Abstract
The Epstein-Barr virus (EBV)-encoded viral protein kinase, EBV-PK (the BGLF4 gene product), is required for efficient nuclear viral egress in 293 cells. However, since EBV-PK phosphorylates a number of different viral and cellular proteins (including lamin A/C), the relative importance of each target during lytic viral replication remains unclear. We show here that an EBV PK mutant (PKmut; containing stop codons at residues 1 and 5 in EBV-PK) is highly defective for release of infectious virus from 293 cells but not 293T cells. Furthermore, the phenotype of the PKmut in 293 cells is substantially reversed by expression of the simian virus 40 (SV40) large (T) and small (t) T antigens. Efficient rescue requires the presence of both SV40 T/t proteins. We show that 293T cells have a much higher level of constitutive lamin A/C phosphorylation than do 293 cells over residues (S22 and S392) that promote phosphorylation-dependent nuclear disassembly and that both large T and small t contribute to enhanced lamin A/C phosphorylation. Finally, we demonstrate that knockdown of lamin A/C expression using small interfering RNA also rescues the PKmut phenotype in 293 cells. These results suggest that essential roles of EBV-PK during lytic viral replication include the phosphorylation and dispersion of lamin A/C.
The Epstein-Barr virus (EBV) BGLF4 gene product, EBV-PK, is homologous to the cytomegalovirus (CMV) UL97 kinase and belongs to a conserved family of herpesviral protein kinases. EBV-PK is the only protein kinase encoded by the EBV genome (6) and is expressed as an early lytic viral protein during viral reactivation (11). EBV-PK is also packaged into the virion tegument and thus is delivered in an active form to cells immediately after virus penetration (3). In the context of the intact virus, several recent studies indicate that a major and essential role of EBV-PK is to promote viral egress from the nucleus during lytic infection (10, 13, 41). Although EBV-PK phosphorylates a number of different viral and cellular proteins in vitro and when overexpressed in cells, the relative importance of the potential EBV-PK cellular and viral targets in the context of the intact viral genome has not been well studied.
The nuclear EBV-PK protein is a serine/threonine kinase that shares many targets with cyclin-dependent cellular kinases. Indeed, half of the EBV-PK targets identified by a protein array in a recent study were also found to be in vitro substrates for the cellular kinase CDK1/cyclin B (63). Overexpression of EBV-PK in cells has been shown to result in phosphorylation of a number of different cellular proteins, including the cell cycle regulatory proteins p27 (19) and pRB (C. V. Kuny and R. F. Kalejta, unpublished data); nuclear lamin A/C (32); interferon regulatory factor 3 (IRF3) (54); cellular translational elongation factor 1δ (24, 26); MCM4 (part of the MCM4-MCM6-MCM7 cellular replication origin binding complex) (29); and histone protein H2AX (52). The ability of EBV-PK to phosphorylate and inactivate two key cell cycle progression inhibitors, p27 and pRB, may provide a mechanism for inducing an S-phase-like environment in terminally differentiated nondividing cells, thus promoting the nucleotide synthesis required for lytic viral replication. Phosphorylation of nuclear lamin A/C by EBV-PK over serine residues 22, 390, and 392 promotes the disassembly of nuclear lamina (32), which could contribute to viral nuclear egress. EBV-PK phosphorylation of IRF3 inhibits its function and may thus decrease the innate immune response to the virus (54). Phosphorylation of MCM4 by EBV-PK inhibits its helicase activity and may play a role in blocking cellular DNA replication during lytic viral infection (29). Overexpression of EBV-PK in cells also leads to unscheduled chromosome condensation and stress fiber rearrangements independent of cellular DNA replication and cellular kinase CDK1/cyclin B activity (31), and activates a DNA damage response (by inducing H2AX phosphorylation) that may contribute to lytic viral replication (52).
A number of different viral targets of EBV-PK have also been identified, including the viral DNA polymerase processivity factor, BMRF1 (6, 12, 55); the latent viral proteins EBNA1 (63), EBNA2 (62), and EBNA-LP (25); and the lytic switch immediate-early protein, BZLF1 (3). Phosphorylation of EBNA2 and EBNA-LP by EBV-PK has been shown to decrease their transcriptional activation function (25, 60, 62), while phosphorylation of EBNA1 inhibits its ability to support the latent form of viral replication (63). EBV-PK phosphorylation of BMRF1 is not required for its replication function but appears to decrease its transcriptional function (58). Phosphorylation of BZLF1 by the EBV-PK is reported to inhibit its ability to activate its own promoter (4). In addition, EBV-PK may increase the expression level of two viral proteins important for nuclear egress (BFRF1 and BFLF2), although the mechanism for this effect is not clear (10, 13).
In the present study, we have examined the phenotype of a PK-mutant virus (PKmut; containing stop codons inserted at residues 1 and 5 in the EBV-PK open reading frame), in both 293 cells and 293T cells. In agreement with previously published findings (10, 13, 41), we find that the PKmut virus is severely impaired for release of infectious viral particles in 293 cells, although its DNA replication is not affected. Somewhat surprisingly, we find that wild-type (WT) and PKmut viruses release a similar amount of infectious virus in 293T cells. Furthermore, we show that the combination of large and small simian virus 40 (SV40) T antigens in 293 cells substantially rescues the phenotype of the PKmut virus and that both proteins contribute to this rescue. We demonstrate that 293T cells but not 293 cells have a high level of constitutive lamin A/C phosphorylation and that both SV40 T antigens contribute to this effect. Importantly, knockdown of lamin A/C using small interfering RNA (siRNA) also efficiently rescues the PKmut phenotype in 293 cells.
These results indicate that the requirement for EBV-PK function during lytic EBV infection is cell line dependent and that SV40 virus encoded proteins (perhaps in conjunction with the adenovirus E1A/B proteins constitutively expressed in 293 cells) can at least partially substitute for EBV-PK during lytic EBV replication. Furthermore, our results suggest that the major rescue effect of the SV40 proteins in EBV PKmut-infected 293 cells may be mediated through enhanced nuclear egress via effects on lamin A/C.
MATERIALS AND METHODS
Construction of an EBV-PK mutant.
EBV B95.8 bacmid p2089 (a gift from W. Hammerschmidt) has the complete genome of B95.8 strain EBV, plus inserted green fluorescent protein (GFP) and hygromycin resistance genes, as previously described (9). The shuttle vector pGS284 has been described and was a gift from W. Hammerschmidt (37). Stop codons were inserted in residues 1 and 5 in the EBV-PK open reading frame in the EBV bacterial artificial chromosome by site-directed mutagenesis as described previously (51, 61). The wild-type EBV sequence (positions 122904 to 124359) flanking the EBV PK start site was PCR-amplified (using the primers 5′-GCGGATCCCTTTAGCCGCACATCCAGCATCTT-3′; and 5′-GCTCTAGATACCCACTGCGGTTTATACACCAT-3′) and cloned into pSP65 to make pSP65-PKreg. Site-directed mutagenesis was performed on pSP65-PKreg to convert the EBV-PK start codon to a stop codon (using the primers 5′-CTCGAGCCATTTGAGGAACTGAGATGTGAATATGGCTGCGGAG-3′ and 5′-CTCCGCCAGCCATATTCACATCTCAGTTCCTCAAATGGCTCGAG-3′) according to the manufacturer's protocol (Stratagene). A second stop mutation was subsequently introduced to change the fifth amino acid of EBV-PK from a methionine to a stop codon using site-directed mutagenesis (primers 5′-GAGGAACTGAGATGTGAATTGAGCTGCGGAGTTGAGCCCGAC-3′ and 5′-GTCGGGCTCAACTCCGCAGCTCAATTCACATCTCAGTTCCTC-3′). The mutated EBV-PK open reading frame was cut out of pSP65 and ligated into the shuttle vector pGS284 to yield pGS284-PKStop. pGS284-PKStop in S17λpir Escherichia coli was conjugated with the wild-type EBV Bacmid p2089 in G500 E. coli. Cointegrates were selected in LB containing carbenicillin and chloramphenicol. Cultures were then recovered in LB containing chloramphenicol only and plated on LB plates containing 5% sucrose and chloramphenicol. Colonies were screened by PCR and further screened by DNA sequencing of the EBV-PK region, and restriction enzyme analysis was performed comparing the PK-Stop BACmid with the wild-type BACmid DNA (using BamHI-, HindII-, SalI-, and EcoRI-independent digestions) to make certain that no deletions or rearrangements of the viral genome had occurred.
