Abstract
Human adenovirus serotype 35 (HAdV-35; here referred to as Ad35) causes kidney and urinary tract infections and infects respiratory organs of immunocompromised individuals. Unlike other adenoviruses, Ad35 has a low seroprevalence, which makes Ad35-based vectors promising candidates for gene therapy. Ad35 utilizes CD46 and integrins as receptors for infection of epithelial and hematopoietic cells. Here we show that infectious entry of Ad35 into HeLa cells, human kidney HK-2 cells, and normal human lung fibroblasts strongly depended on CD46 and integrins but not heparan sulfate and variably required the large GTPase dynamin. Ad35 infections were independent of expression of the carboxy-terminal domain of AP180, which effectively blocks clathrin-mediated uptake. Ad35 infections were inhibited by small chemicals against serine/threonine kinase Pak1 (p21-activated kinase), protein kinase C (PKC), sodium-proton exchangers, actin, and acidic organelles. Remarkably, the F-actin inhibitor jasplakinolide, the Pak1 inhibitor IPA-3, or the sodium-proton exchange inhibitor 5-(N-ethyl-N-isopropyl) amiloride (EIPA) blocked endocytic uptake of Ad35. Dominant-negative proteins or small interfering RNAs against factors driving macropinocytosis, including the small GTPase Rac1, Pak1, or the Pak1 effector C-terminal binding protein 1 (CtBP1), potently inhibited Ad35 infection. Confocal laser scanning microscopy, electron microscopy, and live cell imaging showed that Ad35 colocalized with fluid-phase markers in large endocytic structures that were positive for CD46, αν integrins, and also CtBP1. Our results extend earlier observations with HAdV-3 (Ad3) and establish macropinocytosis as an infectious pathway for species B human adenoviruses in epithelial and hematopoietic cells.
Adenoviruses circulate widely in the human population, and most adults have been exposed to them (13). Currently, there are more than 54 human adenovirus (HAdV) serotypes known, and they have partly divergent entry pathways (for taxonomic details, see http://www.vmri.hu/∼harrach/AdVtaxlong.htm). HAdVs are classified into six species, A to F. Clinical manifestations of HAdV vary considerably but commonly include cold symptoms, pharyngitis, tonsilitis, otitis, and pharyngoconjunctival fever. Less common are severe pneumonia, conjunctivitis, cystitis, encephalitis, and meningitis (49, 80). In immunocompromised patients and young military recruits, HAdVs cause life-threatening infections (48).
The entry of the species C HAdV-2 (Ad2) and HAdV-5 (Ad5) is the best characterized. Ad2/5 bind to the coxsackie B virus-adenovirus receptor CAR (8) and use αν-integrins as secondary receptors to induce receptor-mediated endocytosis involving clathrin, clathrin adaptors, and the large GTPase dynamin (31, 33, 62, 101, 104). These viruses also trigger accessory dynamin-independent macropinocytosis, which is not used to internalize Ad2/5 into cultured cells (62). Macropinocytosis is, however, an important infectious pathway into epithelial cells for the species B1 HAdV-3 (Ad3) (3). It is also an entry pathway for an increasing number of viruses from other families, such as vaccinia virus (66), echovirus 1 (42), Kaposi's sarcoma herpes simplex virus (82), and Ebola virus (81). Macropinocytosis has been associated with human immunodeficiency virus (HIV) type 1 infections in different cell types (27, 57, 59), but dynamin-dependent endocytosis of HIV has also been reported (69).
Macropinocytosis is a form of endocytosis occurring on a large scale. It leads to the formation of large vacuoles, predominantly at the cellular periphery (96). It often but not always involves ruffling protrusions from the plasma membrane that fuse either with themselves or with the cell membrane and thereby engulf extracellular material (21). Macropinocytosis significantly contributes to antigen presentation in immune cells (65, 103) and is used by viral and bacterial pathogens to reduce immune responses (67, 96).
The small GTPase Rac1 and dynamic actin filaments invariably control macropinocytosis. Macropinocytosis also strongly requires the p21-activated kinase Pak1 (20), which binds and activates Rac1 (45), and variably depends on phosphatidylinositol-3-kinase (PI3K), Ras, and Src activities downstream of activated receptors. In addition, macropinocytosis requires C-terminal binding protein 1 (CtBP1), which is phosphorylated by Pak1 and supports membrane fission or stabilization of the emerging macropinosomal vesicle (34, 55). It is strongly blocked by inhibitors of sodium/proton exchangers (102), such as amiloride or an amiloride analogue, 5-(N-ethyl-N-isopropyl) amiloride (EIPA) (36), which decrease cytosolic pH and thereby inhibit the activation of Rac1 and Cdc42 GTPases in submembranous zones (47). Another hallmark of macropinocytosis is the dependency on protein kinase C (PKC) in both macrophages and epithelial cells (4, 5, 62).
Ad35 is a member of the species B2 adenoviruses, which use CD46 as a primary receptor in epithelial and hematopoietic cells (26, 30) and integrins as coreceptors for infection of hematopoietic cells (71). This virus was initially isolated from the kidney of an immunocompromised individual (72). Species B2 adenoviruses naturally infect the kidney and urinary tracts and are sometimes fatal in immunocompromised individuals, possibly due to reemergence from a latent state (37, 52). In addition, Ad35 is a promising vector for clinical gene transfer and vaccination, in part due to the low seroprevalence of neutralizing antibodies in the population (100). For example, an Ad35-based malaria vaccine was shown to protect mice from Plasmodium falciparum sporozoites (77) and induced potent T-cell immunity (83). In addition, Ad35 vectors injected into tissues of nonhuman primates gave rise to specific gene expression at sites of injection in most organs, indicating the high versatility of Ad35 (86, 87).
Here we examined the infectious entry pathway for Ad35 into human epithelial cells and human kidney 2 (HK-2) cells and compared the results with host requirements for infectious entry of species C Ad2/5. Unlike Ad2/5, Ad35 uses macropinocytosis as an infectious uptake pathway. It requires CD46, integrins, PKC, a sodium/proton exchanger, actin, Rac1, Pak1, and CtBP1 but not heparan sulfate, which had been suggested to be involved in virus attachment to Chinese hamster ovary cells (98). These results mirror the infectious pathway into epithelial cells for the species B1 Ad3 (3). This is remarkable, since Ad3 had been suggested to use other receptors besides CD46 (60, 94). Notably, it was recently shown that Ad3 binds to the same region of the terminal short consensus repeat 1 (SCR1) and SCR2 of CD46 as Ad35, albeit with lower affinity than Ad35 (25, 26), and this leads to gene expression in high-CD46-expressing baby hamster kidney (BHK) cells, CHO cells, or malignant glioma cells (35, 94, 99). Our results show that HAdVs of the species B1 and B2 use conserved entry pathways into epithelial cells which depend on CD46.
MATERIALS AND METHODS
Cells and viruses.
Normal diploid human embryonic lung-derived Wi-38 fibroblasts (CCL-75) were purchased from the American Type Cell Culture Collection (ATCC) and grown in Dulbecco's modified Eagle medium (DMEM) (Sigma) containing 10% fetal calf serum (FCS) (Invitrogen). HeLa-ATCC (CCL-2) and HeLa-K, a variant of HeLa-ATCC with particularly high transfection efficiencies, were obtained from U. Kutay (Institute of Biochemistry, ETH Zurich, Switzerland). Human melanoma M21, M21L, and M21L4 cells were grown in DMEM (Sigma) containing 10% FCS (Invitrogen) at a low passage number as described previously (62). M21 (positive for surface-expressed αν-integrins), M21L cells (negative for αν-integrins) and M21L4 transfected with αν-integrin cDNA (24) were obtained from D. Cheresh (Scripps Research Institute, La Jolla, CA). Human kidney HK-2 cells originally isolated from kidney proximal tubular cells and immortalized with the human papillomavirus 16 E6 and E7 proteins (85) was obtained from F. Verrey (Institute of Physiology, University of Zurich, Zurich, Switzerland) and propagated in K1 medium. Human hematopoietic K562 cells were used as described previously (3). CHO cells stably expressing green fluorescent protein (GFP)-labeled CD46 (GFP-CD46) were generated by transfection of CHO-15B6 cells with a pcDNA3.1-neo vector containing the CD46-BC1 sequence, and single clones were selected in G418 (1 mg/ml)-containing medium. Ad35 was isolated from human bronchial epithelial A549 cells. Fluorescent tagging of Ad35 with Texas Red (Molecular Probes, Leiden, Netherlands) was performed as described previously (95). Enhanced GFP (eGFP)-labeled Ad35 (Ad35-eGFP), Ad5-eGFP, and Ad2-ts1 were used as described previously (31, 73, 100). eGFP-labeled herpes simplex virus type 1 (HSV-1-eGFP) was a kind gift from C. Fraefel (Institute of Veterinary Virology, University of Zurich [75]). 3H-labeled Ad35 (3H-Ad35) was generated as described in reference 33.
Viral eGFP transduction experiments.
For infection analyses by wide-field microscopy or confocal laser scanning microscopy (CLSM) analyses, cells were seeded onto glass coverslips in 24-well dishes. Alternatively, infection measurements were carried out in a Safire monochromator-based microplate detection system (Tecan Group Ltd., Switzerland) using 96-well plates. Cells were infected with the indicated eGFP-expressing virus at a multiplicity of infection (MOI) of 5 in warm DMEM-0.2% bovine serum albumin (BSA) (Sigma A9418) for 60 min, washed twice with warm DMEM-0.2% BSA, and further incubated for 7 h, fixed in 3% paraformaldehyde for 25 min, quenched with ammonium chloride, and prepared for analysis as indicated above. Chemical interference with infection was carried out by addition of compounds 30 min prior to infection in DMEM-0.2% BSA at 37°C. Cells were infected in the presence of the inhibitors in DMEM-0.2% BSA for 60 min, washed twice, and further incubated for 7 h.
cDNAs, antibodies, and chemicals.
