability equivalent to that of the wild type (Fig. 2A and 3), was 100-fold less infectious than wild-type virions (Table 1).
Calcium-site-mutated VLPs cannot rescue the infectivity of maturation-defective FHV.
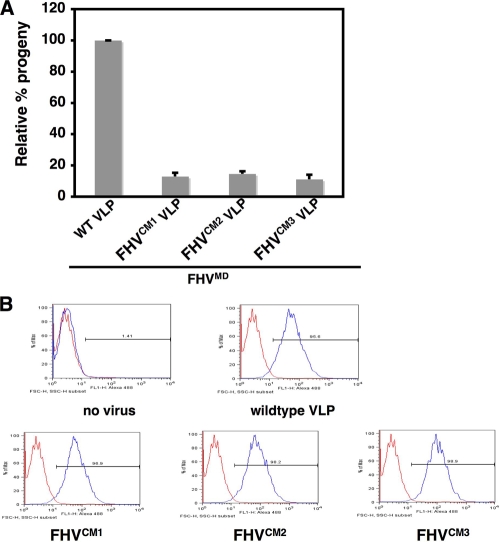
Previous studies have demonstrated that mature wild-type VLPs can rescue the infectivity of noninfectious, maturation-defective FHV (FHVMD), and the extent of rescue can be quantified by specifically labeling the progeny virus produced with 35S (40). The presence of cleaved, full-length gamma peptide in VLPs is essential for rescue (5, 40). The calcium-site-mutated VLPs, although they contained full-length cleaved gamma peptides, could not rescue the infectivity of FHVMD (Fig. 5A).
FIG. 5.
Calcium-site-mutated VLPs bind to DL-1 cells but cannot rescue the infectivity of maturation-defective FHV. (A) Progeny virus produced by coinfecting 1 × 108 Drosophila DL-1 cells with 1.5 × 103 particles/cell of D75N/N363T FHV and 9 × 103 particles/cell of wild-type VLPs or each of the mutated VLPs. [35S]methionine-cysteine-labeled progeny virus was quantified, with the amount of progeny produced during coinfection with D75N/N363T FHV and wild-type VLPs normalized at 100%. (B) FACS analysis of the binding of wild-type or mutated VLPs to 1 × 106 Drosophila DL-1 cells for 1 h at 4°C. Bound particles were detected using a rabbit polyclonal primary antibody against FHV capsid protein and a goat anti-rabbit secondary antibody conjugated with Alexa 488 (Molecular Probes). Five percent BSA was added to cells as a negative control, followed by antibody incubations. Data shown are representative of two separate experiments.
We wanted to ensure that the calcium site mutations in the VLPs did not inhibit their binding to DL-1 cells and therefore prevent rescue at this stage. The extent of binding of mutated VLPs to host cells was determined by labeling the cell-associated capsid protein with a polyclonal antibody conjugated with Alexa 488 and quantifying the fluorescent cells using flow cytometry. Wild-type and mutated VLPs displayed virtually equivalent binding to cells (Fig. 5B), indicating that a different step in virus entry is compromised in the mutated VLPs.
The gamma peptide is more exposed in calcium site mutants.