Plasmids.
pcDNA3.1 was obtained from Invitrogen. The BZLF1 expression plasmid pSG5-Z has BZLF1 genomic sequences downstream of the SV40 promoter (49) (a gift from S. Diane Hayward) in the pSG5 vector (Stratagene). The pcDNA3-BZLF1 expression vector contains BZLF1 cDNA sequences inserted into the pcDNA3 vector as previously described (39). pSG5-R contains genomic BRLF1 sequences downstream of the SV40 promoter in the pSG5 vector (a gift from S. Diane Hayward). The pRK5-BALF4 plasmid expresses the gp110 glycoprotein and was a gift from H. J. Delecluse (42). The C-terminal FLAG-tagged WT EBV-PK expression vector was described previously (11, 34) and was a gift from M. Marschall. An EBV-PK mutant (K102I) that changes lysine residue 102 into an isoleucine in the EBV-PK expression vector (M. Marschall) was a gift from Edward Gershburg. As previously described (55), this mutation inactivates the catalytic function of EBV-PK. The following SV40 vectors were used: pRSVBneodl1440(T), which can produce large T antigen but not small t antigen (53); pRSV-t(t), which contains the SV40 nucleotides 4002 to 5171 (the t cDNA) and expresses small t antigen but not large T antigen (5); an SV40 WT p129 LTag expression vector that can express both SV40 large and small T antigens (T/t) (a gift from Janet Mertz); and pVUCR2, which expresses a mutant large T antigen mutant that does not bind pRB, as well as a wild-type form of small t antigen (a gift from Charles Cole) (21). Plasmid XhoI1.9-Kb, containing unique DNA at the right terminus of EBV in the SP6 vector (45), was a gift from Nancy Raab-Traub and was used in the EBV terminus assay. pCGN-based expression plasmids for N-terminal hemagglutinin (HA)-tagged EBV-PK, CMV UL97, and KSHV ORF36 sequences are described elsewhere (Kuny et al., unpublished).
293 and 293T cell clones.
293 or 293T cells were transfected with EBV WT BAC or the PKmut construct using Lipofectamine 2000 (Invitrogen) as described in the manufacturer's protocol. Selection of stable 293 cell clones that carry the EBV WT or PKmut plasmids was performed in Dulbecco modified Eagle medium with 100 μg of hygromycin/ml. Individual clones were then examined for their ability to produce infectious virus by transfecting them with expression plasmids for BZLF1, BRLF1, and gp110 in the presence or absence of a FLAG-tagged EBV-PK expression vector to induce lytic replication, and the supernatant from cells was used to infect Raji cells. The 293 or 293T cell clones that produced the highest virus titers were frozen at early passage and used for further study.
Virus production assays.
293 or 293T WT and PKmut cells were transfected in six-well plates with BZLF1, BRLF1, and gp110 expression vectors to induce lytic replication. Virus supernatants were harvested, centrifuged and filtered through a 0.8-μm-pore-size filter 3 days after transfection. Virus titer was determined by the Green Raji cell assay as previously described (18). A total of 4 × 105 Raji cells were infected in 24-well plates with serial dilutions of virus supernatant. Raji cells were treated with phorbol-12-myristate-13-acetate (TPA; 20 ng/ml) and sodium butyrate (3 mM) 1 day after infection and scored for GFP expression by using a fluorescence microscope 2 days after infection. The number of green Raji cell units (GRU) per milliliter was used to calculate the concentration of infectious particles in virus stocks.
SV40 virus rescue experiments.
For the SV40 rescue experiments, 293 PKmut cells were cotransfected with the cDNA3-BZLF1, pSG5-R, and gp110 expression vectors in the presence or absence of vectors expressing both the large and the small T antigens (p129 LTag), expressing the large T antigen only [pRSVBneodl1440(T)], or the SV40 small t antigen only [pRSV-t(t)]. A vector containing a mutant form of large T antigen (that does not bind pRB), as well as wild-type small t antigen (pVUCR2), was also used.
siRNA experiments.
293 WT or 293 PKmut cells were transfected with 80 pmol of silencer negative control 2 siRNA (Ambion), p27 siRNA (Santa Cruz), or lamin A/C siRNA (Santa Cruz) using X-treme GENE siRNA transfection reagent (Roche) according to the manufacturer's protocol. The next day, cells were transfected with 0.25 μg of pSG5-Z, 0.1 μg of pSG5-R, and 0.2 μg of pRK5-BALF4, in the presence of 20 pmol of control 2 siRNA, p27 siRNA, or lamin A/C siRNA using Lipofectamine 2000 (Invitrogen). Three days later, virus titer was determined by the green Raji cell assay.
EBV terminus assay.
293 EBV WT or 293 PKmut cells were transfected with a BZLF1 (Z) expression vector, in the presence or absence of a cotransfected EBV-PK expression vector, to induce lytic replication. Three days later genomic DNA was isolated and an EBV terminus assay was performed essentially as described previously (45). Briefly, DNA was digested overnight with BamHI, and 5 μg of DNA was separated by gel electrophoresis (0.8% agarose, 1× Tris-borate-EDTA) for 20 h at 35 V. The gel was incubated in denaturing buffer (1.5 M NaCl, 0.5 N NaOH) for 30 min and then incubated in neutralization buffer (1.5 M NaCl, 0.5 M Tris-HCl [pH 7.0]) for 30 min. The DNA was transferred onto a nitrocellulose membrane by using the TurboBlotter system (Whatman), and prehybridized in hybridization buffer (0.5 M Na2HPO4 [pH 7.2], 1% bovine serum albumin, 7% sodium dodecyl sulfate [SDS], 5 mM EDTA [pH 8.0]) for 30 min at 65°C. The membrane was then hybridized at 65°C overnight with a 32P-labeled DNA probe prepared by labeling EcoRI/HindIII-digested plasmid XhoI1.9-kb (45) (representing a unique DNA sequence at the right EBV terminus) with a random primer labeling system (GE Healthcare). A BamHI/BglII fragment derived from the pSG5-Z plasmid (which contains BZLF1 sequences) was also gel purified and labeled with random primer labeling system. After hybridization, membranes were washed with wash buffer (1% SDS, 20 mM Na2HPO4 [pH 7.2], 1 mM EDTA) three times (10 min for each wash), the membrane was exposed to film at −80°C overnight, and films were developed.
Immunoblot analysis.
293 EBV WT or 293 PKmut cells were transfected with a BZLF1 expression vector, in the presence or absence of a cotransfected EBV-PK expression vector, to induce lytic replication. Cells were harvested 48 h posttransfection, washed once with cold 1× phosphate-buffered saline, and resuspended in a 1:3 mixture of SUMO buffer I (5% SDS, 0.15 M Tris-HCl [pH 6.8], 30% glycerol) and SUMO buffer II (25 mM Tris-HCl [pH 8.3], 50 mM NaCl, 0.5% NP-40, 0.5% deoxycholate, 0.1% SDS) and 1× complete protease inhibitors (Roche). The cells were briefly sonicated and centrifuged, and protein concentration of the supernatant was determined with the SUMO protein assay reagent (Bio-Rad). Equal amounts of protein were separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) denaturing gel. The proteins were transferred onto a nitrocellulose membrane (Protran), blocked in 1× phosphate-buffered saline-5% milk-0.1% Tween 20 (PBST), and incubated with anti-Z (mouse; 1:250 [Argene]), anti-R (mouse; 1:250 [Argene]), anti-EBV diffuse early antigen (BMRF1; mouse; 1:250 [Vector Laboratories]), anti-EBV TK (rabbit; 1:2,000, a gift from Joyce Fingeroth), anti-BFRF1 (rabbit; 1:1,000; a gift from Robert Gonnella), anti-BFLF2 (mouse;1:10; a gift from Robert Gonnella), anti-SM (rabbit; 1:1,000; a gift from Sankar Swaminathan), anti-BcLF1 (rabbit; 1:1,000; a gift from Lindsey Hutt-Fletcher), anti-p27[Kip1](mouse; 1:2,000 [BD Transduction Laboratories]), anti-BGLF4 (C-term; rabbit; 1:1,000 [ABGENT]), anti-phosphorylated lamin A+C (rabbit polyclonal, phospho-lamin A/C S392; 1:2,000 [Abcam]), and (rabbit polyclonal phospho-lamin A/C S22; 1:1,000 [Cell Signaling]), monoclonal anti-α-tubulin clone B5-1-2 (mouse; 1:2,000 [Sigma]), anti-SV40 Tag (mouse; 1:1,000 [Santa Cruz]), anti-EBV VCA-gp125(mouse; 1:1,000 [Chemicon], anti-HA-probe (rabbit; 1:500 [Santa Cruz]) and anti-β-actin antibody (mouse; 1:5,000[Sigma]) for 1 h. After primary antibody incubation, membranes were washed in PBST three times (5 min for each wash) and incubated in the horseradish peroxidase secondary antibody (Thermo Scientific) at a 1:10,000 (anti-rabbit) or 1:5,000 (anti-mouse) dilution. Membranes were then washed with PBST for three times (15 min for each wash) and visualized by ECL treatment (Pierce) and exposure to film.
Lamin A/C fractionation studies.
293 PKmut cells were transfected with 0.5 μg of BZLF1, in the presence or absence of EBV-PK, small t, or large T antigen expression vectors. After transfection, cells were changed to 0.1% fetal bovine serum medium. Cellular fractionation studies were performed 18 h after transfection as previously described (46) with small modifications. Briefly, cells were extracted in cytoskeleton buffer (CSK; 10 mM PIPES [pH 6.8], 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, and 1 mM EGTA, supplemented with 1 mM dithiothreitol, protease inhibitor cocktail, and 0.5% [vol/vol] Triton X-100). The suspension was incubated for 5 min on ice and centrifuged at 7,500 rpm for 3 min. The supernatant was kept as the “soluble” fraction. The pellet was washed with CSK buffer and resuspended with 1 mg of RNase-free DNase I/ml in CSK buffer for 2 h at 37°C. Ammonium sulfate was added to a final concentration of 0.25 M. After 5 min at 4°C, samples were centrifuged again. The supernatant was kept as the “chromatin” fraction. The pellet was washed with 80 μl of 2 M NaCl in CSK buffer and then centrifuged. The remaining pellet was resuspended in 80 μl of 8 M urea in 10 mM Tris (pH 8) and was kept as the “nuclear matrix” fraction.