Human K44A-Dyn2 and Dyn2 expression constructs were obtained from C. Lamaze (Pasteur Institute, Paris, France). Plasmids expressing human Rac1 and T17N-Rac1 were from A. Hall (University College, London, United Kingdom). Expression plasmids encoding CtBP1-S (obtained from A. Colanzi and A. Luini, Department of Cell Biology and Oncology, Sta Maria Imbaro, Italy) were used to construct Myc-tagged CtBP1 and CtBP1-S147A (3). Pak1 and Pak1 autoinhibitory domain (AID) constructs were obtained from J. Chernoff (Fox Chase Cancer Center, Philadelphia, PA). The following antibodies and chemicals were purchased: anti-CtBP1 (BD-Transduction Laboratories, Switzerland), anti-CD46 E4.3 (monoclonal, used for immunofluorescence analyses; BD-Pharmingen), anti-CD46 (polyclonal H-294, for Western blots; Santa Cruz), anti-CD46 (monoclonal MEM-258, used for inhibition experiments; Serotec Ltd., Oxford, United Kingdom), and anti-αν β5-integrin (P1F6, monoclonal; Chemicon). Secondary antibodies were goat antimouse or antirabbit coupled to Cy5 (Pierce). The dynamin inhibitor dynasore (58) was purchased from Sigma and dissolved in dimethyl sulfoxide (DMSO). Latrunculin was from Enzo Life Sciences, and cyclic tripeptide arginine-glycine-aspartate (single amino acid code cRGD) and cRAD (alanine substitution for glycine) peptides were obtained from Peptides International. The PKC inhibitor Gö6976, the sodium/proton exchanger inhibitor EIPA, cytochalasin D, and jasplakinolide were from Calbiochem. The Pak1 inhibitor IPA-3 and its inactive analogon PIR3.5 were obtained from J. Peterson (Basic Science Division, Fox Chase Cancer Center, Philadelphia, PA) (19).
siRNA transfections.
HeLa-ATCC, HK-2, and A549 cells were transfected with 20 or 50 nM small interfering RNA (siRNA) for 72 h or as appropriate using Lipofectamine 2000 (Invitrogen) and the following siRNA. Validated siRNAs were used against CtBP1 (GGAUAGAGACCACGCCAGUUU; Qiagen [9]), Pak1 (catalog no. SI00605696; Qiagen), eGFP (no. 1022064; Qiagen), and CD46 (Qiagen) (as described in reference 30), nonsilencing scrambled siRNA (AGGUAGUGUAAUCGCCUUG dTdT; Microsynth, Switzerland), clathrin heavy chain (AACCUGCGGUCUGGAGUCAAC; Qiagen) (62), and dynamin2 (GACAUGAUCCUGCAGUUCA; Qiagen). K562 cells were transfected with the appropriate siRNA using Nucleofector I (Amaxa, Germany).
Microscopy.
Spinning disc live cell microscopy images were recorded with an UplanApo100X objective (numerical aperture [NA], 1.35) on an Olympus IX81 inverted microscope (Olympus) equipped with a Yokogawa scanning head, QLC100 (Visitron Systems GmbH, Puchheim, Germany), containing a triple-bandpass excitation filter (488 nm/565 nm/647 nm; Chroma) and an NV 40/1CL piezo stepper for objective positioning (Piezosystem Jena). Images were recorded on a Cascade 512 electron multiplying charge-coupled-device camera (Photometrics) with a 16- by 16-μm2 pixel size. Image acquisition was controlled by MetaMorph software (Molecular Devices, Visitron Systems Germany). Confocal laser scanning microscopy was conducted with an inverted Leica SP5 microscope (Leica Microsystems, Switzerland) equipped with a 63× (oil immersion; NA, 1.4) objective, a diode laser (405-nm excitation), an argon laser (458/476/488/496/514-nm excitation), and a helium laser (561/594/633-nm excitation). Wide-field microscopy was carried out with an Olympus IX81 microscope equipped with a 40× UPlanApo 1.00 oil objective. Cell shapes were drawn by hand, and mean intensities of eGFP fluorescence per cell were measured from 16-bit images using the NIH ImageJ software program (http://rsbweb.nih.gov/ij/). Samples for analysis by electron microscopy (EM) were fixed in 1.5% glutaraldehyde-2% formaldehyde in 0.1 m cacodylate buffer (pH 7.4) for 60 min and processed for quantitative transmission EM (TEM) analysis as described previously (31).
Data representation and statistical analyses.
Images were batch processed and contrast enhanced using the Adobe Photoshop software program. Data were represented as the mean values from triplicates of a representative experiment with the indicated number of cells or viruses and the standard deviation. In EM studies, error bars indicate the standard error of the mean. P values were derived from Student t tests.
RESULTS
CD46 and integrin-dependent but not HSPG-dependent Ad35 infection of HeLa-ATCC and HK-2 cells.
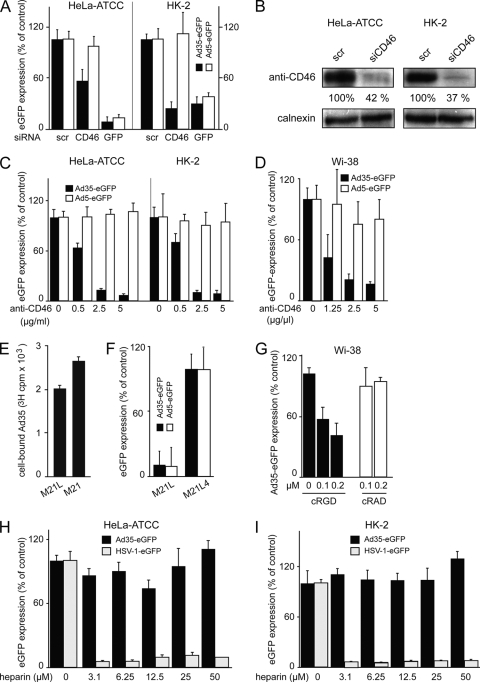
We first tested the receptor requirements for Ad35 for infection of HeLa-ATCC and human kidney (HK-2) cells (85) by small interfering RNA (siRNA)-mediated CD46 knockdown. A 45% or 72% reduction in the CD46 levels in HeLa-ATCC and HK-2 cells inhibited Ad35-mediated transduction of eGFP by 50% and 78%, respectively (Fig. 1A and B). This result was confirmed with an Ad35 infection-neutralizing monoclonal antibody directed against short consensus repeat region (SCR) 1 of CD46 (26), which gave a robust dose-dependent inhibition of Ad35 but not Ad5-mediated eGFP transduction in HeLa-ATCC, HK-2, and Wi-38 normal lung fibroblasts (Fig. 1C and D). This provided confirmatory evidence that CD46 has a major role for infection of both HeLa-ATCC and HK-2 cells, in agreement with earlier results from HeLa cells, human bronchial epithelial A549 cells, and hematopoietic cells (25, 26, 30).
FIG. 1.
CD46 and αν integrins are required for Ad35 infection, HSPG is not. (A) HeLa-ATCC (left) or HK-2 (right) cells were transfected with 20 nM siRNAs against CD46, GFP, or nonsilencing scrambled siRNA (scr) for 72 h and infected with Ad35-eGFP (MOI of 5) or Ad5-eGFP (MOI of 5) for 8 h, followed by Safire fluorescence analysis. (B) Quantification of knockdown efficiency by Western blots against CD46, normalized against calnexin. (C and D) HeLa-ATCC (left) or HK-2 (right) cells (C) or Wi-38 cells (D) were preincubated with an inhibiting antibody against CD46 (MEM-258) in the cold for 30 min and infected for 8 h or 15 h, respectively. (E) 3H-Ad35 was bound to human melanoma M21L cells lacking αν integrin or to M21 αν integrin-positive cells. (F) M21L or M21 cells were infected with Ad35-eGFP or Ad5-eGFP and analyzed by Safire fluorescence analyses. (G) Wi-38 cells were preincubated with cRGD or cRAD peptides in the cold for 30 min and infected with Ad35-eGFP for 15 h. (H and I) Ad35-eGFP (MOI of 5) or HSV-1-eGFP (MOI of 5) were preincubated for 30 min with different concentrations of heparin as indicated, followed by inoculation of HeLa-ATCC or HK-2 cells for 8 h, and infection analyses were done with a Safire2 plate reader after normalization to the cell numbers determined by 4′,6-diamidino-2-phenylindole (DAPI) staining (see Materials and Methods). Note that Ad35-eGFP transduction was not affected whereas HSV-1-eGFP transduction was strongly reduced in both cell lines.