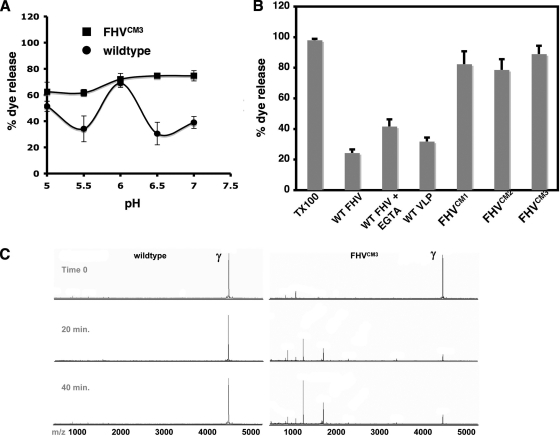
We next determined if calcium-site-mutated VLPs were able to disrupt membranes. The capacity of FHVCM3 VLPs to rupture artificial liposomes and release packaged fluorescent dye (sulforhodamine B) was quantified at pHs ranging from 5.0 to 7.0. At pH 6.0, after a 15-min incubation, FHVCM3 disrupted liposomes comparably to wild-type FHV (Fig. 6A). But while the capacity of wild-type VLPs to disrupt membranes decreased dramatically at a neutral pH, FHVCM3 VLPs maintained comparable membrane activities at all pHs tested (Fig. 6A). Membrane disruption by FHV particles is due to the activity of the gamma peptide, which is primarily sequestered inside the capsid at a neutral pH, with only occasional externalization (7, 26). Gamma becomes fully exposed in the low-pH environment inside early endosomes, which correlates with increased membrane disruption demonstrated by wild-type FHV at pH 6.0 in vitro (5, 26). The pattern of membrane disruption by FHVCM3 VLPs indicates that the exposure of gamma in this particle is no longer controlled by pH conditions. FHVCM1 and FHVCM2 VLPs contain similarly exposed gamma, as evidenced by the fact that they disrupted liposomes as efficiently as FHVCM3 VLPs at a neutral pH (Fig. 6B).
FIG. 6.
The gamma peptide is more exposed in calcium site mutants. (A) Relative amounts of sulforhodamine B released from DOPC liposomes after 15 min of incubation with 6.37 × 1011 particles of wild-type FHV (circles) or FHVCM3 (squares) VLPs, at different pHs. The level of fluorescence dequenching achieved upon the addition of 0.1% Triton X-100 (TX100) to DOPC liposomes is considered 100%. (B) Relative amount of sulforhodamine B released from liposomes after 1 h of incubation with 6.37 × 1011 particles of each virus or VLP as indicated. The dye release by 0.1% TX100 from DOPC liposomes is considered 100%. (C) MALDI-TOF mass analysis of a time course proteolysis reaction comparing wild-type and FHVCM3 VLPs. Peptides are released more rapidly from the mutant particles (series on the right), indicating that they are more dynamic. In this reaction the enzyme-to-FHV protein ratio was 1:4,000 (wt/wt). Reactions using more enzyme with the wild-type particles confirmed that the cleavage pattern was the same.
Wild-type virions, upon EGTA treatment, demonstrated a somewhat increased capacity for liposome disruption at pH 7.0 (Fig. 6B), and wild-type VLPs behaved in a similar manner (data not shown). This indicates that the removal of calcium ions from the FHV capsid, either by treatment with metal chelators or by the introduction of mutations at calcium-binding sites, leads to a greater exposure of gamma peptides at a neutral pH. Mutations at the calcium-binding sites have a quantitatively greater effect than EGTA treatment (Fig. 6B), possibly due to the prevalence of divalent metal ions in the buffers and lipids utilized in the assay.
The presence of a more dynamic capsid and gamma peptide in the calcium-site-mutated particles was also demonstrated by time-resolved, limited protease digestion and mass spectrometry (Fig. 6C). This method can identify the protease-accessible and dynamic regions of virus capsids (7, 8). Wild-type and FHVCM3 VLPs were treated with trypsin, and the proteolytic fragments were analyzed using MALDI-MS at specific time points. Gamma peptide was the only significant ion detected in the low-mass region of either sample before the addition of protease (Fig. 6C). After the addition of trypsin, peptide fragments were detected only in reaction mixtures with FHVCM3 VLPs. After continued proteolysis for 20 to 40 min, gamma fragments were barely detectable in FHVCM3, although they were still present in the trypsin digestion product of wild-type VLPs (Fig. 6C). This indicates that the protein capsid of the mutant is more dynamic than that of wild-type FHV.
Calcium site mutations do not affect the overall fold of FHV but do alter the conformation of gamma.