RESULTS
Establishment of 293 or 293T cell clones stably infected with WT and PKmut EBV.
To create an EBV-PK mutant virus, stop codons were inserted in residues 1 and 5 in the EBV-PK open reading frame in the EBV bacterial artificial chromosome by site-directed mutagenesis as described previously and validated by sequencing and restriction enzyme analysis (51, 61). EBV WT or PKmut bacmids were then transfected into 293 and 293T cells, and stable GFP+ clones were obtained by using hygromycin selection. WT and PKmut cell clones (at least 30 clones for each construct in each cell type) were induced into the lytic form of viral replication by BZLF1/BRLF1/gp110 transfection (plus a cotransfected EBV-PK expression vector in the PKmut clones) and virus titers determined as described in Materials and Methods. Clones that produced high titers of virus were frozen at early passage and used for all further studies. Three different clones from each virus in both 293 and 293T cells were expanded and analyzed. Similar results were obtained with each mutant clone in a particular cell type.
To confirm that the PKmut virus does not express EBV-PK, WT or PKmut cells were transfected with a BZLF1 (Z) expression vector to induce lytic infection in the presence or absence of a cotransfected wt EBV-PK expression vector, and immunoblotting was performed to examine the expression of BMRF1, EBV-PK, BZLF1, and β-actin. BZLF1 transfection induced endogenous EBV-PK expression in 293 WT cells but not in 293 PKmut cells (Fig. 1A). We also compared the hyperphosphorylated form of BMRF1 protein in the WT versus PKmut-infected cell lines, since it is well known that EBV-PK expression is required for BMRF1 to be converted to a hyperphosphorylated form that can be easily seen on immunoblots (6, 12). The hyperphosphorylated form of BMRF1 was observed in the 293 WT cells but not in the 293 PKmut cells, and expression of the EBV-PK in trans in 293 PKmut cells restored the hyperphosphorylated form of BMRF1 (Fig. 1B). These results indicate that cells infected with the PKmut virus do not have detectable EBV-PK function.
FIG. 1.
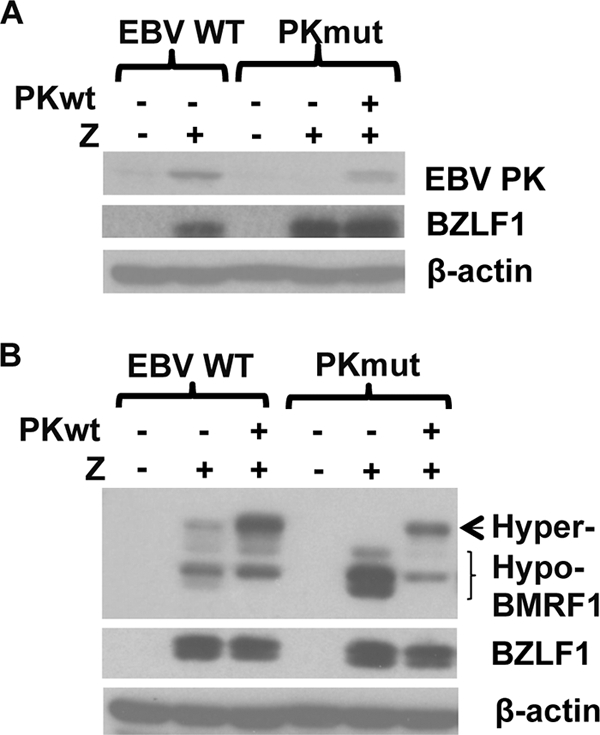
Creation of stable 293 and 293T cell lines latently infected with WT or PKmut viruses. (A) 293 cells latently infected with WT or PK-mutant EBV (PKmut) were transfected with a BZLF1 (Z) expression vector to induce lytic infection, in the presence or absence of a cotransfected EBV-PK expression vector, and immunoblots performed to examine expression of EBV-PK, BZLF1, and β-actin. (B) 293 cells latently infected with WT or PKmut were transfected with a BZLF1 (Z) expression vector to induce lytic infection, in the presence or absence of a cotransfected EBV-PK expression vector, and immunoblotting was performed to examine the expression of BMRF1, BZLF1, and β-actin. The hypo- and hyperphosphorylated forms of BMRF1 are indicated.
The PKmut virus is not impaired for lytic viral protein expression or viral DNA replication in either 293 or 293T cells.
To compare viral protein expression in cells infected with the WT and PKmut viruses during lytic viral infection, cells were transfected with a BZLF1 expression vector, and immunoblots were performed 2 days later to quantitate expression of immediate-early, early, and late viral proteins. Similar amounts of lytic viral protein expression (including BZLF1, BRLF1, viral thymidine kinase [BXLF1], BFRF1, BFLF2, SM, and BcLF1) were found in cells infected with WT and PKmut viruses in both 293 cells and 293T cells (Fig. 2 and data not shown). In contrast to the results of a study that used siRNA to decrease EBV-PK expression (13), we did not find that loss of EBV-PK affects the expression level of the essential viral egress proteins, BFRF1 and BFLF2.
FIG. 2.
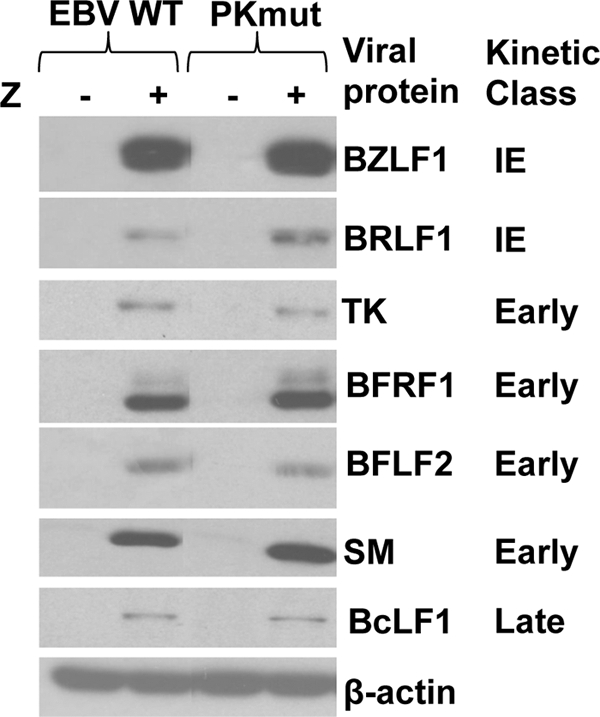
The WT and PKmut viruses have a similar level of lytic viral protein expression following BZLF1 transfection in 293 and 293T cells. 293 cells latently infected with WT or PKmut EBV were transfected with a BZLF1 (Z) expression vector to induce lytic replication. The expression level of the indicated lytic viral proteins was determined by performing immunoblot assays on cellular lysates 2 days after transfection. The kinetic class (immediate-early [IE], early, or late) is indicated next to each viral protein examined. β-Actin was used as a loading control.
To determine whether loss of EBV-PK expression affects the amount of viral DNA replication in either 293 cells or 293T cells, we performed the “terminus assay.” In this Southern blot based assay with an EBV terminus probe, the fused form of the EBV termini is derived from both the latent episome and lytically replicated concatemer DNA, while the cleaved forms are derived exclusively from lytically replicated (linear) DNA. After BZLF1 transfection, the 293 cells infected with PKmut virus supported at least as much lytic viral replication as the cells infected with the WT virus (Fig. 3); similar results were obtained in the 293T cell clones (data not shown). These results indicate that the PKmut is not defective for viral DNA replication in 293 cells or 293T cells.
FIG. 3.
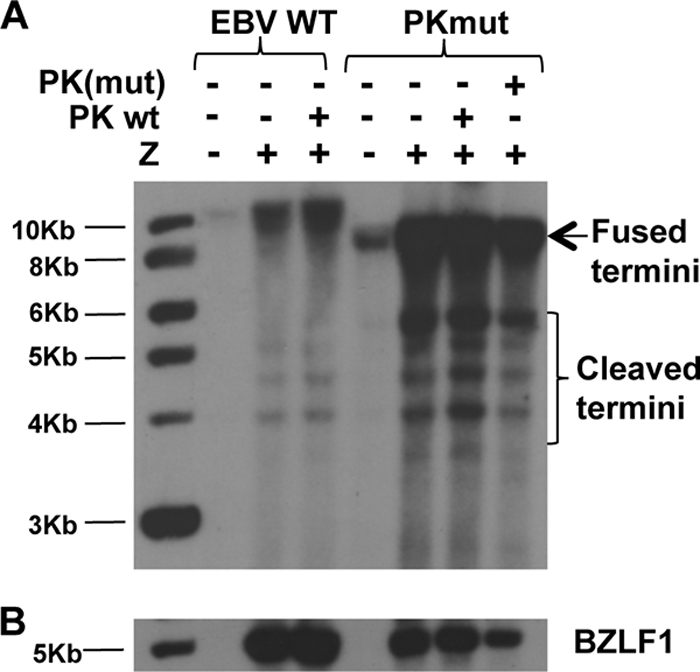
Viral DNA replication of the PKmut virus is not impaired in 293 cells. 293 WT or PKmut cells were transfected with a BZLF1 (Z) expression vector, in the presence or absence of a cotransfected WT or mutant (catalytically inactive) EBV-PK expression vector. Genomic DNA was isolated 3 days later and an EBV “terminus assay” was performed to detect the fused versus linear forms of the viral genome. (A) Southern blot hybridization was performed with a probe to the EBV termini. The positions of the fused viral termini (which are derived from both the fused termini in the latent viral episome plus the fused termini in the replicated viral concatemer DNA), and the cleaved termini (derived only from the replicated linear genome) are indicated. (B) Hybridization was performed with a probe against BZLF1 to document equal level of transfected BZLF1.