All sequenced HAdV serotypes, except for Ad40 and Ad41, which infect the digestive tract (2), contain an arginine-glycine-aspartate (single amino acid code RGD) motif which binds integrin and is contained in extracellular matrix proteins (84). Therefore, we investigated the involvement of integrins in Ad35 infection. The RGD motif in the Ad2 penton base had been shown to bind soluble αν beta 5 integrin heterodimers (12). The attachment of 3H-radiolabeled Ad35 to human melanoma M21L cells lacking αν-integrins (24) was not significantly inhibited compared results for αν-positive M21 cells (Fig. 1E). Both Ad35- and Ad5-mediated eGFP transduction of M21L cells, however, was strongly reduced compared to results with M21L4 cells transfected with αν-integrin cDNA or native M21 cells (not shown), indicating that αν-integrins support Ad35 transduction of epithelial cells (Fig. 1F). A role for integrins was further strengthened by the finding that Ad35-eGFP expression in Wi-38 cells was significantly reduced by the soluble integrin ligand cRGD but not cRAD peptides (Fig. 1G). These results were in agreement with an earlier report showing that integrins are involved in Ad35 transduction of human hematopoietic cells (71).
It was recently suggested that Ad35 uses heparan sulfate proteoglycans (HSPGs) for attachment to CHO cells in the absence of CD46 receptors, as concluded from competition experiments with soluble heparin (98). To address if HSPGs were involved in Ad35 infection of HeLa-ATCC or HK-2 cells, we preincubated Ad35-eGFP with increasing concentrations of heparin for 30 min, added the mixture to HeLa-ATCC or HK-2 cells for 8 h at a MOI of 5, and measured eGFP transgene expression. Unexpectedly, we did not observe any significant inhibition or stimulation of eGFP expression with Ad35 in either cell type (Fig. 1H and I). We did, however, find a strong block of herpes simplex virus 1 (HSV-1)-mediated eGFP transduction, as reported earlier (106). We concluded that HSPGs are not involved in Ad35 infection of the CD46-positive HeLa and HK-2 cells.
Cell type-dependent requirement of dynamin for Ad35 infection.
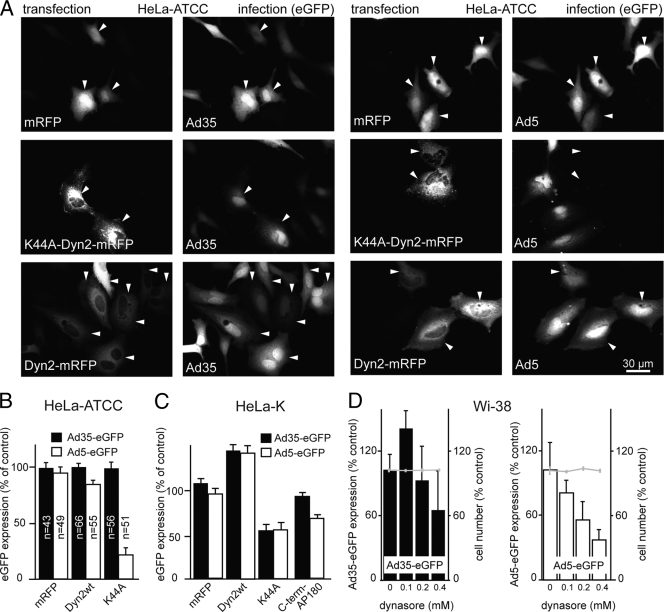
Ligation of CD46 by antibodies or Ad3 has been shown to trigger clathrin-mediated endocytosis or dynamin-independent macropinocytosis, depending on the degree of CD46 cross-linking (3, 16, 94). By transfecting cells with dominant-negative K44A-Dyn2 tagged with monomeric red fluorescent protein (mRFP) for 24 h followed by inoculation of the cells with Ad35-eGFP, we found that Ad35 transduction of HeLa-ATCC cells was independent of K44A-Dyn2, in contrast to the case with Ad5-eGFP (Fig. 2A and B). Ad35 infection of HeLa-K cells, however, was dynamin dependent (Fig. 2C), exactly as reported for Ad3 (3). To test if dynamin-dependent Ad35 infection of HeLa-K cells required a clathrin-related pathway, we overexpressed the carboxy-terminal clathrin heavy chain binding domain of AP180 (amino acids [aa] 530 to 915), which blocks clathrin recruitment to the plasma membrane (28) and inhibits clathrin-mediated endocytosis (70). It also inhibits infectious clathrin and dynamin-dependent endocytosis of Ad2 (31). We found that Ad35 infection of HeLa-K cells was not inhibited by the C-terminal domain of AP180, unlike Ad5-eGFP transduction (Fig. 2C). In support of this, the dynamin inhibitor dynasore (58) had no significant effects on Ad35 transduction but affected Ad5 transduction of Wi-38 cells (Fig. 2D). This suggested that Ad35 entry into HeLa-K cells was at least partly dependent on dynamin and most likely clathrin and that Ad35 entry into HeLa-ATCC and Wi-38 cells was dynamin independent.
FIG. 2.
Cell type-specific requirements of dynamin. HeLa-ATCC or HeLa-K cells were transfected with dominant-negative K44A-Dyn2-mRFP, wild-type Dyn2-mRFP, or mRFP for 24 h and infected with Ad35-eGFP or Ad5-eGFP (MOI of 5) for 8 h. (A) Representative images of transfected and infected HeLa-ATCC cells, fixed and recorded for mRFP and GFP fluorescence in a wide-field microscope. (B) NIH ImageJ quantification of total fluorescence intensities. (C) HeLa-K cells transfected as for panel A and in addition with the carboxy-terminal domain of AP180, infected and assayed by fluorescence-activated cell sorting. Dominant-negative K44A-Dyn2 inhibited both Ad35-eGFP and Ad5-eGFP, whereas the carboxy-terminal domain of AP180 had no effects on Ad35-eGFP but inhibited Ad5-eGFP by 40%. (D) Dynasore-independent transduction of Wi-38 cells by Ad35-eGFP (left) and dynasore-dependent transduction by Ad5-eGFP (right panel) in the absence of cell toxicity, indicated by cell number measurements shown with gray line graphs, with 100% representing noninfected non-drug-treated conditions.
Macropinocytosis inhibitors block infectious Ad35 uptake into epithelial cells.
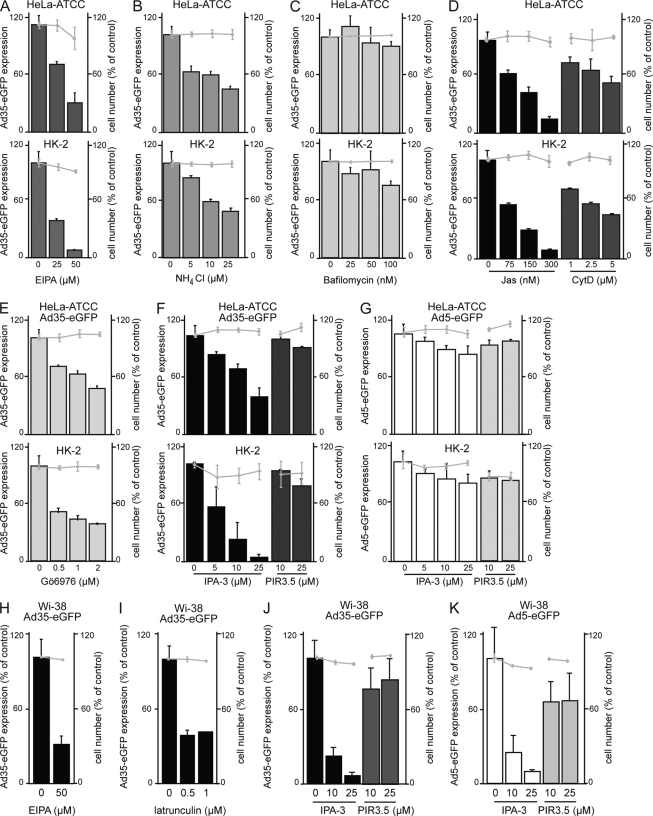
To analyze the dynamin-independent infection pathway of Ad35, we treated HeLa-ATCC, HK-2, or Wi-38 cells with pharmacological inhibitors of macropinocytosis. The classical inhibitors of macropinocytosis, amiloride and its derivative 5-(N-ethyl-N-isopropyl) amiloride (EIPA), block the sodium/proton exchangers, lead to mild acidification of the cytosol, alter the subcellular localization of early and late endosomes, and possibly raise the lumenal pH of mildly acidic organelles (29). Likewise, millimolar concentrations of NH4Cl inhibit macropinocytosis (15), possibly by acidification of the cytosol and effects on actin dynamics (38). We found that low micromolar concentrations of EIPA inhibited Ad35-eGFP transduction of HeLa-ATCC, HK-2, and Wi-38 cells (Fig. 3A and H) similarly to millimolar concentrations of NH4Cl in HeLa-ATCC, HK2, or Wi-38 cells (Fig. 3B and data not shown). In contrast, the proton ATPase inhibitor bafilomycin A1 (Baf) did not affect Ad35-eGFP transduction of HeLa or HK-2 cells except at the very highest concentration of 100 nM, where it decreased Ad35-eGFP transduction by 10 to 25% (Fig. 3C). It did not affect Ad35-eGFP transduction of Wi-38 cells (not shown). In contrast, Baf (50 nM) reduced human rhinovirus serotype 16 infection of HeLa cells at least 20-fold compared to results for control cells and blocked acidification of endosomal organelles (C. Neugebauer, A. Jurgeit, and U. F. Greber, unpublished observations), suggesting that vacuolar ATPases are not required for infectious Ad35 entry.
FIG. 3.