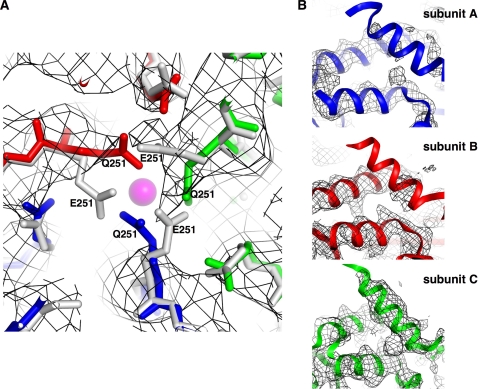
FHVCM3 VLPs were crystallized, and the structure was determined to a resolution of 3.6 Å using molecular replacement. A polyalanine model of the wild-type FHV X-ray structure (17) (PDB ID 2z2q) served as an initial phasing model for structure determination (crystallography and refinement statistics are presented in Table 2). A comparison of the wild-type and FHVCM3 structures indicated that the overall capsid architectures are virtually identical. No ordered density corresponding to calcium ions is detectable in the refined map for the mutant, although density corresponding to sulfate ions is present at the quasi-3-fold axes of symmetry and at the interfaces between subunits, as in wild-type FHV. Closer inspection revealed that the density corresponding to some of the mutated calcium-coordinating residues had shifted away from the metal-binding sites (Fig. 7A). The map for FHVCM3 contained some disordered areas that are well ordered in wild-type FHV. Density corresponding to the gamma amphipathic helix (residues 364 to 381) is highly disordered in subunits A and B of FHVCM3 (Fig. 7B). Only 3 to 4 residues out of 21 could be fitted in the density based on the known positions of these residues in the wild-type structure; otherwise, the density for the A- and B-subunit gamma peptides was not interpretable. The density corresponding to the C-subunit gamma allowed fitting of the main chain and side chain residues (Fig. 7B). However, the temperature factors corresponding to the C-subunit gamma residues are up to 1.7 times higher than the capsid protein average, indicating that this region is also partially disordered, but not to the extent seen in the A and B subunits. This overall lack of order in the gamma peptides correlates with the greater dynamic character demonstrated in the biochemical experiments (Fig. 6A to C).
TABLE 2.
Crystallographic statistics
| Statistica | Valueb |
|---|---|
| Data processing | |
| Space group | C2 |
| Unit cell | a = 477.1 Å, b = 404.9 Å, c = 476.2 Å |
| β = 90.6° | |
| Resolution range (Å) | 40-3.6 (3.73-3.6) |
| Measured reflections | 474,211 |
| Unique reflections | 372,968 |
| Completeness (%) | 35.9 (17.5) |
| Rmerge (%) | 10.3 (29.0) |
| 〈I/σ(I)〉 | 5.73 (1.37) |
| Multiplicity | 1.3 (1.0) |
| CC(ave) (%) | 78.8 |
| Rave (%) | 44.6 |
| Refinement | |
| Noncrystallographic symmetry | 60 |
| Rcryst (%) | 34.7 (54.5) |
| RMS deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.49 |
| Average B factor (Å2) | |
| Entire capsid | 182.7 |
| Capsid proteins (β) | 178.1 |
| Gamma peptides | 237.7 |
| RNA | 331.7 |
| Ramachandran plot (%) | |
| Favored | 95.5 |
| Allowed | 4.2 |
| Outliers | 0.3 |
Rmerge, (Σh Σi(Ihi − <Ih>)/Σh Σi Ih) × 100, where <Ih> is the mean of the Ihi observations of reflection h; <I/σ(I)>, average signal-to-noise ratio of measured intensities (I); CC(ave), Σ((|Fo| − <|Fo|>)(|Fc| − <|Fc|>)/(Σ(|Fo| − <Fo>)2 Σ(|Fc| − <|Fc|>)2)1/2; Rcryst, (Σh|Fo − Fc|/Σh Fo), where Fo and Fc are the observed and calculated structure factors, respectively; Rave, same as Rcryst except that Fc is calculated from a 60-fold NCS-averaged electron density map; RMS, root mean square.