The PKmut virus is highly impaired for release of infectious viral particles in 293 cells, and this defect was not rescued by the HCMV or KSHV kinases.
We next compared the amount of infectious virus released after lytic induction of 293 cells infected with the WT versus PKmut viruses using the green Raji cell assay to titer virus production as previously described (18). Similar to the results of two previous studies examining EBV-PK function in 293 cells (one which used siRNA to knock down EBV-PK expression [13] and one that examined the phenotypes of a PK mutant virus [41]), we found that 293 cells infected with the PKmut virus released only 1% as much infectious virus as the WT-virus-infected cells (Fig. 4). This defect in virus production was rescued when the PKmut cells were cotransfected with a WT EBV-PK expression vector but not with a kinase-dead EBV-PK expression vector (Fig. 4), indicating that the loss of virus production in PKmut-infected 293 cells is specifically due to the loss of EBV-PK activity. These results confirm that EBV-PK is required for efficient release of infectious viral particles in 293 cells.
FIG. 4.
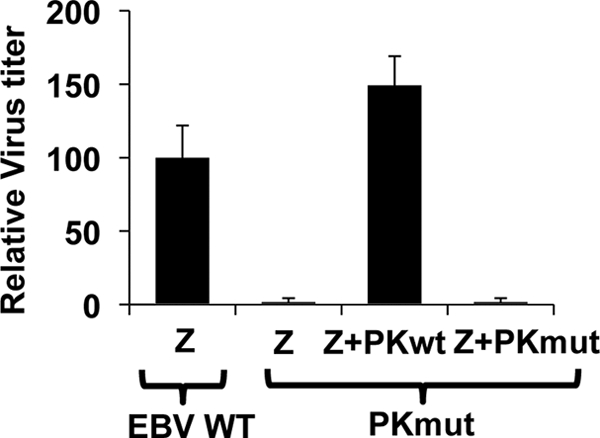
The PKmut is highly impaired for release of infectious viral particles in 293 cells and this defect is rescued by expression of EBV-PK in trans. 293 WT or PKmut cells were transfected with BZLF1/BRLF1/gp110 expression vectors to induce lytic viral replication, in the presence or absence of a cotransfected WT PK expression vector or catalytically inactive (PK K102I) vector. The virus titer was quantitated by infecting Raji cells with various amounts of the supernatant at 72 h posttransfection and counting the number of GFP-positive cells by using a fluorescence microscope. The results from three independent experiments are shown. The average virus titer of WT virus-infected 293 cells transfected with BZLF1/BRLF1/gp110 is set as 100 (± the standard deviation).
We sought to determine whether the homologous protein kinases encoded by two other herpesviruses, human cytomegalovirus (HCMV) and Kaposi's sarcoma herpesvirus (KSHV), can rescue the replication defect of PKmut in 293 cells. For this study, we compared the viral rescue using three different viral kinase expression vectors in which an HA tag was fused to the amino terminus of the EBV, CMV (UL97), and KSHV (ORF36) kinases, respectively. Although the EBV-PK vector as expected rescued viral replication in 293 PKmut cells, neither the HCMV nor KSHV kinase vectors substantially rescued viral replication (Fig. 5A), although each kinase was expressed in the 293 PKmut cells (Fig. 5B). These results suggest that one or more essential EBV-PK targets are not shared by the other viral kinases or that one or more HCMV/KSHV PK substrates (not shared by the EBV-PK) act to inhibit EBV replication.
FIG. 5.
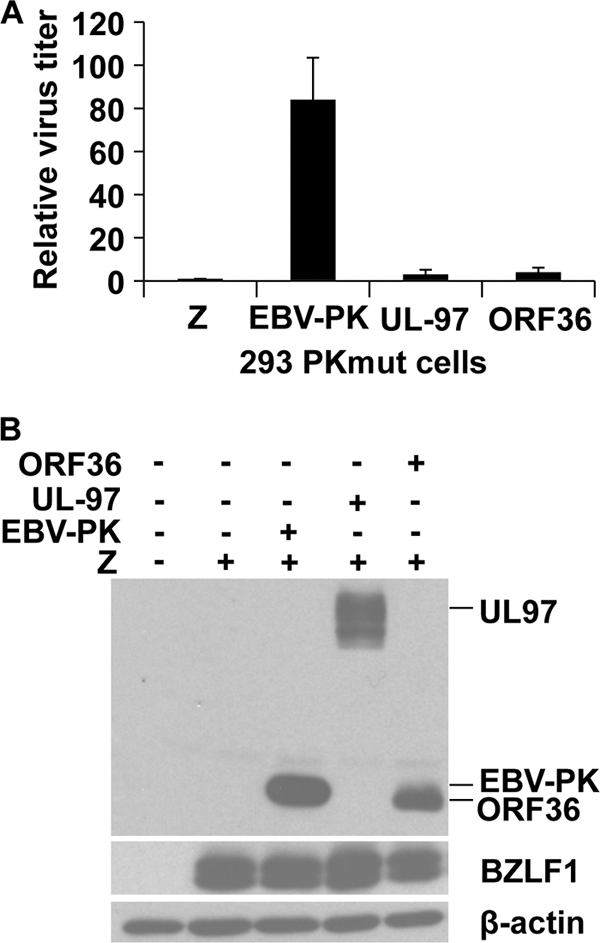
The HCMV and KSHV kinases do not rescue the phenotype of the PKmut in 293 cells. (A) 293 PKmut cells were transfected with BZLF1/BRLF1/gp110 expression vectors to induce lytic viral replication, in the presence or absence of a cotransfected HA-tagged expression vectors for EBV-PK, CMV-PK (UL97), or KSHV-PK (ORF36). The titer of virus was determined as described before. The average virus titer of 293 PKmut cells transfected with BZLF1/BRLF1/gp110 is set as 1. (B) Immunoblot analysis of the transfected HA-tagged EBV-PK, CMV UL97, and KSHV ORF36 proteins in the 293 cells used to derive the virus in the experiments in panel A is shown, along with transfected BZLF1 and cellular β-actin levels.
The PKmut virus is not impaired for viral release in 293T cells.
We also compared the amount of infectious virus released by lytically induced 293T cells infected with the WT or PKmut virus. Surprisingly, the WT and PKmut viruses released a similar amount of infectious virus in 293T cells (Fig. 6A), which are derived from 293 cells and express the SV40 large T and small t antigens (30, 57). A similar result was observed in several different independently derived 293T PKmut and WT clones (data not shown).
FIG. 6.
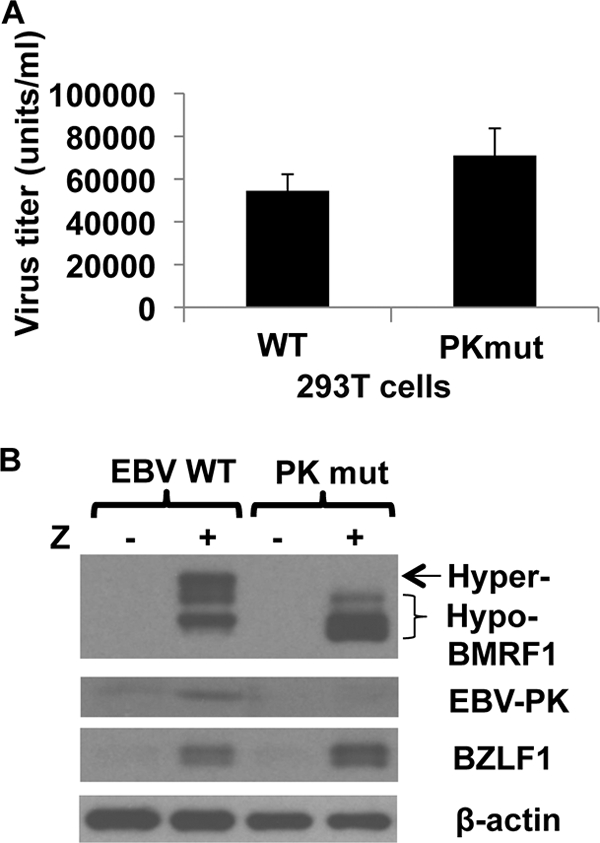
The PKmut is not defective for release of infectious virus in 293T cells. (A) 293T cells infected with WT or PKmut virus were transfected with BZLF1/BRLF1/gp110 expression vectors to induce lytic viral replication, and the titer of virus was determined 3 days later. The results from two independent experiments are shown (expressed as the number of infectious viral particles released per ml of supernatant as measured by the green Raji cell assay). (B) Immunoblots were performed on 293T EBV WT or 293T PKmut cells, transfected with or without BZLF1, to examine the level of BMRF1 hyperphosphorylation and endogenous EBV-PK expression. β-Actin was used as a loading control.