Inhibitors against Pak1, PKC, sodium/proton exchanger, and actin reduce Ad35-eGFP transduction. HeLa-ATCC and HK-2 cells were preincubated with the indicated concentrations of drugs for 30 min and infected with Ad35-eGFP for 8 h. Cells were analyzed on a Safire2 plate reader, and eGFP intensities were normalized to the DAPI signal of the cell nuclei, representing cell numbers. Results for EIPA are shown in panel A, NH4Cl in B, bafilomycin (Baf) in C, jasplakinolide (Jas) and cytochalasin D (CytD) in D, the PKC inhibitor Gö6976 in E, and the Pak1 inhibitor IPA-3 and its inactive derivative PIR3.5 in F and G for both Ad35-eGFP and Ad5-eGFP, respectively. Panels H to K show results with macropinocytic interference in Wi-38 cells transduced with Ad35-eGFP or Ad5-eGFP. Note the absence of significant cell toxicity, as indicated by cell number measurements shown with gray line graphs, with 100% representing noninfected non-drug-treated conditions.
Another class of commonly used inhibitors of macropinocytosis are actin-directed compounds. Macropinosome formation involves filamentous actin (F-actin) and is sensitive to pharmacological inhibitors (64, 67), including jasplakinolide (Jas), which stabilizes actin polymers, cytochalasin D (CytD), which blocks actin polymerization at the barbed ends of F-actin, and the G-actin binding marine macrolide latrunculin B (LatB) (10, 18, 79). Both Jas and CytD inhibited Ad35-eGFP transduction of HeLa-ATCC and HK-2 cells in a dose-dependent manner (Fig. 3D), and LatB inhibited Ad35-eGFP transduction of Wi-38 cells (Fig. 3I).
A further requirement for macropinocytosis is the activation of calcium and diacylglycerol-dependent protein kinase C (PKC) family members (4, 68). PKC activators induce ruffling and fluid uptake by various mechanisms involving signal transduction at the plasma membrane, which can be inhibited, for example, by the calcium-dependent PKC inhibitor Gö6976 (17, 62). The treatment of HeLa-ATCC or HK-2 cells with Gö6976 led to a dose-dependent inhibition of Ad35-eGFP transduction (Fig. 3E), similar to the case with Ad3, which uses PKC-dependent macropinocytosis for infectious entry into epithelial cells (3). Interestingly, Gö6976 had no effect on Ad35-eGFP transduction of Wi-38 cells, suggesting a cell type-variable involvement of PKC in Ad35 infection (data not shown).
An important regulator of macropinocytosis is the serine/threonine kinase p21-activated kinase (Pak) (20, 67). We treated cells with the Pak inhibitor IPA-3, which allosterically blocks autoinhibited Pak1 and related isoforms of this kinase (19). Ad35 transduction of HeLa-ATCC, HK-2, or Wi-38 cells was inhibited by IPA-3 in a dose-dependent manner but not by an inactive IPA-3-related compound, PIR3.5 (Fig. 3F and J). In HeLa-ATCC or HK-2 cells, IPA-3 treatment specifically inhibited Ad35-eGFP without affecting Ad5-eGFP transduction (Fig. 3G), although Ad5 transduction of Wi-38 cells was sensitive to IPA-3 at low micromolar concentrations (Fig. 3K). It is possible that Paks are overexpressed and/or hyperactivated in HeLa cells, consistent with the notion that signaling pathways in human tumor cells differ from those in normal tissue (23). Ad5 could thus circumvent Pak-1 inhibition in cancer cells by using other signaling pathways, which are not upregulated in normal human diploid fibroblasts Wi-38 cells. Notably, our earlier data showed that in cancer cells, Ad2/5 depends on dynamin and clathrin-mediated pathways (31), activates Pak1, and triggers accessory Pak1-dependent macropinocytosis, as indicated by RNA interference (3, 62). We conclude that Ad35 transduction is sensitive to inhibition of macropinocytosis.
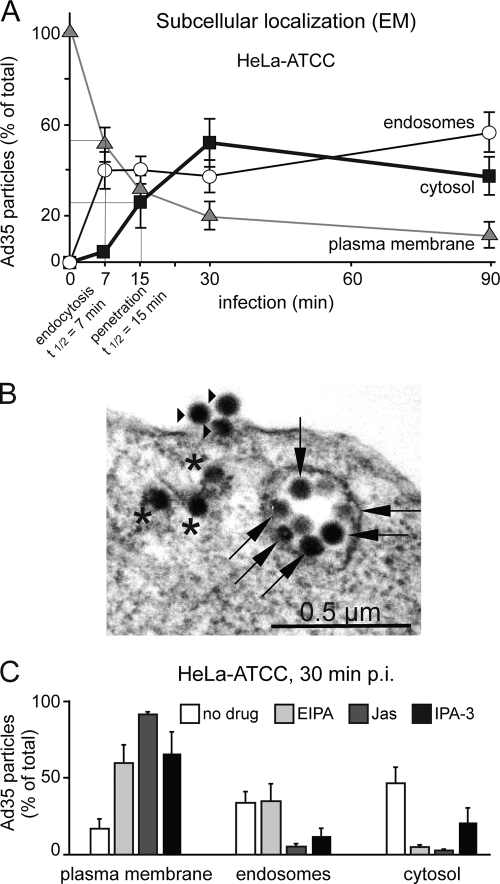
We next used electron microscopy (EM) to determine the subcellular localization of Ad35 in epithelial HeLa-ATCC cells. Time course analyses showed that Ad35 particles were rapidly and efficiently cleared from the plasma membrane, and more than 50% of the virus particles localized to the cytosol at 30 min postinfection (p.i.) (Fig. 4A; for a representative image, see Fig. 4B). This indicated rapid viral uptake and endosomal escape, with estimated half-maximal times of 7 and 15 min, respectively. EIPA, Jas, and IPA-3 delayed viral uptake into cells and strongly reduced Ad35 localization in the cytosol 30 min p.i. (Fig. 4C). This suggested that infectious Ad35 endocytosis required sodium-proton exchangers, dynamic F-actin, and Pak1.
FIG. 4.
EM analysis of Ad35 entry into HeLa-ATCC cells. (A) Cells on glass coverslips were incubated with doubly CsCl-purified Ad35 particles for 90 min in the cold at high a MOI of 5,000, washed extensively, warmed to 37°C in growth medium containing 0.2% BSA for the indicated times, fixed, and processed for quantitative transmission EM analyses of virus particles at the plasma membrane, in endosomes, and in the cytosol. Half-maximal time points for endocytic uptake and penetration into the cytosol were 7 and 15 min, respectively. (B) Electron micrograph of an Ad35-infected HeLa-ATCC cell 30 min p.i. Stars depict virus particles in the cytosol, arrows depict particles in endosomes, and arrowheads depict particles at the plasma membrane. (C) The same experiment was repeated with cells treated or not treated with the sodium/proton inhibitor EIPA (50 μM), the F-actin stabilizer Jas (300 nM), or the Pak1 inhibitor IPA-3 (25 μM) and infected for 30 min.
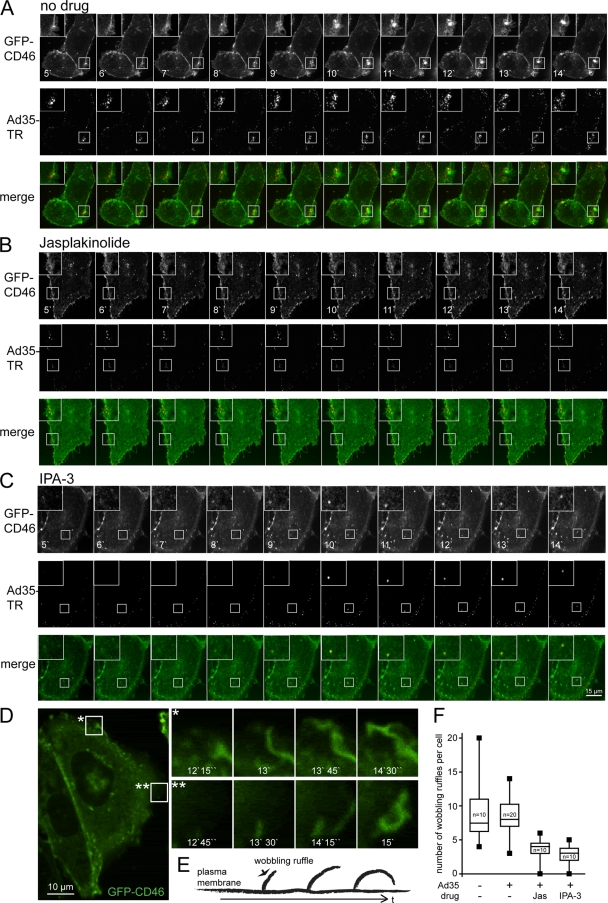
We next analyzed if actin inhibition by Jas, or Pak1 inhibition by IPA-3, affected the dynamics of the plasma membrane during entry of Texas Red-labeled Ad35 (Ad35-TR). For this, we monitored the localization of the eGFP-tagged human CD46 BC1 splice form in stably transfected Chinese hamster ovary cells (CHO-GFP-CD46) in warm infected cells 5 to 15 min p.i. CHO cells are CD46 negative and resistant to Ad35 infection, whereas GFP-CD46 expression in these cells (CHO-CD46) facilitates Ad35 transduction similarly to CD46 expression (data not shown, and 25, 26, 30). Control infected or noninfected cells showed extensive dynamics of the plasma membrane marker GFP-CD46, including membrane ruffling and protrusions, which we call here collectively “wobbling ruffles,” and frequent events of virus and CD46 colocalizations were observed (Fig. 5A [see also Movie S1 in the supplemental material] and Fig. 5D [see also Movie S4]). In contrast, the wobbling ruffles of GFP-CD46 at the plasma membrane were strongly suppressed in cells treated with Jas, although Ad35-TR particles readily associated with the cell periphery and remained confined there throughout the observation period (Fig. 5B; see Movie S2). Likewise, the Pak1 inhibitor IPA-3 suppressed to a large extent the peripheral wobbling of GFP-CD46 (Fig. 5C and 5F; see Movie S3). Significantly, the virus particles in the cell periphery remained confined and were largely immobile, suggesting that IPA-3 inhibited viral uptake into cells (see Fig. 4B). Interestingly, viruses that were attached to protruding filopodium-like extensions of control or IPA-3-treated cells moved toward the cell body. Such movements were not observed in Jas-treated cells, where filopodium-associated viruses remained stationary. This is consistent with earlier results showing that filopodial dynamics can be blocked by Jas, which freezes F-actin (10, 92). Pak1 apparently does not contribute to filopodial surfing of Ad35. Whether filopodial surfing of Ad35 contributes to infection remains to be analyzed.