Values in parentheses are for the outer shell resolution bin (3.73 to 3.6).
FIG. 7.
Crystal structure of the FHVCM3 VLP. (A) Density corresponding to the calcium-binding site at the quasi-3-fold axes of the FHVCM3 VLP (dark gray mesh), with the crystal structures for FHVCM3 VLP and wild-type FHV (including the calcium ion) built in. The A, B, and C subunits of the FHVCM3 VLP are shown in blue, red, and green, respectively. The subunits of wild-type FHV are in gray, and the calcium ion present in the quasi-3-fold axes of wild-type FHV is shown as a magenta sphere. (B) Density corresponding to the amphipathic gamma helices (residues 364 to 381) in subunits A, B, and C of the FHVCM3 VLP, with the wild-type FHV crystal structure built in. The A, B, and C subunits of wild-type FHV are shown in blue, red, and green, respectively. Density for the gamma peptides is mostly disordered in subunits A and B of the FHVCM3 VLP.
DISCUSSION
The importance of capsid-bound divalent cations has been studied in nonenveloped viruses with host specificities ranging from plants to insects and animals (23, 35, 43). Here we designed a calcium-free version of FHV to mimic the structural changes expected to occur in the virus capsid in the endosomal compartments during cellular entry. Calcium-site mutated particles were produced as authentic virions as well as virus-like particles (VLPs). The expression of VLPs was essential, since poorly infectious virions could not be produced in sufficient quantity for biophysical studies. The mutated VLPs were utilized as surrogates for obtaining a mechanistic explanation of the biological behavior displayed by the corresponding authentic virions.
We found that mutations at the calcium-binding sites drastically reduce FHV infectivity by specifically affecting host membrane disruption during virus entry. The membrane-active region of FHV, the gamma peptide, is in an altered structural state in mutated particles, which inhibits its biological activity. The pattern of membrane disruption by FHVCM1, FHVCM2, and FHVCM3 VLPs in vitro clearly indicates that the gamma peptides in these particles are exposed in a pH-independent manner. Further evidence for this comes from limited proteolysis and MALDI-MS, where the accessibility of gamma to trypsin at a neutral pH is higher in FHVCM3 VLPs than in the wild type. In wild-type particles, gamma is sequestered inside the capsid at a neutral pH, with occasional exposure for “sampling” the environment (7, 8). Full exposure of gamma, which imposes a temporal control on the initiation of cellular membrane disruption during virus entry, is expected to be attained inside early endosomes during entry (26). Premature release of the lytic peptides from calcium mutants, probably at the plasma membrane, diminishes the quantity of peptides available for interacting with endosomal membranes. The inability of mutated VLPs to rescue the infectivity of FHVMD underscores the altered biological behavior of gamma specifically during entry. From our previous studies, it is known that rescue occurs during the early stages of the virus-host interaction (5). We show here that the initial step—binding to host Drosophila cells—remains unaltered in the mutated VLPs. Rescue of the infectivity of FHVMD is dependent on the supply of wild-type, cleaved gamma from VLPs (5), indicating that the chief role of VLPs during rescue is membrane disruption. The altered pattern of membrane disruption by mutated VLPs in vitro probably reflects poor endosomal membrane disruption in vivo. The reduced infectivity of virions with the same mutations can be similarly attributed to the ineffectiveness of gamma in promoting endosomal membrane disruption during entry.
