Abstract
Substantial evidence for prion transmission via blood transfusion exists for many transmissible spongiform encephalopathy (TSE) diseases. Determining which cell phenotype(s) is responsible for trafficking infectivity has important implications for our understanding of the dissemination of prions, as well as their detection and elimination from blood products. We used bioassay studies of native white-tailed deer and transgenic cervidized mice to determine (i) if chronic wasting disease (CWD) blood infectivity is associated with the cellular versus the cell-free/plasma fraction of blood and (ii) in particular if B-cell (MAb 2-104+), platelet (CD41/61+), or CD14+ monocyte blood cell phenotypes harbor infectious prions. All four deer transfused with the blood mononuclear cell fraction from CWD+ donor deer became PrPCWD positive by 19 months postinoculation, whereas none of the four deer inoculated with cell-free plasma from the same source developed prion infection. All four of the deer injected with B cells and three of four deer receiving platelets from CWD+ donor deer became PrPCWD positive in as little as 6 months postinoculation, whereas none of the four deer receiving blood CD14+ monocytes developed evidence of CWD infection (immunohistochemistry and Western blot analysis) after 19 months of observation. Results of the Tg(CerPrP) mouse bioassays mirrored those of the native cervid host. These results indicate that CWD blood infectivity is cell associated and suggest a significant role for B cells and platelets in trafficking CWD infectivity in vivo and support earlier tissue-based studies associating putative follicular B cells with PrPCWD. Localization of CWD infectivity with leukocyte subpopulations may aid in enhancing the sensitivity of blood-based diagnostic assays for CWD and other TSEs.
Chronic wasting disease (CWD) is an infectious protein-misfolding disease, or transmissible spongiform encephalopathy (TSE), affecting cervids in North America (59, 76-79) and one Asian country (41, 68). CWD is unique among prion diseases in affecting free-ranging wildlife populations (deer, elk, and moose). Early and subsequent observations made by Williams and Miller (58, 79) related CWD transmission to direct contact with clinically affected deer, as well as indirect contact with environments previously populated by infected deer (57). Bioassay studies of white-tailed deer have demonstrated that body fluids and excreta (saliva, urine, feces, and blood) contain infectious prions (53, 54). Both clinical and preclinical CWD-infected deer harbored sufficient infectious prions to produce CWD in naïve white-tailed deer following ingestion of saliva or transfusion of whole blood (53, 54).
The detection of blood-borne infectious prions has important implications for our understanding of the spread of prions among and within individuals, as well as for the elimination of prions from blood products (13, 15, 33, 45), given the evidence for Creutzfeldt-Jakob disease (CJD) transmission via blood transfusion (16, 29, 47, 50, 62, 72, 73). Identifying the cell phenotype or cell-free protein fractions that harbor prion infectivity would contribute importantly to this understanding and to the development of blood-based assays to detect prion infection. We undertook the present studies to address these issues.
MATERIALS AND METHODS
Bioassay studies of white-tailed deer.
White-tailed deer fawns were provided by the Warnell School of Forestry and Natural Resources, University of Georgia, Athens—a region in which CWD has not been detected. The deer fawns were hand raised and human and indoor adapted before overnight transport directly to the Colorado State University (CSU) CWD research indoor isolation facility without contact with the native Colorado environment. The 4-month-old fawns were adapted to the facility housing conditions and diet for 2 months before the study start.
Genotyping.
All white-tailed deer were genotyped to determine their GG/GS (codon 96) status by the laboratory of Katherine O'Rourke, USDA-ARS, Pullman, WA. Deer were allocated into inoculation cohorts (n = 4) without knowledge of their G96 genotypes.
Biocontainment protocols.
Protocols to preclude extraneous exposure and cross contamination between cohorts of animals as previously described (53, 54) incorporated protective shower-in requirements, Tyvek clothing, masks, head covers, and footwear while maintaining stringent husbandry. Tonsil biopsy and terminal sample collections were taken with animal-specific biopsy and sample collection instruments to minimize the possibility of cross contamination. Bedding and liquid waste from each suite were either incinerated or collected in a dedicated outdoor underground holding tank and denatured by alkaline digestion.
Deer inoculation cohorts.