FIG. 5.
Ad35-TR is associated with GFP-CD46 clusters early in infection. Stably transfected CHO-GFP-CD46 cells were infected with Ad35-TR (1 μg/ml) for 5 min and recorded in a spinning disc confocal microscope equipped with a warm chamber at 37°C from 5 min to 15 min p.i. at an acquisition frequency of 0.06 Hz. (A) Control cell with extended wobbling of GFP-CD46 membrane domains and frequently overlapping signals of CD46 and Ad35-TR. B Cell pretreated with 300 nM jasplakinolide (Jas) for 30 min. (C) Cell pretreated with 25 μM IPA-3. Time stamps are in min (′). (D) Analyses of GFP-CD46 wobbling ruffles, with schematic drawing shown in panel E of an untreated, infected cell with two zoom-in views marked “*” and “**” with the corresponding time stamp min (′) and seconds (′′) p.i. (F) Quantitative box plot analyses of wobbling ruffles in control noninfected cells, infected cells, and infected cells treated with Jas or IPA-3.
Pak1 and CtBP1 facilitate infectious Ad35 macropinocytosis.
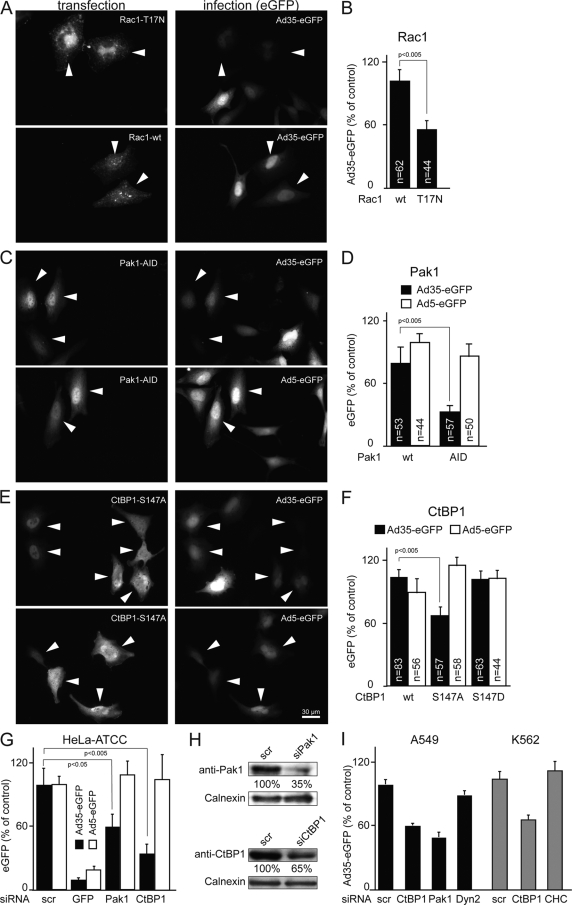
Pak1 is activated by the small GTPase Rac1 (Ras-related C3 botulinum toxin substrate 1) during growth factor-stimulated macropinocytosis, cell adhesion, and motility (40) and is implicated in endocytosis of interleukin-2 receptor (32) and infectious macropinocytosis of Ad3 and vaccinia virus (3, 66). The expression of dominant-negative Rac1 (with threonine 17 mutated to asparagine [T17N]) inhibited the expression of Ad35-eGFP by about 50% (Fig. 6A and B). Likewise, the autoinhibitory domain of Pak1 reduced Ad35-eGFP levels by about 70% but did not affect Ad5-eGFP (Fig. 6C and D), supporting the notion that Pak1 is involved in species B but not species C adenovirus infections (3).
FIG. 6.
Rac1, Pak1, and CtBP1 are required for Ad35-eGFP transduction of HeLa-ATCC cells. Cells were transfected with the indicated constructs for 24 h and infected for 8 h, fixed, recorded in a wide-field microscope, and analyzed with ImageJ. (A) Representative images of dominant-negative mRFP-T17N-Rac1- or Rac-wt-transfected cells. (B) Quantification of the mean fluorescence intensity per cell with indicated number of cells (n). (C and D) Representative images of Pak1 autoinhibitory domain (AID)-transfected cells infected with Ad35-eGFP or Ad5-eGFP (C) and quantification of the mean fluorescence intensity per cell (D). (E) Representative images of phosphorylation-defective CtBP1-S147A-transfected cells infected with Ad35-eGFP or Ad5-eGFP. (F) Quantification of the mean fluorescence intensities of GFP expression per cell in wild-type (wt) CtBP1 and the S147A and S147D CtBP1 mutants. (G) Cells were transfected with 50 nM siRNA against indicated targets for 72 h, infected for 8 h, and analyzed with Safire2. Cell numbers were normalized to the DAPI signal. (H) Knockdown quantification of Pak1 and CtBP1 by Western blotting, normalized against calnexin. (I) A549 cells were transfected with 20 nM siRNA against CtBP1, Pak1, or dynamin2 for 48 h, infected with Ad35-eGFP (16 h), and analyzed by flow cytometry. K562 cells were transfected with 20 nM siRNA against CtBP1 or clathrin heavy-chain (CHC) as described previously (3), infected with Ad35-eGFP (16 h), and analyzed by flow cytometry.
Activated Pak1 phosphorylates the transcriptional repressor C-terminal binding protein 1 (CtBP1) at serine 147 in the nucleus and recruits CtBP1 into the cytoplasm (7). There are two splice forms of CtBP1, a short form truncated by 11 amino acids (also called CtBP3/BARS) and a long form (CtBP1). Both forms control dynamin-independent endocytosis (9), membrane fission, and endocytic cup formation (3, 42, 55). We found that the expression of dominant-negative, phosphorylation-defective CtBP1 (serine 147-to-alanine mutation [S147A]) but not the phosphomimetic S147D mutant reduced Ad35-eGFP by about 35% without affecting Ad5-eGFP (Fig. 6E and F). These results were corroborated by siRNA experiments against Pak1 and CtBP1 in HeLa-ATCC and human alveolar epithelial A549 cells. Knockdown of Pak1 by 65% and CtBP1 by 35% (measured by Western blotting in HeLa cells) reduced Ad35 transduction between 40 and 60%, depending on the cell type, but without affecting Ad5-eGFP expression (Fig. 6G and H and data not shown). Control siRNAs against eGFP inhibited both Ad35- and Ad5-eGFP expression (Fig. 6G). Similar to the case with epithelial cells, Ad35-eGFP transduction of hematopoietic K562 cells was inhibited by CtBP1 siRNA but not by clathrin siRNA, analogous to an earlier report for Ad3 (3). We concluded that CtBP1 supports Ad35 infection.
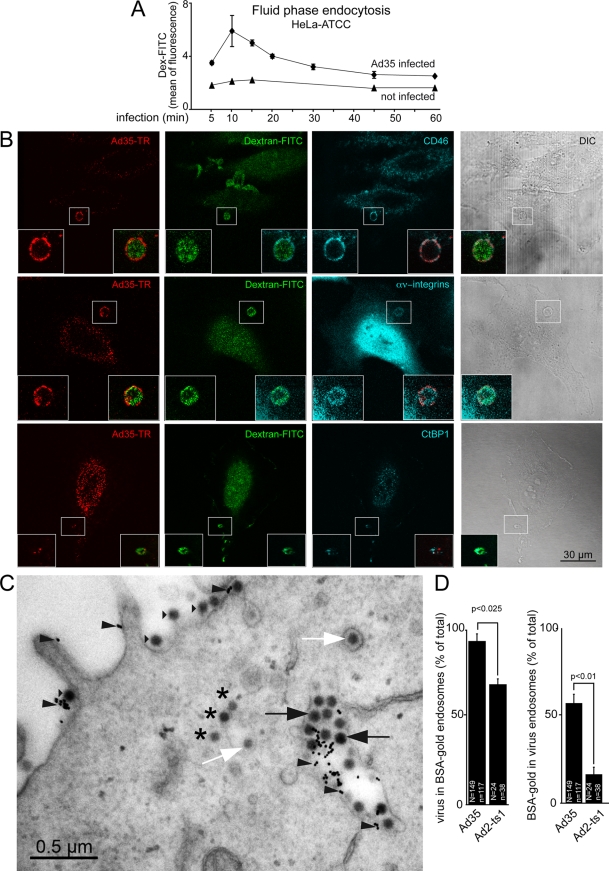
Both Pak1 and CtBP1 are involved in macropinocytosis, in which cells engulf a large amount of fluids. We tested if Ad35 induced the endocytic uptake of fluid phase into HeLa-ATCC cells. Ad35 was bound to HeLa-ATCC in the cold, free virus was washed off, and cells were incubated in warm medium and pulsed with dextran-fluorescein isothiocyanate (FITC) for 5 min prior to analyses of acid-washed cells for intracellular dextran by flow cytometry. We found a peak of fluid-phase uptake 10 min postwarming, indicating that Ad35 transiently induced dextran uptake (Fig. 7A), similar to the case with Ad2/5 and Ad3 (3, 39, 62, 63). Multichannel confocal immunofluorescence analyses revealed that dextran and Ad35-TR-positive endosomes contained CD46, αν integrins, and to a lesser degree also CtBP1 at 10 min p.i. (Fig. 7B). Noninfected cells did not contain detectable dextran-FITC-positive endosomes under these pulse-labeling conditions, indicating that the dextran-positive endosomal structures described in Fig. 7B were virus induced (see Fig. S1 in the supplemental material). Quantitative analyses using wide-field fluorescence microscopy showed that 91% of the dextran-filled vesicles in Ad35-infected cells were positive for CD46, essentially all were αν integrin positive, and 77% were positive for CtBP1 (see Fig. S2).