A crystal structure of FHVCM3 VLP at 3.6 Å provided supporting evidence for the increased exposure and altered behavior of gamma. Although the overall fold and organization of the FHVCM3 capsid were very similar to those of wild-type particles, there were differences in the gamma region. The refined map for the mutant contained very little ordered density corresponding to gamma in two out of three subunits in the capsid. This disorder correlates well with the biochemical evidence demonstrating that gamma is more exposed in these particles. The N arms (residues 21 to 31 of capsid protein α) of the A subunits, which are ordered in wild-type FHV and, along with ordered duplex RNA, form the flat contacts at the icosahedral 2-fold axes of the capsid (17), are also somewhat disordered in the mutant (data not shown). It is possible that lack of metal binding causes a “loosening” of the capsid, leading to disorder in the more-flexible regions of the capsid protein, such as the gamma peptides and the N arms, although the cores of the subunits remain well ordered. A recent cryo-EM reconstruction of calcium-site-mutated particles of simian virus 40 (SV40) also demonstrates subtle changes from the native form, with the overall structures being very similar (21). This mutation at the metal-binding site of SV40 capsid protein produces a similar pattern of slight adjustments in structure accompanied by a major change in infectivity. However, it is possible that a degree of charge repulsion between the asparagine and glutamine residues introduced at the metal-binding sites could be responsible for introducing some additional instability into the overall structure and behavior of FHVCM3, apart from its lack of metal binding.
Calcium site mutations also affected the stability of FHV particles—with the thermally induced structural transitions in FHVCM2 and FHVCM3 VLPs shifting to lower temperatures than those for wild-type VLPs. The major thermal transition in wild-type virions also shifted to a lower temperature upon EGTA treatment, as in FHVCM2 and FHVCM3 VLPs. Indeed, the decreased stability and increased dynamic character of the FHVCM3 VLPs are reflected in an overall high average temperature factor for the crystal structure. In addition to the N arms and the gamma peptides, the packaged cellular RNA is also partially disordered in the FHVCM3 VLPs and becomes visible only at low contour levels relative to the wild-type particle (data not shown). The decreased stability and disorder indicates that during virus entry, release of metal ions from the capsid could represent an essential first step in FHV disassembly. In the endosomes, a combination of low pH and low Ca2+ concentrations (28) could result in the protonation of the acidic side chains coordinating the divalent cations, lowering their binding affinity for the cations and promoting the release of calcium ions from the capsid. This could lead to a global increase in the dynamic character and instability of the capsid, accompanied by the externalization of the flexible regions of the capsid, including the gamma peptides. Thus, removal of metal ions could provide the trigger for conformational changes in the capsid, leading to the initiation of membrane penetration, as well as the stepwise disassembly of the particles and release of the genome. A line of evidence including the increase in the membrane disruption activity of EGTA-treated FHV and its reduced stability, as well as the biochemical behavior of FHVCM3 VLPs, supports this theory. The FHVCM3 VLPs thus structurally and functionally mimic a crucial intermediate in the entry pathway of FHV.
The behavior of mutated virions and VLPs can be used to distinguish the contributions of separate calcium-binding sites to FHV infectivity and stability. Mutations at the quasi-3-fold axes of symmetry did not produce any changes in the stability of VLPs within the range of experimental error, but the infectivity of the corresponding virions (FHVCM1) was decreased 100-fold from that of the wild type. The acidic residues forming this metal-binding site are conserved in most members of the family Nodaviridae, and it is possible that metal binding at the quasi-3-fold axes is essential for RNA release and virus infectivity, as is predicted for T=3 icosahedral plant viruses, such as CCMV (34, 35). The metal-binding sites at subunit interfaces contribute both to capsid stability and to virus infectivity. The FHVCM2 and FHVCM3 virions are 100-fold less infectious than FHVCM1 particles, and it can be argued that in these particles, decreased capsid stability further decreases virus infectivity.