To confirm that 293T cells infected with PKmut are actually missing EBV-PK function, we examined the level of BMRF1 hyperphosphorylation and endogenous EBV-PK expression. In contrast to the 293T EBV-WT cells, the 293T PKmut cells did not have the hyperphosphorylated form of BMRF1 or express endogenous EBV-PK, after BZLF1 transfection (Fig. 6B). These results indicate that the replication defect of the PKmut virus is cell line dependent and suggest the possibility that one or more SV40-encoded proteins can complement the functions of EBV-PK in 293 cells.
SV40 T/t antigen expression partially rescues the defect of the PKmut in 293 cells.
If the SV40 small t and/or large T antigens contribute to the ability of PKmut to produce infectious virus in 293T cells, then they should be able to (at least partially) complement the defect of PKmut in 293 cells. To examine this, 293 PKmut cells were lytically induced in the presence or absence of a vector expressing the SV40 small t and large T, and the amount of infectious virus released was quantitated. The SV40 T/t antigen vector increased PKmut virus production by 45-fold in 293 cells (Fig. 7A), indicating that one or both SV40 T/t antigens complement the PKmut phenotype. To determine whether both large T and small t antigen are required for PKmut virus rescue, 293 PKmut cells were induced into lytic infection in the presence or absence of vectors that express only the SV40 large T or small t antigen. Although the expression level of the cotransfected EBV BALF4 (gp110), BZLF1 and BRLF1 proteins was similarly modestly increased in cells transfected with either large T antigen alone or both the large and small (T/t) antigens together (presumably reflecting the presence of the SV40 origin in the EBV vectors) (Fig. 7B), only the combination of both large and small T/t antigens could efficiently rescue EBV virus production (Fig. 7A). Expression of SV40 small t antigen alone was not sufficient to rescue the PKmut defect in 293 cells (Fig. 7A). These results suggest that both the SV40 large T and small t antigens contribute to the ability of PKmut to produce virus in 293T cells.
FIG. 7.
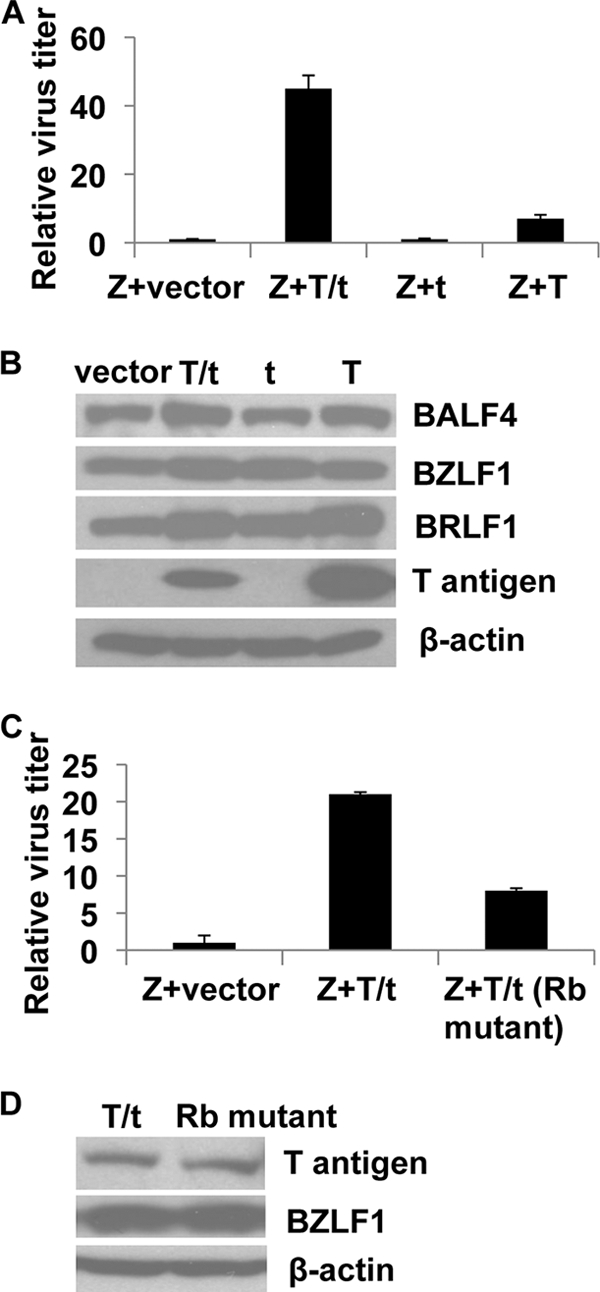
The PKmut phenotype in 293 cells is partially rescued by the SV40 large T/small t combination. (A) 293 PKmut cells were transfected with BZLF1/BRLF1/gp110 expression vectors in the presence or absence of a vector expressing both large and small SV40 T antigens (p129 LTag [T/t]), a vector that only expresses the SV40 small t antigen [pRSV-t(t)], or a vector that only expresses the large T antigen [pRSVBneodl1440(T)]. Virus titers were quantitated after 3 days. Values are the average green Raji units per ml ± the standard deviation relative to cells transfected with BZLF1/BRLF1/gp110 (set as 1). (B) The level of BALF4 (gp110), BZLF1, BRLF1, large T antigen, and β-actin in the 293 PKmut cells transfected with various vectors in Fig. A was examined by immunoblotting. (C) 293 PKmut cells were transfected with BZLF1/BRLF1/gp110 expression vectors in the presence or absence of a vector expressing WT SV40 large T and small t antigen (T/t) or a T/t vector in which the pRB-binding domain of large T antigen has been mutated (8). Virus titers were quantitated after 3 days. Values are the average green Raji units per ml ± the standard deviation relative to cells transfected with BZLF1/BRLF1/gp110 alone (set as 1). (D) The levels of large T antigen, BZLF1, and β-actin in the transfected cells in panel C are shown.
Since the SV40 large T antigen binds to and inactivates pRB (8, 16), and EBV-PK phosphorylates and inactivates RB (Kuny and Kalejta, unpublished), we also sought to determine whether the ability of T antigen to inactivate pRB function contributes to T/t rescue of the PKmut phenotype. As shown in Fig. 7C and D, removal of the T antigen pRB binding domain (amino acids 107 to 112) considerably reduced the ability of the T/t combination to rescue PKmut virus production, although the expression level of the WT and mutant T antigens was similar. These results indicate that the ability of large T antigen to inactivate pRB function is partially responsible for the rescue effect of the T/t combination.
Knockdown of p27 also increases production of PKmut virus in 293 cells.
Since EBV-PK was recently shown to phosphorylate p27 and led to its degradation (19) and the SV40 small t antigen has likewise been reported to decrease p27 function (44), we examined the effect of p27 knockdown on viral production of the WT versus PKmut viruses in 293 cells (Fig. 8). Although treatment of 293 WT virus-infected cells with p27 siRNA only slightly increased virus production, it led to a 15-fold increase in virus production (versus control siRNA) in 293 cells infected with the PKmut virus (Fig. 8A). Treatment with p27 siRNA decreased p27 protein in both 293 WT and 293 PKmut cells (Fig. 8B). As previously reported, we found that overexpression of EBV-PK decreased the total p27 level in 293 PKmut cells (Fig. 8C). These results suggest that the ability of EBV-PK to inhibit p27 function in lytically infected cells also contributes to viral production.
FIG. 8.
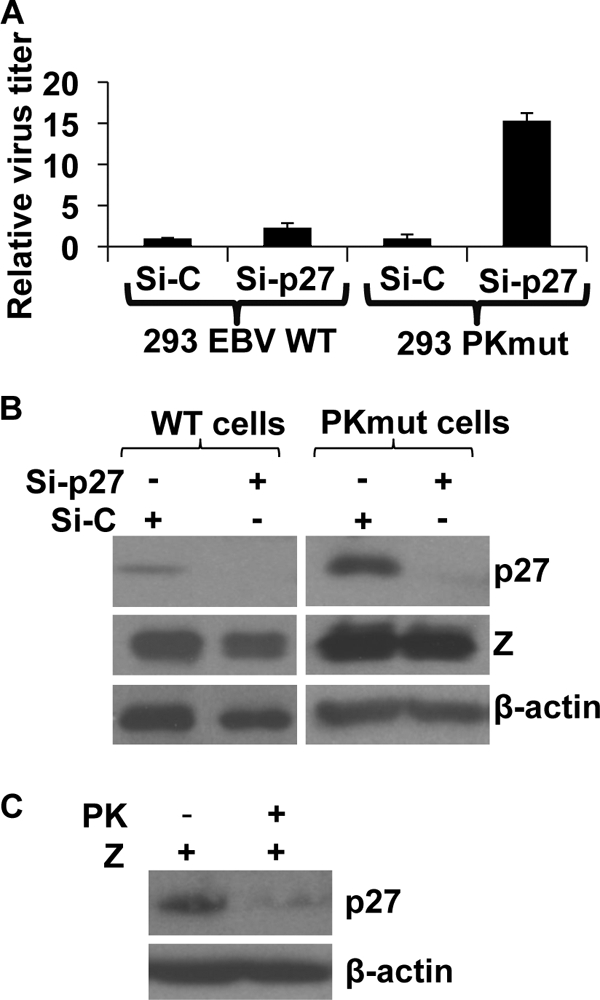
Knockdown of cell cycle regulatory protein p27 increases production of PKmut virus in 293 cells. (A) 293 cells infected with WT or PKmut were treated twice with control siRNA (Si-C), or siRNA directed against the p27 protein (Si-p27) prior to transfecting the cells with BZLF1/BRLF1/gp110 to induce lytic infection. The virus titer for each condition is shown (normalized to 1 for the amount of each virus induced in the presence of the control siRNA). (B) Western blot of the total p27 level in 293 WT and PKmut cells (note that 5 μg of total cell protein was loaded on the WT cell p27 blot, whereas 20 μg was loaded on the PKmut cell p27 blot). The level of BZLF1 and β-actin was also determined by immunoblotting analysis. (C) 293 cells infected with PKmut EBV were transfected with BZLF1 in the presence or absence of an EBV-PK expression vector. Western blotting was performed to examine the expression of p27 and β-actin.