FIG. 7.
Ad35-TR induces fluid phase uptake and colocalizes with CD46, integrins, and CtBP1 in dextran-filled macropinosomes. (A) Ad35 (2 mg/ml) was cold bound to HeLa-ATCC cells for 1 h. Cells were washed, pulsed with dextran-FITC (0.5 mg/ml) in warm medium (37°C) containing BSA 5 min before the indicated time points, and prepared for flow cytometric analysis. (B) Cells were cold bound with Ad35-TR (1 μg/ml) for 1 h, washed, and incubated at 37°C in the presence of dextran-FITC (0.5 mg/ml) for 10 min, fixed, stained for the indicated antigens, and analyzed by confocal laser scanning microscopy with corresponding differential interference contrast (DIC) images. Fluorescence images represent single sections, and insets are magnifications of the white boxed areas. (C) Electron micrograph of an Ad35-infected HeLa-ATCC cell (cold synchronized infection at a MOI of 5,000), pulsed in the presence of BSA-nanogold for 10 min. Arrowheads indicate BSA-gold particles, black arrows show Ad35 in BSA-gold-positive endosomes, white arrows show virus particles in endosomes without BSA-gold, small triangles indicate viruses at the plasma membrane, and stars depict Ad35 particles in the cytosol. (D) Quantification of panel C, including HeLa-ATCC cells inoculated with Ad2-ts1, which is defective in endosomal escape. “N” indicates the number of virus particles, and “n” indicates the number of BSA-gold particles.
Transmission electron microscopy showed that at 10 min p.i., Ad35 particles were in large endosomes several micrometers in diameter (Fig. 7C). These endosomes contained cointernalized BSA-gold. To test if macropinocytosis contributed to the levels of BSA-gold in Ad35-positive endosomes, we compared the amounts of BSA-gold in endosomes containing Ad2-ts1. Ad2-ts1 enters cells by dynamin and clathrin-mediated endocytosis in the absence of macropinocytic stimulation (31, 39). More than 90% of the endosomal Ad35 particles were in BSA-gold-positive vesicles, whereas about 65% of the Ad2-ts1 particles were in gold-positive endosomes (Fig. 7D). We found that about 60% of the total BSA-gold particles were in Ad35-positive endosomes, whereas about 15% of the total BSA-gold was in Ad2-ts1-positive endosomes. This strongly supported the conclusion that Ad35 was predominantly in fluid-enriched endosomes 10 min p.i. Together the data indicate that Ad35 induces CD46 and integrin-dependent macropinocytosis for infectious uptake into epithelial HeLa-ATCC and kidney HK-2 cells, as well as hematopoietic cells (Fig. 8 shows a schematic overview).
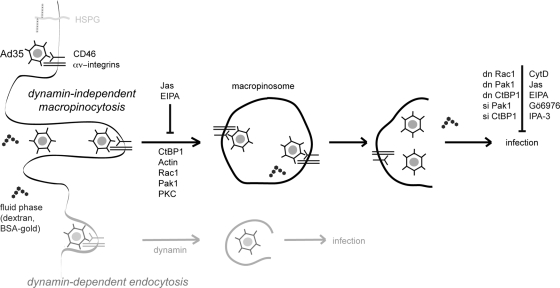
FIG. 8.
Schematic model of infectious macropinocytosis of Ad35 in human epithelial cells. Ad35 binds to CD46 and αν integrins independently of heparan sulfate proteoglycans (HSPGs). Ad35 is internalized in an actin-, Rac1-, Pak1-, CtBP1-, and PKC-dependent manner and localizes to dextran-filled vesicles with its receptors CD46, αν integrins, and also CtBP1. Ad35 escapes to the cytosol by an unknown mechanism and traffics to the nucleus for infection. dn, dominant negative; si, small interfering.
DISCUSSION
Adenoviruses cause infectious disease and significant health problems across the world. Therapeutic forms of adenoviruses are increasingly being developed to treat human diseases, including cancer and immune disorders (91, 97). Research in the past years has shown that both virulent and therapeutic viruses strongly depend on host factors for eliciting therapeutic or disease phenotypes (for overviews, see references 46, 91, and 93). Here we analyzed the early interactions of Ad35 with cultured human cells and showed that they involve macropinocytosis of CD46-associated Ad35 particles. Unlike the case in an earlier study of CHO cells (98), we found no requirement of heparan sulfate proteoglycans for Ad35 infection of HeLa or human kidney HK-2 cells, which emphasizes the importance of CD46 for infectious entry of Ad35 (30). This is in line with earlier findings showing that the species B1 Ad3 uses macropinocytosis for infectious uptake into epithelial and hematopoietic cells (3). Notably, Ad35 is one of several species B human adenoviruses which have been shown to bind with their fiber knobs to CD46 (25, 26, 30, 78). Other CD46-tropic adenoviruses include Ad3 (94), Ad11 (90), Ad14, Ad16, Ad21, and Ad50 (30) and also the species D Ad37 (105) and Ad49 (53). It is possible that members of the species B HAdVs use CD46 with different affinities or by unknown mechanisms or bind to additional receptors.
In addition to binding to the species B HAdVs, CD46 also binds the Edmonston strain of measles virus (22, 74), human herpes virus 6 (88), bovine viral diarrhea virus (61), and various bacteria, including uropathogenic Escherichia coli (54, 56). The selection of CD46 as a receptor for numerous pathogens suggests that there are advantages for pathogens in binding to CD46, e.g., dampening or suppression of innate immune responses (43, 76). The ubiquitously expressed human CD46 is involved in the control of complement lysis and inhibition of T-cell effector functions (6, 44). The cytoplasmic splice variant 1 of CD46 controls the induction of autophagic degradation (41), which can lead to enhanced presentation of antigenic peptides to major histocompatibility complexes of class II and promote adaptive immune responses and inflammation (89). Although both cytoplasmic splice variants 1 and 2 of CD46 have been found to bind Ad3 and Ad35 (30, 94), it is unknown if binding of HAdVs to CD46 induces autophagy.
Our data here indicate that Ad35 induced infectious CD46-dependent macropinocytosis, similar to Ad3 in epithelial cells (3), and this could be related to integrin-dependent Ad35 infection of hematopoietic cells (71). The pathway that we delineate for Ad35 infection involves αν integrins and is independent of clathrin-mediated endocytosis, as concluded from insensitivity against dynamin inhibition by dynasore, dominant-negative constructs, or siRNA in HeLa-ATCC cells, Wi-38 human lung fibroblasts, A549 cells, or hematopoietic K562 cells. Interestingly, K44A-dynamin inhibited the transduction of Ad35 in HeLa-K cells, a variant of HeLa-ATCC cells, but infection of these cells was not affected by the C-terminal fragment of AP180, which binds to clathrin and blocks clathrin-mediated endocytosis (28). This is similar to Ad3 transduction described earlier (3). Since dynamin interference inhibited the uptake of transferrin, a classical ligand for clathrin-mediated endocytosis, to a similar extent in both HeLa-ATCC and HeLa-K cells, we speculate that in certain cell types, such as HeLa-K, dynamin supports Ad3 and Ad35 transduction at the level of endosomal trafficking (50, 51). This speculation could be supported by an earlier finding that the clathrin binding protein CALM (clathrin assembly lymphoid myeloid) is required for infection with wild-type Ad2 but not the endosomal escape-defective mutant Ad2-ts1 (39).
The requirements for infectious endocytosis of Ad35 by human epithelial cells closely reflect those for macropinocytosis, as indicated by drug sensitivity and morphological analyses at the level of light microscopy and EM. We found a strong but cell type-dependent requirement of Ad35 transduction for PKC and a strong cell type-independent requirement for the sodium/proton exchanger, actin, and Pak1 in HeLa-ATCC, HK-2, and Wi-38 cells, as well as the actin modulator Rac1 and the Pak1 effector CtBP1 in HeLa-ATCC and HK-2 cells. CtBP1 is commonly acting as a transcriptional repressor and membrane organizer (11, 14). Ad35 colocalized with its receptors CD46 and αν-integrins in dextran-filled macropinosomes and was associated with dynamic GFP-CD46 clusters early in infection. These endosomes were positive for CtBP1, unlike noninfected cells, suggesting that CtBP1 was recruited to Ad35-positive membrane domains, possibly from the nucleus upon phosphorylation by Pak1 (7). This was supported by the finding that dominant-negative phosphorylation-defective CtBP1 inhibited Ad35 transduction. Since macropinocytosis has been implicated in immune suppression, for example in the uptake of apoptotic bodies (1), it is possible that Pak1/CtBP1-dependent macropinocytosis silences immune responses in certain cell types.