Surprisingly, calcium site mutations did not prevent the assembly or maturation of FHV capsids. The yield of mature mutated VLPs was similar to that of mature wild-type VLPs. It is possible that the strong interaction of the highly basic N termini of the FHV capsid protein (25) with the phosphate backbone of RNA can compensate for the stability conferred by metal ion binding at the assembly stage. Removal of metal ions by treatment with chelators did not alter the overall morphology of the authentic virions. This distinguishes alphanodaviruses, such as FHV, from the closely related betanodaviruses, which can be disassembled by treatment with metal chelators (43). Our result also differs from that of a previous study where FHV particles purified in the presence of EDTA were found to be noninfectious provirions that had not undergone maturation cleavage (30). It was previously speculated that calcium binding might be necessary for stabilizing and tightening the capsid and for bringing a catalytic residue (aspartate-75) proximal to the site of cleavage in capsid protein α, allowing maturation cleavage to proceed (44). It is possible that an electrostatic charge repulsion among the metal-coordinating acidic side chains leads to a prominent conformational change in EDTA-treated FHV, which inhibits autoproteolytic cleavage during maturation. This conformational alteration may not be completely replicated in capsids with acidic-to-neutral amino acid mutations at the calcium-binding sites. The crystal structure of FHVCM3 has no discernible gaps, and its overall fold and quaternary structure are indistinguishable from those of wild-type FHV. EGTA- or EDTA-treated FHV is morphologically similar to FHVCM3 capsids upon negative-stain electron microscopy (data not shown), but there may be differences at higher resolutions.
Capsid assembly and disassembly are unique processes specific for individual virus types, but more often than not, common themes can be detected across families. The requirement for metal ions during the assembly of several nonenveloped viruses is well established; however, we show that metal binding could also be essential during virus entry. The membrane-disrupting peptides required for entry are usually sequestered inside the capsid, until low pH conditions or receptor binding alters the conformation of the particle, allowing the buried regions to come into contact with cellular membranes (4). The spatial and temporal control is essential for virus infectivity, since premature membrane interaction can impede entry. Our data show that the inclusion of metal ions in the FHV capsid is essential for correct temporal exposure of membrane-interacting peptides, which in turn is required for virus infectivity. There is also evidence for metal ion-dependent structural alterations in biological systems apart from virus capsids. It was recently demonstrated that the removal of calcium ions from the LR5 repeat of the low-density lipoprotein (LDL) receptor in the endosomes is crucial for the unfolding of the repeat and the release of LDL from its receptor (3). It is possible that the inclusion of metal ions in virus capsids during assembly provides the necessary structural elasticity for stepwise exposure of the membrane-lytic peptides and disassembly of the capsid, once the ions have been removed.
Acknowledgments
This work was supported by NIH grant 4R37-GM34220 to J.E.J. B.B. and P.P.A. were supported by funding from the Center for BioInspired Nanomaterials (ONR N000140710645) and the NSF NIRT program (CBET 0709358).
We thank Amy L. Odegard for technical help with FACS analysis, Duane Prasuhn, Jr., and M. G. Finn for calcium quantification studies, and Steven Edgcomb and Jamie R. Williamson for access to the scanning calorimeter.
Footnotes
Published ahead of print on 17 February 2010.
REFERENCES
- 1.Adamec, T., Z. Palkova, K. Velkova, J. Stokrova, and J. Forstova. 2005. Point mutation in calcium-binding domain of mouse polyomavirus VP1 protein does not prevent virus-like particle formation, but changes VP1 interactions with Saccharomyces cerevisiae cell structures. FEMS Yeast Res. 5:331-340. [DOI] [PubMed] [Google Scholar]