Decreasing lamin A/C expression also enhances production of the PKmut virus in 293 cells.
Phosphorylation of lamin A/C at residues S22 and S392 by the cellular CDK1/cyclin B kinase is required for the normal nuclear disassembly during mitosis (17, 43, 56). EBV-PK phosphorylates lamin A/C in vitro over the same residues, and overexpression of the EBV-PK in HeLa cells results in displacement of a GFP-lamin A/C protein from the nuclear envelope (32). However, whether EBV-PK phosphorylation of lamin A/C is required for viral nuclear egress in the context of the intact virus is unknown. To further examine this, we used siRNA to decrease lamin A/C expression in 293 PKmut cells or 293 WT cells and examined the effect on viral production. As shown in Fig. 9A, lamin A/C siRNA only slightly increased WT virus production, but increased PKmut production by 40-fold. Lamin A/C siRNA decreased lamin A/C expression in both WT and PKmut cells to a similar degree (Fig. 9B). These results suggest that the ability of EBV-PK to phosphorylate lamin A/C, and thus dislocate it from the nuclear envelope, plays a key role in promoting viral release.
FIG. 9.
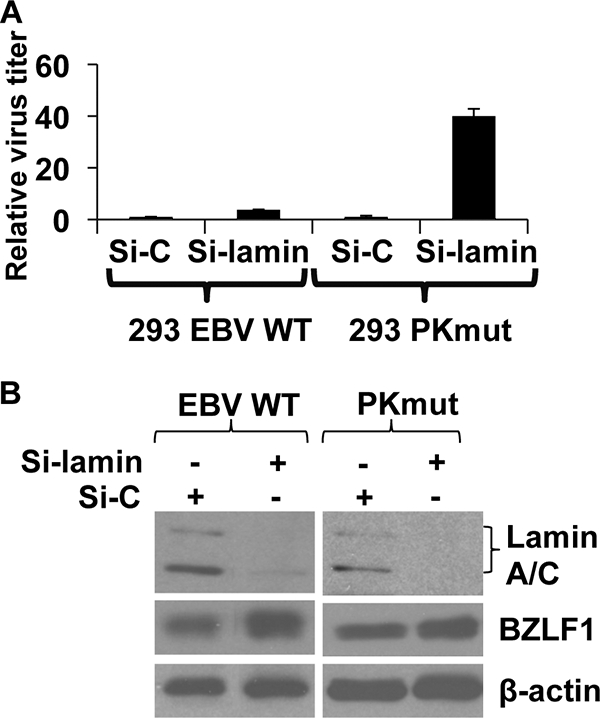
Decreasing lamin A/C greatly enhances production of the PKmut virus in 293 cells. (A) 293 cells infected with WT or PKmut EBV were treated twice with control siRNA (Si-C), or siRNA directed against the lamin A/C proteins (Si-lamin) prior to transfecting the cells with BZLF1/BRLF1/gp110 to induce lytic infection. The virus titer for each condition is shown (normalized to 1 for the amount of virus induced in the presence of the control siRNA). (B) Western blot analysis of total lamin A/C, BZLF1, and β-actin in control siRNA- and lamin A/C siRNA-treated cells.
SV40 small and large T antigens induce lamin A/C phosphorylation.
To further investigate the mechanism(s) by which the SV40 T/t antigens rescue the PKmut phenotype in 293 cells, we compared the level of lamin A/C phosphorylation in 293 cells versus 293T cells using phospho-specific antibodies. As shown in Fig. 10A, lamin A/C was found to be constitutively phosphorylated at both the S22 and S392 residues in 293T cells but not in 293 cells. The total amount of lamin A/C was similar in both cell lines. These results suggest that the ability of EBV-PK to phosphorylate lamin A/C is not required for viral release in PKmut-infected 293T cells because lamin A/C is already efficiently phosphorylated by cellular kinases.
FIG. 10.
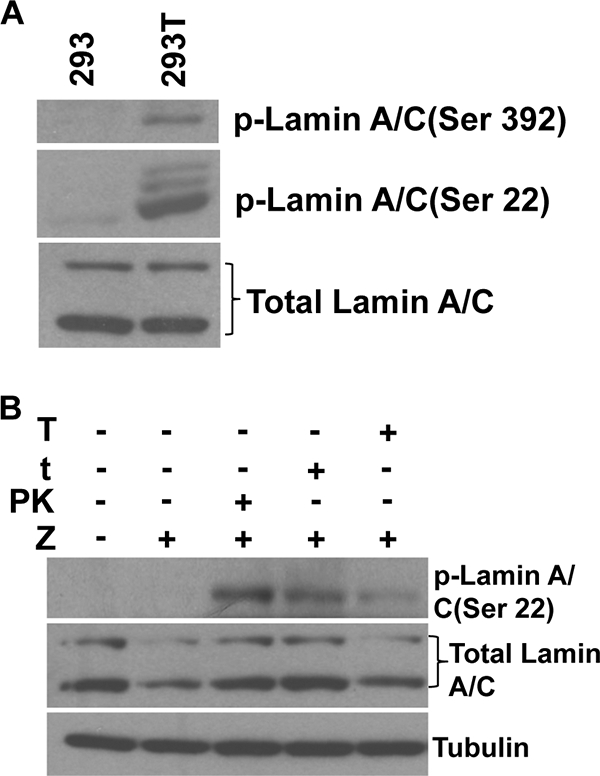
Lamin A/C is constitutively phosphorylated in 293T cells, but not 293 cells. (A) Equal amounts of whole-cell lysates from 293 or 293T cells were subjected to immunoblotting analysis with the phosphorylation-specific anti-lamin A/C antibodies (Ser-22 or Ser-392) or anti-total lamin A/C antibody. (B) 293 EBV PKmut cells were transfected with expression vectors for BZLF1, EBV-PK, SV40 small t antigen, or SV40 large T antigen as indicated. After transfection, cells were cultured in 0.1% serum medium for 18 h. Cell lysates were fractionated as described in Materials and Methods. The protein from the soluble fraction of cells was analyzed by immunoblotting assays with antibodies directed against phosphorylated lamin A/C (Ser-22) and tubulin. An antibody that recognizes total lamin A/C was used for immunoblot analysis of the nuclear matrix fractions.
Although neither the large nor small SV40 T/t antigens have been reported to enhance the amount of phosphorylated lamin A/C in cells, small t antigen inhibits the activity of protein phosphatase 2A (2, 7) and thus could potentially increase lamin A/C phosphorylation by this mechanism. Large T antigen directly interacts with the lamin A/C proteins (27), although the functional effect of this interaction is unknown. To determine whether either large and/or small T antigens can induce lamin A/C phosphorylation, and/or enhance lamin A/C solubilization, in 293 cells, we transfected 293 PKmut cells with a BZLF1 vector in the presence or absence of an EBV-PK expression vector or vectors expressing the large or small SV40 T antigen. Cellular lysates were fractionated as previously described (46) to separate the nuclear matrix, chromatin, and soluble fractions, and the level of total and phosphorylated lamin A/C was examined in the soluble versus nuclear matrix fractions. 293 PKmut cells cotransfected with the BZLF1 and EBV-PK vectors clearly had an increased amount of phosphorylated lamin A/C in the soluble fraction compared to cells transfected with BZLF1 alone (Fig. 10B), a finding consistent with the previously described ability of EBV-PK to directly phosphorylate lamin A/C over residues that led to its disassociation from the nuclear envelope. Overexpression of the SV40 small t antigen and, to a lesser extent, the large T antigen also increased the amount of phosphorylated lamin A/C within the soluble fraction of the extracts. Equal amounts of tubulin were found in each soluble extract, confirming that separation of the soluble component in each condition was successful. The level of total lamin A/C in the nuclear matrix fraction was similar in all conditions (Fig. 10B). These results suggest that both the small and large forms of the SV40 T antigens contribute to the enhanced lamin A/C phosphorylation observed in 293T cells.