Together, our results provide supportive evidence that cross-linking of CD46 by multivalent Ad35 particles leads to the formation of macropinocytic vesicles, similar to cross-linking induced by anti-CD46 antibodies or measles virus which triggered the formation of pseudopodia and macropinocytic engulfment of CD46 (16). This indicates that macropinocytosis is an infectious uptake route exploited by an increasing number of pathogens and has implications for gene delivery and vaccination strategies.
Supplementary Material
Acknowledgments
We thank Nicola Imelli for expert help in EM analyses, Jeff Peterson for IPA-3, and Corinne Wilhelm for excellent cell culture and support.
This project was supported by the Swiss National Science Foundation and in part by the Swiss SystemsX.ch initiative, grant LipidX-2008/011 to U.F.G.
Footnotes
Published ahead of print on 17 March 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Albert, M. L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4:223-231. [DOI] [PubMed] [Google Scholar]
- 2.Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64:125-136. [DOI] [PubMed] [Google Scholar]
- 3.Amstutz, B., M. Gastaldelli, S. Kälin, N. Imelli, K. Boucke, E. Wandeler, J. Mercer, S. Hemmi, and U. F. Greber. 2008. Subversion of CtBP1 controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27:956-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amyere, M., B. Payrastre, U. Krause, P. V. Smissen, A. Veithen, and P. J. Courtoy. 2000. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 11:3453-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki, N., M. T. Johnson, and J. A. Swanson. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astier, A. L. 2008. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology 124:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, C. J., R. K. Vadlamudi, S. K. Mishra, R. H. Jacobson, F. Li, and R. Kumar. 2003. Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat. Struct. Biol. 10:622-628. [DOI] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 9.Bonazzi, M., S. Spano, G. Turacchio, C. Cericola, C. Valente, A. Colanzi, H. S. Kweon, V. W. Hsu, E. V. Polishchuck, R. S. Polishchuck, M. Sallese, T. Pulvirenti, D. Corda, and A. Luini. 2005. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7:570-580. [DOI] [PubMed] [Google Scholar]
- 10.Burckhardt, C. J., and U. F. Greber. 2009. Virus movements on the plasma membrane support infection and transmission between cells. PLoS Pathog. 5:e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinnadurai, G. 2007. Transcriptional regulation by C-terminal binding proteins. Int. J. Biochem. Cell Biol. 39:1593-1607. [DOI] [PubMed] [Google Scholar]
- 12.Chiu, C. Y., P. Mathias, G. R. Nemerow, and P. L. Stewart. 1999. Structure of adenovirus complexed with its internalization receptor, alpha(v)beta 5 integrin. J. Virol. 73:6759-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, J., and W. Powderly. 2004. Infectious diseases, 2nd ed. Mosby, New York, NY.
- 14.Corda, D., A. Colanzi, and A. Luini. 2006. The multiple activities of CtBP/BARS proteins: the Golgi view. Trends Cell Biol. 16:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Cosson, P., I. de Curtis, J. Pouyssegur, G. Griffiths, and J. Davoust. 1989. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J. Cell Biol. 108:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crimeen-Irwin, B., S. Ellis, D. Christiansen, M. J. Ludford-Menting, J. Milland, M. Lanteri, B. E. Loveland, D. Gerlier, and S. M. Russell. 2003. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J. Biol. Chem. 278:46927-46937. [DOI] [PubMed] [Google Scholar]
- 17.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayel, M. J., and R. D. Mullins. 2004. Activation of Arp2/3 complex: addition of the first subunit of the new filament by a WASP protein triggers rapid ATP hydrolysis on Arp2. PLoS Biol. 2:E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deacon, S. W., A. Beeser, J. A. Fukui, U. E. Rennefahrt, C. Myers, J. Chernoff, and J. R. Peterson. 2008. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15:322-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharmawardhane, S., A. Schurmann, M. A. Sells, J. Chernoff, S. L. Schmid, and G. M. Bokoch. 2000. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11:3341-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty, G. J., and H. T. McMahon. 2009. Mechanisms of endocytosis. Annu. Rev. Biochem. 78:857-902. [DOI] [PubMed] [Google Scholar]
- 22.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 23.Dummler, B., K. Ohshiro, R. Kumar, and J. Field. 2009. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 28:51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felding-Habermann, B., B. M. Mueller, C. A. Romerdahl, and D. A. Cheresh. 1992. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J. Clin. Invest. 89:2018-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischli, C., D. Sirena, G. Lesage, M. J. Havenga, R. Cattaneo, U. F. Greber, and S. Hemmi. 2007. Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J. Gen. Virol. 88:2925-2934. [DOI] [PubMed] [Google Scholar]
- 26.Fleischli, C., S. Verhaagh, M. Havenga, D. Sirena, W. Schaffner, R. Cattaneo, U. F. Greber, and S. Hemmi. 2005. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J. Virol. 79:10013-10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontenot, D. R., P. den Hollander, E. M. Vela, R. Newman, J. K. Sastry, and R. Kumar. 2007. Dynein light chain 1 peptide inhibits human immunodeficiency virus infection in eukaryotic cells. Biochem. Biophys. Res. Commun. 363:901-907. [DOI] [PubMed] [Google Scholar]
- 28.Ford, M. G., B. M. Pearse, M. K. Higgins, Y. Vallis, D. J. Owen, A. Gibson, C. R. Hopkins, P. R. Evans, and H. T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291:1051-1055. [DOI] [PubMed] [Google Scholar]
- 29.Fretz, M., J. Jin, R. Conibere, N. A. Penning, S. Al-Taei, G. Storm, S. Futaki, T. Takeuchi, I. Nakase, and A. T. Jones. 2006. Effects of Na+/H+ exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein transduction domains HIV-TAT peptide and octaarginine. J. Control Release 116:247-254. [DOI] [PubMed] [Google Scholar]
- 30.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 31.Gastaldelli, M., N. Imelli, K. Boucke, B. Amstutz, O. Meier, and U. F. Greber. 2008. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 9:2265-2278. [DOI] [PubMed] [Google Scholar]
- 32.Grassart, A., A. Dujeancourt, P. B. Lazarow, A. Dautry-Varsat, and N. Sauvonnet. 2008. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 9:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 34.Haga, Y., N. Miwa, S. Jahangeer, T. Okada, and S. Nakamura. 2009. CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis. EMBO J. 28:1197-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall, K., M. E. Blair Zajdel, and G. E. Blair. 2009. Defining the role of CD46, CD80 and CD86 in mediating adenovirus type 3 fiber interactions with host cells. Virology 392:222-229. [DOI] [PubMed] [Google Scholar]
- 36.Harris, C., and L. Fliegel. 1999. Amiloride and the Na(+)/H(+) exchanger protein: mechanism and significance of inhibition of the Na(+)/H(+) exchanger (review). Int. J. Mol. Med. 3:315-321. [DOI] [PubMed] [Google Scholar]
- 37.Hierholzer, J. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5:262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huotari, V., J. Vaaraniemi, V. P. Lehto, and S. Eskelinen. 1996. Regulation of the disassembly/assembly of the membrane skeleton in Madin-Darby canine kidney cells. J. Cell. Physiol. 167:121-130. [DOI] [PubMed] [Google Scholar]
- 39.Imelli, N., Z. Ruzsics, D. Puntener, M. Gastaldelli, and U. F. Greber. 2009. Genetic reconstitution of the human adenovirus type 2 temperature-sensitive 1 mutant defective in endosomal escape. Virol. J. 6:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 41.Joubert, P. E., G. Meiffren, I. P. Gregoire, G. Pontini, C. Richetta, M. Flacher, O. Azocar, P. O. Vidalain, M. Vidal, V. Lotteau, P. Codogno, C. Rabourdin-Combe, and M. Faure. 2009. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe 6:354-366. [DOI] [PubMed] [Google Scholar]
- 42.Karjalainen, M., E. Kakkonen, P. Upla, H. Paloranta, P. Kankaanpaa, P. Liberali, G. H. Renkema, T. Hyypia, J. Heino, and V. Marjomaki. 2008. A Raft-derived, Pak1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol. Biol. Cell 19:2857-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 44.Kemper, C., and J. P. Atkinson. 2007. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 7:9-18. [DOI] [PubMed] [Google Scholar]
- 45.Knaus, U. G., Y. Wang, A. M. Reilly, D. Warnock, and J. H. Jackson. 1998. Structural requirements for PAK activation by Rac GTPases. J. Biol. Chem. 273:21512-21518. [DOI] [PubMed] [Google Scholar]
- 46.Knipe, D. M., and P. M. Howley. 2007. Fields virology, 5th ed., vol. 1 and 2. Lippincott Williams & Wilkins, Philadelphia, PA.