- 2.Aramayo, R., C. Merigoux, E. Larquet, P. Bron, J. Perez, C. Dumas, P. Vachette, and N. Boisset. 2005. Divalent ion-dependent swelling of tomato bushy stunt virus: a multi-approach study. Biochim. Biophys. Acta 1724:345-354. [DOI] [PubMed] [Google Scholar]
- 3.Arias-Moreno, X., A. Velazquez-Campoy, J. C. Rodriguez, M. Pocovi, and J. Sancho. 2008. Mechanism of low density lipoprotein (LDL) release in the endosome: implications of the stability and Ca2+ affinity of the fifth binding module of the LDL receptor. J. Biol. Chem. 283:22670-22679. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, M., and J. E. Johnson. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept. Sci. 9:16-27. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee, M., R. Khayat, H. E. Walukiewicz, A. L. Odegard, A. Schneemann, and J. E. Johnson. 2009. Dissecting the functional domains of a nonenveloped virus membrane penetration peptide. J. Virol. 83:6929-6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothner, B., X. F. Dong, L. Bibbs, J. E. Johnson, and G. Siuzdak. 1998. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 273:673-676. [DOI] [PubMed] [Google Scholar]
- 8.Bothner, B., A. Schneemann, D. Marshall, V. Reddy, J. E. Johnson, and G. Siuzdak. 1999. Crystallographically identical virus capsids display different properties in solution. Nat. Struct. Biol. 6:114-116. [DOI] [PubMed] [Google Scholar]
- 9.Brünger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54:905-921. [DOI] [PubMed] [Google Scholar]
- 10.Bubeck, D., D. J. Filman, N. Cheng, A. C. Steven, J. M. Hogle, and D. M. Belnap. 2005. The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. J. Virol. 79:7745-7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran, K., and M. L. Nibert. 2003. Animal cell invasion by a large nonenveloped virus: reovirus delivers the goods. Trends Microbiol. 11:374-382. [DOI] [PubMed] [Google Scholar]
- 12.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50:760-763. [DOI] [PubMed] [Google Scholar]
- 13.Conway, J. F., N. Cheng, P. D. Ross, R. W. Hendrix, R. L. Duda, and A. C. Steven. 2007. A thermally induced phase transition in a viral capsid transforms the hexamers, leaving the pentamers unchanged. J. Struct. Biol. 158:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dryden, K. A., G. Wang, M. Yeager, M. L. Nibert, K. M. Coombs, D. B. Furlong, B. N. Fields, and T. S. Baker. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122:1023-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emsley, P., and K. Cowtan. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60:2126-2132. [DOI] [PubMed] [Google Scholar]
- 16.Fabiola, F., A. Korostelev, and M. S. Chapman. 2006. Bias in cross-validated free R factors: mitigation of the effects of non-crystallographic symmetry. Acta Crystallogr. D Biol. Crystallogr. 62:227-238. [DOI] [PubMed] [Google Scholar]
- 17.Fisher, A. J., and J. E. Johnson. 1993. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature 361:176-179. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, A. J., B. R. McKinney, A. Schneemann, R. R. Rueckert, and J. E. Johnson. 1993. Crystallization of viruslike particles assembled from Flock House virus coat protein expressed in a baculovirus system. J. Virol. 67:2950-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 64:1934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerasimenko, J. V., A. V. Tepikin, O. H. Petersen, and O. V. Gerasimenko. 1998. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 8:1335-1338. [DOI] [PubMed] [Google Scholar]
- 21.Kawano, M. A., L. Xing, H. Tsukamoto, T. Inoue, H. Handa, and R. H. Cheng. 2009. Calcium-bridge triggers capsid disassembly in the cell entry process of simian virus 40. J. Biol. Chem. 284:34703-34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleywegt, G. J., and T. A. Jones. 1994. From first map to final model, p. 59-66. SERC Daresbury Laboratory, Warrington, United Kingdom.