DISCUSSION
The EBV-encoded protein kinase, EBV-PK, phosphorylates cellular substrates that are common to a number of different herpesvirus protein kinases, including EF-1δ (24), lamin A/C (32), and pRB (Kuny and Kalejta, unpublished). The phenotypes of EBV mutants missing the viral PK gene (10, 41) are similar to that of HCMV UL97 mutants, since in both cases the mutant viruses are defective for nuclear egress. Nevertheless, it remains unclear which of the growing number of reported cellular and viral EBV-PK targets are actually important for promoting lytic replication in the context of the virus and whether the defects of EBV-PK mutants are cell type dependent. In the present study, we show that a mutant EBV virus containing inserted stop codons in the EBV-PK open reading frame is highly impaired for the release of infectious virus in 293 cells but is surprisingly not impaired in 293T cells. Our results suggest that both the large and small SV40 T antigens contribute to the rescue of the EBV PK mutant virus in 293T cells and that a critical factor in this rescue is the ability of SV40 proteins to enhance phosphorylation of lamin A/C over residues involved in the regulation of nuclear disassembly. Furthermore, we show that knockdown of lamin A/C expression by siRNA in 293 cells also rescues the PKmut phenotype.
A surprising and interesting aspect of our results was the unexpected discovery that the EBV-PK mutant is not impaired for viral production in 293T cells. The importance of SV40 large and small T antigens in this rescue was confirmed by our finding that coexpression of the small and large T antigens in 293 cells increased the titer of the EBV-PK mutant virus by 45-fold. Furthermore, we showed that both the small and large forms of the SV40 T antigens contribute to rescue of the EBV-PK mutant virus in 293 cells. Thus, rescue the PKmut phenotype in 293 cells by SV40 encoded proteins likely involves several different mechanisms.
A recent report showed that the phenotype of a HCMV UL97 mutant can be partially rescued by expression of the human papillomavirus E7 protein and that this rescue is largely due to the ability of E7 to inactivate pRB function (23). However, the major effect of the E7 protein in regard to the rescue of the UL97-null HCMV virus occurred at the level of viral DNA replication, and this rescue was much more impressive in nondividing versus dividing cells (23). In contrast, the major effect of the SV40 T/t proteins in rescuing the phenotype of the EBV-PK mutant in 293 cells likely occurs at the level of viral nuclear egress, since the viral DNA replication of the mutant EBV virus is not impaired in this cell type. The lack of a viral replication defect of the EBV-PK mutant in 293 cells may be due to their constitutive expression of adenoviral proteins (E1A/E1B) that inhibit pRB function. Nevertheless, our finding that a large SV40 T antigen mutant that is impaired for inhibition of pRB function was also impaired for rescue of the PKmut virus in 293 cells suggests that pRB may not be totally inactivated by adenovirus proteins in 293 cells. Alternatively, the same region of SV40 large T antigen may also be required for some other function that contributes to PKmut rescue. In addition, we also found that knockdown of the cyclin-dependent kinase (CDK) inhibitor p27 (using siRNA) enhances the replication of the PKmut virus much more dramatically than it affects replication of the WT virus. This finding, combined with the recent report showing that EBV-PK phosphorylates and degrades p27 (19), suggests that the previously described ability of the SV40 small T antigen to inhibit p27 function (44) may be important for its ability to rescue the EBV PK mutant in conjunction with the large SV40 T antigen.
Another unexpected finding was our discovery that knockdown of lamin A/C expression in 293 cells is sufficient to enhance the replication of the EBV PKmut virus by 40-fold, while having only a 4-fold effect on replication of the WT virus. This result confirms that the ability of EBV-PK to phosphorylate lamin A/C over key residues that result in disassembly of the nuclear network is a critical aspect of EBV-PK function. Herpesviruses replicate and assemble nucleocapsids in the cell nucleus and exit by budding through the inner nuclear membranes. The nuclear lamina, composed of a meshwork of 10-nm lamin filaments that immediately underlie the inner membrane of the nuclear envelope (1), poses a barrier for transit of large DNA viruses. Nuclear lamins are involved in the assembly of the nuclear envelope (14), and disassembly of lamins is required during the stage of nuclear egress in which the nucleocapsids exit the nucleus (36). During normal cellular mitosis, cdc2/CDK1 disassembles the nuclear lamina by phosphorylation of the lamins (43).
The ability to induce nuclear lamin disruption is shared by HSV-1, HCMV, and EBV. The HSV-1-encoded protein kinase, Us3, directly phosphorylates lamin A/C (39) and contributes to the ability of HSV-1 to induce nuclear lamin disruption, although the UL31 and UL34 egress proteins are also required for efficient rearrangement of nuclear lamin (50). HSV-1 Us3 kinase activity partially alleviates the lamin A/C-dependent impediment to HSV-1 viral replication (40). The HCMV kinase, UL97, phosphorylates lamin A/C on Ser-22 (15), and UL97 is required for alterations in the nuclear lamina during HCMV replication (28). Likewise, EBV-PK was recently shown to phosphorylate lamin A/C in vitro at Ser-22, Ser-390, and Ser-392 (as well as some additional sites) and to induce disassembly of the nuclear lamina when overexpressed in HeLa cells (32). These disruptions presumably allow access of EBV nucleocapsids to the inner nuclear membrane for primary envelopment and budding into the space between the inner and outer nuclear membranes, and thus nuclear egress.
Consistent with the finding that the EBV PKmut can replicate in 293T cells, but not 293 cells, we found that lamin A/C is constitutively phosphorylated at residues S22 and S392 in 293T cells but not 293 cells. Furthermore, our results suggest that both the small and large SV40 T antigens can enhance the level of phosphorylated lamin A/C in 293 cells. Small t antigen binds to and inhibits PP2A by displacing low-affinity regulatory subunits of the PP2A enzyme (22, 59), suggesting an obvious mechanism by which small t antigen enhances the level of phosphorylated lamin A/C in cells. In the case of the SV40 large T antigen, the increased level of lamin A/C phosphorylation may simply reflect the known ability of large T to promote cell cycle entry (48) and hence increase cellular cdc2/CDK1 activity. In addition, SV40 large T antigen has been shown to interact directly with lamin A/C (27), although the role of this interaction is not clear. Although it is not currently understood exactly how the SV40 virus egresses the nucleus during productive SV40 infection, our results suggest that the ability of the large and small T antigens to enhance lamin A/C phosphorylation may promote SV40 nuclear egress.
Interestingly, lamin A/C not only serves as a key structural component of the nuclear envelope, but it has also been shown to directly interact with and regulate both pRB and p107 activity. Lamin A/C proteins tether the pRB and p107 proteins to perinucleolar foci that are required for their function (20, 33). Furthermore, cells missing lamin A/C are phenotypically similar to cells missing pRB (20). Hence, the efficient rescue of the PKmut phenotype by lamin A/C siRNA in 293 cells likely reflects not only the disruption of the normal nuclear membrane structure, but the inhibition of pRB/p107 function (20).
Although EBV-PK was reported to partially complement the replication defect of an HCMV mutant with UL97 deleted (47), we show here that the reverse is not true, since our EBV-PK mutant was not effectively rescued by either the HCMV or KSHV kinases in 293 cells. This result suggests that EBV-PK recognizes one or more specific and essential substrates not recognized by the HCMV and KSHV kinases. It is clear that whereas some of the targets of the EBV, HCMV, and KSHV kinases are shared in common, other targets are unique. For example, we found that while the EBV kinase induces hyperphosphorylation of the EBV BMRF1 protein, neither the HCMV nor the KSHV kinases can induce this hyperphosphorylation (data not shown). In addition, EBV-PK induces nuclear disassembly more efficiently than either the UL97 or the ORF36 kinases (32), and EBV-PK phosphorylates more sites on lamin A/C than does UL97 (32). Alternatively, EBV-PK may have a role in EBV replication that is independent of its kinase function and not shared by the other viral kinases. It is also possible that certain phosphorylated cellular targets of the HCMV and KSHV kinases are actually detrimental to EBV lytic replication.
Finally, the discovery of a cell line that efficiently supports the lytic form of EBV-PK mutant virus replication has provided us with a mechanism to ask what role, if any, EBV-PK plays in conferring antiviral susceptibility of EBV to the nucleoside analogues, acyclovir and ganciclovir. Although it is known that the HCMV UL97 kinase is required for HCMV susceptibility to ganciclovir and that the UL97 kinase can directly phosphorylate this nucleoside analogue, it has remained unclear in the case of EBV whether the EBV-PK and/or the EBV-encoded thymidine kinase primarily mediates viral susceptibility to ganciclovir. In an accompanying study, we used the 293T PKmut cell line to show that the PKmut virus is highly resistant to not only ganciclovir but also acyclovir, whereas a viral thymidine kinase-deleted EBV mutant remains highly susceptible to both drugs (35). Thus, EBV-PK shares with the HCMV UL97 PK the ability to activate certain nucleoside analogues.
Acknowledgments
We thank our collaborators Xianming Yu and Janet Mertz at the University of Wisconsin-Madison for SV40 expression vectors and help in constructing the EBV-PK mutant. We also thank Henri-Jacques Delecluse and Jim Pipas for providing valuable reagents.
This study was supported by grants R01-CA58853, R01-CA66519, R01-H6064851, P01-CA022443, and P01-CA019014 to S.C.K.; training grant T32-AI078985 to C.V.K. and S.R.H.; and R01-AI074984 to R.F.K. R.F.K. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Published ahead of print on 10 February 2010.