- 47.Koivusalo, M., C. Welch, H. Hayashi, C. C. Scott, M. Kim, T. Alexander, N. Touret, K. M. Hahn, and S. Grinstein. 2010. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188:547-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13:155-171. [DOI] [PubMed] [Google Scholar]
- 49.Kosulin, K., C. Haberler, J. A. Hainfellner, G. Amann, S. Lang, and T. Lion. 2007. Investigation of adenovirus occurrence in pediatric tumor entities. J. Virol. 81:7629-7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreitzer, G., A. Marmorstein, P. Okamoto, R. Vallee, and E. Rodriguez-Boulan. 2000. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat. Cell Biol. 2:125-127. [DOI] [PubMed] [Google Scholar]
- 51.Lajoie, P., and I. R. Nabi. 2007. Regulation of raft-dependent endocytosis. J. Cell Mol. Med. 11:644-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leen, A. M., and C. M. Rooney. 2005. Adenovirus as an emerging pathogen in immunocompromised patients. Br. J. Haematol. 128:135-144. [DOI] [PubMed] [Google Scholar]
- 53.Lemckert, A. A., J. Grimbergen, S. Smits, E. Hartkoorn, L. Holterman, B. Berkhout, D. H. Barouch, R. Vogels, P. Quax, J. Goudsmit, and M. J. Havenga. 2006. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol. 87:2891-2899. [DOI] [PubMed] [Google Scholar]
- 54.Li, K., M. J. Feito, S. H. Sacks, and N. S. Sheerin. 2006. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J. Immunol. 177:2543-2551. [DOI] [PubMed] [Google Scholar]
- 55.Liberali, P., E. Kakkonen, G. Turacchio, C. Valente, A. Spaar, G. Perinetti, R. A. Bockmann, D. Corda, A. Colanzi, V. Marjomaki, and A. Luini. 2008. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27:970-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindahl, G., U. Sjobring, and E. Johnsson. 2000. Human complement regulators: a major target for pathogenic microorganisms. Curr. Opin. Immunol. 12:44-51. [DOI] [PubMed] [Google Scholar]
- 57.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macia, E., M. Ehrlich, R. Massol, E. Boucrot, C. Brunner, and T. Kirchhausen. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839-850. [DOI] [PubMed] [Google Scholar]
- 59.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 79:14429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maurer, K., T. Krey, V. Moennig, H. J. Thiel, and T. Rumenapf. 2004. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 78:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier, O., M. Gastaldelli, K. Boucke, S. Hemmi, and U. F. Greber. 2005. Early steps of clathrin-mediated endocytosis involved in phagosomal escape of Fcgamma receptor-targeted adenovirus. J. Virol. 79:2604-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meier, O., and U. F. Greber. 2003. Adenovirus endocytosis. J. Gene Med. 5:451-462. [DOI] [PubMed] [Google Scholar]
- 65.Mellman, I. 2005. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv. Exp. Med. Biol. 560:63-67. [DOI] [PubMed] [Google Scholar]
- 66.Mercer, J., and A. Helenius. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531-535. [DOI] [PubMed] [Google Scholar]
- 67.Mercer, J., and A. Helenius. 2009. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510-520. [DOI] [PubMed] [Google Scholar]
- 68.Miyata, Y., E. Nishida, S. Koyasu, I. Yahara, and H. Sakai. 1989. Protein kinase C-dependent and -independent pathways in the growth factor-induced cytoskeletal reorganization. J. Biol. Chem. 264:15565-15568. [PubMed] [Google Scholar]
- 69.Miyauchi, K., Y. Kim, O. Latinovic, V. Morozov, and G. B. Melikyan. 2009. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motley, A., N. A. Bright, M. N. Seaman, and M. S. Robinson. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murakami, S., F. Sakurai, K. Kawabata, N. Okada, T. Fujita, A. Yamamoto, T. Hayakawa, and H. Mizuguchi. 2007. Interaction of penton base Arg-Gly-Asp motifs with integrins is crucial for adenovirus serotype 35 vector transduction in human hematopoietic cells. Gene Ther. 14:1525-1533. [DOI] [PubMed] [Google Scholar]
- 72.Myerowitz, R. L., H. Stalder, M. N. Oxman, M. J. Levin, M. Moore, J. D. Leith, N. M. Gantz, J. C. Hierholzer, and J. C. Hierholzer. 1975. Fatal disseminated adenovirus infection in a renal transplant recipient. Am. J. Med. 59:591-598. [DOI] [PubMed] [Google Scholar]
- 73.Nagel, H., S. Maag, A. Tassis, F. O. Nestle, U. F. Greber, and S. Hemmi. 2003. The alphavbeta5 integrin of hematopoietic and nonhematopoietic cells is a transduction receptor of RGD-4C fiber-modified adenoviruses. Gene Ther. 10:1643-1653. [DOI] [PubMed] [Google Scholar]
- 74.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nunez, R., M. Ackermann, Y. Saeki, A. Chiocca, and C. Fraefel. 2001. Flow cytometric assessment of transduction efficiency and cytotoxicity of herpes simplex virus type 1-based amplicon vectors. Cytometry 44:93-99. [DOI] [PubMed] [Google Scholar]
- 76.Oliaro, J., A. Pasam, N. J. Waterhouse, K. A. Browne, M. J. Ludford-Menting, J. A. Trapani, and S. M. Russell. 2006. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc. Natl. Acad. Sci. U. S. A. 103:18685-18690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ophorst, O. J., K. Radosevic, M. J. Havenga, M. G. Pau, L. Holterman, B. Berkhout, J. Goudsmit, and M. Tsuji. 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Persson, B. D., S. Muller, D. M. Reiter, B. B. Schmitt, M. Marttila, C. V. Sumowski, S. Schweizer, U. Scheu, C. Ochsenfeld, N. Arnberg, and T. Stehle. 2009. An arginine switch in the species B adenovirus knob determines high-affinity engagement of cellular receptor CD46. J. Virol. 83:673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peterson, J. R., and T. J. Mitchison. 2002. Small molecules, big impact: a history of chemical inhibitors and the cytoskeleton. Chem. Biol. 9:1275-1285. [DOI] [PubMed] [Google Scholar]
- 80.Pickering, L. 2006. Red book: 2006 report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, IL.
- 81.Quinn, K., M. A. Brindley, M. L. Weller, N. Kaludov, A. Kondratowicz, C. L. Hunt, P. L. Sinn, P. B. McCray, Jr., C. S. Stein, B. L. Davidson, R. Flick, R. Mandell, W. Staplin, W. Maury, and J. A. Chiorini. 2009. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J. Virol. 83:10176-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raghu, H., N. Sharma-Walia, M. V. Veettil, S. Sadagopan, and B. Chandran. 2009. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J. Virol. 83:4895-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez, A., J. Goudsmit, A. Companjen, R. Mintardjo, G. Gillissen, D. Tax, J. Sijtsma, G. J. Weverling, L. Holterman, D. E. Lanar, M. J. Havenga, and K. Radosevic. 2008. Impact of recombinant adenovirus serotype 35 priming versus boosting of a Plasmodium falciparum protein: characterization of T- and B-cell responses to liver-stage antigen 1. Infect. Immun. 76:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 85.Ryan, M. J., G. Johnson, J. Kirk, S. M. Fuerstenberg, R. A. Zager, and B. Torok-Storb. 1994. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 45:48-57. [DOI] [PubMed] [Google Scholar]
- 86.Sakurai, F., S. Nakamura, K. Akitomo, H. Shibata, K. Terao, K. Kawabata, T. Hayakawa, and H. Mizuguchi. 2008. Transduction properties of adenovirus serotype 35 vectors after intravenous administration into nonhuman primates. Mol. Ther. 16:726-733. [DOI] [PubMed] [Google Scholar]
- 87.Sakurai, F., S. I. Nakamura, K. Akitomo, H. Shibata, K. Terao, K. Kawabata, T. Hayakawa, and H. Mizuguchi. 2009. Adenovirus serotype 35 vector-mediated transduction following direct administration into organs of nonhuman primates. Gene Ther. 16:297-302. [DOI] [PubMed] [Google Scholar]
- 88.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 89.Schmid, D., and C. Munz. 2007. Innate and adaptive immunity through autophagy. Immunity 27:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma, A., X. Li, D. S. Bangari, and S. K. Mittal. 2009. Adenovirus receptors and their implications in gene delivery. Virus Res. 143:184-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherer, N. M., and W. Mothes. 2008. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 18:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh, R., and K. Kostarelos. 2009. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol. 27:220-229. [DOI] [PubMed] [Google Scholar]
- 94.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kaelin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suomalainen, M., M. Y. Nakano, K. Boucke, S. Keller, R. P. Stidwill, and U. F. Greber. 1999. Microtubule-dependent minus and plus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144:657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swanson, J. A. 2008. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 9:639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tuve, S., H. Wang, J. D. Jacobs, R. C. Yumul, D. F. Smith, and A. Lieber. 2008. Role of cellular heparan sulfate proteoglycans in infection of human adenovirus serotype 3 and 35. PLoS Pathog. 4:e1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ulasov, I. V., M. A. Tyler, S. Zheng, Y. Han, and M. S. Lesniak. 2006. CD46 represents a target for adenoviral gene therapy of malignant glioma. Hum. Gene Ther. 17:556-564. [DOI] [PubMed] [Google Scholar]
- 100.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.West, M. A., M. S. Bretscher, and C. Watts. 1989. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.West, M. A., R. P. Wallin, S. P. Matthews, H. G. Svensson, R. Zaru, H. G. Ljunggren, A. R. Prescott, and C. Watts. 2004. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 305:1153-1157. [DOI] [PubMed] [Google Scholar]
- 104.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 105.Wu, E., S. A. Trauger, L. Pache, T. M. Mullen, D. J. von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.