- 23.Li, P. P., A. Naknanishi, M. A. Tran, K. Ishizu, M. Kawano, M. Phillips, H. Handa, R. C. Liddington, and H. Kasamatsu. 2003. Importance of Vp1 calcium-binding residues in assembly, cell entry, and nuclear entry of simian virus 40. J. Virol. 77:7527-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, P. P., A. P. Nguyen, Q. Qu, Q. H. Jafri, S. Aungsumart, R. H. Cheng, and H. Kasamatsu. 2007. Importance of calcium-binding site 2 in simian virus 40 infection. J. Virol. 81:6099-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall, D., and A. Schneemann. 2001. Specific packaging of nodaviral RNA2 requires the N-terminus of the capsid protein. Virology 285:165-175. [DOI] [PubMed] [Google Scholar]
- 26.Odegard, A. L., M. H. Kwan, H. E. Walukiewicz, M. Banerjee, A. Schneemann, and J. E. Johnson. 2009. Low endocytic pH and capsid protein autocleavage are critical components of Flock House virus cell entry. J. Virol. 83:8628-8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 28.Saito, M., P. I. Hanson, and P. Schlesinger. 2007. Luminal chloride-dependent activation of endosome calcium channels: patch clamp study of enlarged endosomes. J. Biol. Chem. 282:27327-27333. [DOI] [PubMed] [Google Scholar]
- 29.Schneemann, A., R. Dasgupta, J. E. Johnson, and R. R. Rueckert. 1993. Use of recombinant baculoviruses in synthesis of morphologically distinct viruslike particles of Flock House virus, a nodavirus. J. Virol. 67:2756-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneemann, A., T. M. Gallagher, and R. R. Rueckert. 1994. Reconstitution of Flock House provirions: a model system for studying structure and assembly. J. Virol. 68:4547-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 66:6728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotti, P. D., S. Dearing, and D. W. Mossop. 1983. Flock House virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae). Arch. Virol. 75:181-189. [DOI] [PubMed] [Google Scholar]
- 33.Sorger, P. K., P. G. Stockley, and S. C. Harrison. 1986. Structure and assembly of turnip crinkle virus. II. Mechanism of reassembly in vitro. J. Mol. Biol. 191:639-658. [DOI] [PubMed] [Google Scholar]
- 34.Speir, J. A., B. Bothner, C. Qu, D. A. Willits, M. J. Young, and J. E. Johnson. 2006. Enhanced local symmetry interactions globally stabilize a mutant virus capsid that maintains infectivity and capsid dynamics. J. Virol. 80:3582-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speir, J. A., S. Munshi, G. Wang, T. S. Baker, and J. E. Johnson. 1995. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure 3:63-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang, L., K. N. Johnson, L. A. Ball, T. Lin, M. Yeager, and J. E. Johnson. 2001. The structure of Pariacoto virus reveals a dodecahedral cage of duplex RNA. Nat. Struct. Biol. 8:77-83. [DOI] [PubMed] [Google Scholar]
- 37.Tong, L., and M. G. Rossmann. 1997. Rotation function calculations with GLRF program. Methods Enzymol. 276:594-611. [PubMed] [Google Scholar]
- 38.Tong, L. A., and M. G. Rossmann. 1990. The locked rotation function. Acta Crystallogr. A 46(Pt 10):783-792. [DOI] [PubMed] [Google Scholar]
- 39.Trask, S. D., and P. R. Dormitzer. 2006. Assembly of highly infectious rotavirus particles recoated with recombinant outer capsid proteins. J. Virol. 80:11293-11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walukiewicz, H. E., M. Banerjee, A. Schneemann, and J. E. Johnson. 2008. Rescue of maturation-defective Flock House virus infectivity with noninfectious, mature, viruslike particles. J. Virol. 82:2025-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walukiewicz, H. E., J. E. Johnson, and A. Schneemann. 2006. Morphological changes in the T=3 capsid of Flock House virus during cell entry. J. Virol. 80:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wery, J. P., V. S. Reddy, M. V. Hosur, and J. E. Johnson. 1994. The refined three-dimensional structure of an insect virus at 2.8 Å resolution. J. Mol. Biol. 235:565-586. [DOI] [PubMed] [Google Scholar]
- 43.Wu, Y. M., C. H. Hsu, C. H. Wang, W. Liu, W. H. Chang, and C. S. Lin. 2008. Role of the DxxDxD motif in the assembly and stability of betanodavirus particles. Arch. Virol. 153:1633-1642. [DOI] [PubMed] [Google Scholar]
- 44.Zlotnick, A., V. S. Reddy, R. Dasgupta, A. Schneemann, W. J. Ray, Jr., R. R. Rueckert, and J. E. Johnson. 1994. Capsid assembly in a family of animal viruses primes an autoproteolytic maturation that depends on a single aspartic acid residue. J. Biol. Chem. 269:13680-13684. [PubMed] [Google Scholar]