REFERENCES
- 1.Aebi, U., J. Cohn, L. Buhle, and L. Gerace. 1986. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323:560-564. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo, J. D., and W. C. Hahn. 2005. Involvement of PP2A in viral and cellular transformation. Oncogene 24:7746-7755. [DOI] [PubMed] [Google Scholar]
- 3.Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai, R., A. Kato, and Y. Kawaguchi. 2009. Epstein-Barr virus protein kinase BGLF4 interacts with viral transactivator BZLF1 and regulates its transactivation activity. J. Gen. Virol. 90:1575-1581. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L. S., M. M. Pater, N. I. Hutchinson, and G. di Mayorca. 1984. Transformation by purified early genes of simian virus 40. Virology 133:341-353. [DOI] [PubMed] [Google Scholar]
- 6.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y., Y. Xu, Q. Bao, Y. Xing, Z. Li, Z. Lin, J. B. Stock, P. D. Jeffrey, and Y. Shi. 2007. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14:527-534. [DOI] [PubMed] [Google Scholar]
- 8.DeCaprio, J. A., J. W. Ludlow, J. Figge, J. Y. Shew, C. M. Huang, W. H. Lee, E. Marsilio, E. Paucha, and D. M. Livingston. 1988. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 54:275-283. [DOI] [PubMed] [Google Scholar]
- 9.Delecluse, H. J., T. Hilsendegen, D. Pich, R. Zeidler, and W. Hammerschmidt. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. U. S. A. 95:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feederle, R., A. M. Mehl-Lautscham, H. Bannert, and H. J. Delecluse. 2009. The Epstein-Barr Virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production. J. Virol. 83:10877-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershburg, E., M. Marschall, K. Hong, and J. S. Pagano. 2004. Expression and localization of the Epstein-Barr virus-encoded protein kinase. J. Virol. 78:12140-12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershburg, E., S. Raffa, M. R. Torrisi, and J. S. Pagano. 2007. Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J. Virol. 81:5407-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman, R. D., Y. Gruenbaum, R. D. Moir, D. K. Shumaker, and T. P. Spann. 2002. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16:533-547. [DOI] [PubMed] [Google Scholar]
- 15.Hamirally, S., J. P. Kamil, Y. M. Ndassa-Colday, A. J. Lin, W. J. Jahng, M. C. Baek, S. Noton, L. A. Silva, M. Simpson-Holley, D. M. Knipe, D. E. Golan, J. A. Marto, and D. M. Coen. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, K. F., J. B. Christensen, and M. J. Imperiale. 1996. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J. Virol. 70:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heald, R., and F. McKeon. 1990. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61:579-589. [DOI] [PubMed] [Google Scholar]
- 18.Hong, G. K., H. J. Delecluse, H. Gruffat, T. E. Morrison, W. H. Feng, A. Sergeant, and S. C. Kenney. 2004. The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1. J. Virol. 78:4983-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwahori, S., T. Murata, A. Kudoh, Y. Sato, S. Nakayama, H. Isomura, T. Kanda, and T. Tsurumi. 2009. Phosphorylation of p27Kip1 by Epstein-Barr virus protein kinase induces its degradation through SCFSkp2 ubiquitin ligase actions during viral lytic replication. J. Biol. Chem. 284:18923-18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, B. R., R. T. Nitta, R. L. Frock, L. Mounkes, D. A. Barbie, C. L. Stewart, E. Harlow, and B. K. Kennedy. 2004. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc. Natl. Acad. Sci. U. S. A. 101:9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalderon, D., and A. E. Smith. 1984. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology 139:109-137. [DOI] [PubMed] [Google Scholar]
- 22.Kamibayashi, C., R. Estes, R. L. Lickteig, S. I. Yang, C. Craft, and M. C. Mumby. 1994. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 269:20139-20148. [PubMed] [Google Scholar]
- 23.Kamil, J. P., A. J. Human, I. Jurak, K. Munger, R. F. Kalejta, and D. M. Coen. 2009. Human papillomavirus 16 E7 inactivator of retinoblastoma family proteins complements human cytomegalovirus lacking UL97 protein kinase. Proc. Natl. Acad. Sci. U. S. A. 106:16823-16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 25.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klawitz, I., U. Preuss, and K. H. Scheidtmann. 2001. Interaction of SV40 large T antigen with components of the nucleo/cytoskeleton. Int. J. Oncol. 19:1325-1332. [DOI] [PubMed] [Google Scholar]
- 28.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudoh, A., T. Daikoku, Y. Ishimi, Y. Kawaguchi, N. Shirata, S. Iwahori, H. Isomura, and T. Tsurumi. 2006. Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication. J. Virol. 80:10064-10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebkowski, J. S., S. Clancy, and M. P. Calos. 1985. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature 317:169-171. [DOI] [PubMed] [Google Scholar]
- 31.Lee, C. P., J. Y. Chen, J. T. Wang, K. Kimura, A. Takemoto, C. C. Lu, and M. R. Chen. 2007. Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J. Virol. 81:5166-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, C. P., Y. H. Huang, S. F. Lin, Y. Chang, Y. H. Chang, K. Takada, and M. R. Chen. 2008. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J. Virol. 82:11913-11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini, M. A., B. Shan, J. A. Nickerson, S. Penman, and W. H. Lee. 1994. The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. U. S. A. 91:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 35.Meng, Q., S. R. Hagemeier, J. D. Fingeroth, E. Gershberg, J. S. Pagano, and S. C. Kenney. 2010. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral replication. J. Virol. 84:4534-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9:423-429. [DOI] [PubMed] [Google Scholar]
- 37.Moorman, N. J., D. O. Willer, and S. H. Speck. 2003. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 77:10295-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison, T. E., and S. C. Kenney. 2004. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 328:219-232. [DOI] [PubMed] [Google Scholar]
- 39.Mou, F., T. Forest, and J. D. Baines. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81:6459-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mou, F., E. G. Wills, R. Park, and J. D. Baines. 2008. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J. Virol. 82:8094-8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murata, T., H. Isomura, Y. Yamashita, S. Toyama, Y. Sato, S. Nakayama, A. Kudoh, S. Iwahori, T. Kanda, and T. Tsurumi. 2009. Efficient production of infectious viruses requires enzymatic activity of Epstein-Barr virus protein kinase. Virology 389:75-81. [DOI] [PubMed] [Google Scholar]
- 42.Neuhierl, B., R. Feederle, W. Hammerschmidt, and H. J. Delecluse. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. U. S. A. 99:15036-15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peter, M., J. Nakagawa, M. Doree, J. C. Labbe, and E. A. Nigg. 1990. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61:591-602. [DOI] [PubMed] [Google Scholar]
- 44.Porras, A., S. Gaillard, and K. Rundell. 1999. The simian virus 40 small-t and large-T antigens jointly regulate cell cycle reentry in human fibroblasts. J. Virol. 73:3102-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 46.Reyes, J. C., C. Muchardt, and M. Yaniv. 1997. Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J. Cell Biol. 137:263-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romaker, D., V. Schregel, K. Maurer, S. Auerochs, A. Marzi, H. Sticht, and M. Marschall. 2006. Analysis of the structure-activity relationship of four herpesviral UL97 subfamily protein kinases reveals partial but not full functional conservation. J. Med. Chem. 49:7044-7053. [DOI] [PubMed] [Google Scholar]
- 48.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 49.Sarisky, R. T., Z. Gao, P. M. Lieberman, E. D. Fixman, G. S. Hayward, and S. D. Hayward. 1996. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J. Virol. 70:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson-Holley, M., R. C. Colgrove, G. Nalepa, J. W. Harper, and D. M. Knipe. 2005. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J. Virol. 79:12840-12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 97:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarakanova, V. L., V. Leung-Pineda, S. Hwang, C. W. Yang, K. Matatall, M. Basson, R. Sun, H. Piwnica-Worms, B. P. Sleckman, and H. W. Virgin IV. 2007. Gammaherpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thimmappaya, B., and T. Shenk. 1979. Nucleotide sequence analysis of viable deletion mutants lacking segments of the simian virus 40 genome coding for small t antigen. J. Virol. 30:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, J. T., S. L. Doong, S. C. Teng, C. P. Lee, C. H. Tsai, and M. R. Chen. 2009. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 83:1856-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, J. T., P. W. Yang, C. P. Lee, C. H. Han, C. H. Tsai, and M. R. Chen. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J. Gen. Virol. 86:3215-3225. [DOI] [PubMed] [Google Scholar]
- 56.Ward, G. E., and M. W. Kirschner. 1990. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 61:561-577. [DOI] [PubMed] [Google Scholar]
- 57.Yang, C. S., M. J. Vitto, S. A. Busby, B. A. Garcia, C. T. Kesler, D. Gioeli, J. Shabanowitz, D. F. Hunt, K. Rundell, D. L. Brautigan, and B. M. Paschal. 2005. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol. Cell. Biol. 25:1298-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, P. W., S. S. Chang, C. H. Tsai, Y. H. Chao, and M. R. Chen. 2008. Effect of phosphorylation on the transactivation activity of Epstein-Barr virus BMRF1, a major target of the viral BGLF4 kinase. J. Gen. Virol. 89:884-895. [DOI] [PubMed] [Google Scholar]
- 59.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, X., Z. Wang, and J. E. Mertz. 2007. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS Pathog. 3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yue, W., E. Gershburg, and J. S. Pagano. 2005. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J. Virol. 79:5880-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu, J., G. Liao, L. Shan, J. Zhang, M. R. Chen, G. S. Hayward, S. D. Hayward, P. Desai, and H. Zhu. 2009. Protein array identification of substrates of the Epstein-Barr virus protein kinase BGLF4. J. Virol. 83:5219-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]