Groups of 6-month-old fawns (usually four per group) (Table 1) were housed in separate isolation suites throughout the study. Suite-dedicated protective clothing, utensils, and waste disposal were incorporated to exclude cross contamination by fomites, bedding, food, excretions, or contact. Deer cohorts 1 to 6 were inoculated by the intravenous (i.v.) route with blood components from CWD-infected donor deer housed in the CSU CWD isolation facility as follows: cohort 1, whole blood (250 ml); cohort 2, blood mononuclear cell fraction (1 × 107 to 1.24 × 108 white blood cells [WBC] plus platelets); cohort 3, cell-free plasma fraction (140 to 150 ml) recovered from 250 ml citrated whole blood; cohort 4, B cells (1 × 106 to 5 × 106) separated by Dynatec magnetic bead separation (98% purity) using anti-sheep B-cell monoclonal antibody (MAb) 2-104, which identifies peripheral blood B cells and may identify follicular dendritic cells (FDCs) in lymphoid germinal centers (80); cohort 5, CD41/61+ platelets (6 × 109 to 35 × 109) magnetically separated to 99% purity using MAb CAPPA 2A (VMRD, Pullman, WA); cohort 6, CD14+ cells (4 × 105) magnetically separated to 98% purity using anti-sheep CD14 MAb clone VPM65 (Fitzgerald Industries Inc., Concord, MA); cohort 7, naïve white-tailed deer inoculated with blood from CWD− deer, which served as negative controls for the study and were housed in a separate suite at the same facility.
TABLE 1.
White-tailed deer cohorts i.v. inoculated with blood components
| Cohort | No./cohort | Donor status | Inoculum source | Inoculum |
|---|---|---|---|---|
| 1 | 8 | CWD+ | Whole blood | 250 ml CWD+ blood |
| 2 | 4 | CWD+ | Blood mononuclear cells | 1 × 107-1.24 × 108 WBC + platelets |
| 3 | 4 | CWD+ | Cell-free plasma | 140-150 ml recovered from 250 ml blood |
| 4 | 4 | CWD+ | B cells | 1 × 106-5 × 106 2-104+ cells (98% pure) |
| 5 | 4 | CWD+ | Platelets | 6 × 109-35 × 109 CD41/61+ cells (99% pure) |
| 6 | 4 | CWD+ | Monocytes | 4 × 105 CD14+ cells (98% pure) |
| 7 | 2 | CWD− | Negative control | 250 ml CWD− blood |
Blood donor deer.
Six experimentally inoculated CWD+ and two CWD− deer housed at the CSU indoor research facility were recruited from previously described studies (54) for use as blood donors for these studies (Table 2).
TABLE 2.
CWD+/CWD− blood cell component donor history
| Recipient cohort(s) | Donor status | No. of donors | Donor source history | Donor incubation time/clinical status | Donor codon 96 G/S |
|---|---|---|---|---|---|
| 1, 2, 3 | CWD+ | 2 | i.c. inoculated with brain homogenate from naturally infected CWD+ deer TS-989-09147 | 14 months p.i.a/terminal clinical disease | G/S |
| 1, 4, 5, 6 | CWD+ | 2 blood pool | i.v. transfused with 250 ml whole blood from inoculated CWD+ deer TS989-CSU112 | 24 months p.i./late-stage clinical disease | G/G |
| 1, 4, 5, 6 | CWD+ | 2 brain pool | p.o. inoculated (2 g/day for 5 days) with CWD+ brain from naturally infected CWD+ deer TS-989-09147 or WDNR (1 each) | 23 months p.i./late-stage clinical disease | 1 G/G, 1 G/S |
| 7 | CWD− | 2 | i.v. infused with 250 ml CWD− whole blood from deer residing at Warnell School of Forestry and Natural Resources, Athens, GA | CWD− | G/G |
p.i., postinfection.
Blood donors for cohorts 1, 2, and 3.
Two CWD-infected deer previously inoculated intracranially (i.c.) with 1 g whole brain homogenate collected from a naturally infected CWD+ deer (TS-989-09147) were the source animals.
Blood donors for cohorts 1, 4, 5, and 6.
Four CWD-infected deer served as blood donors for cohorts 1, 4, 5, and 6. Two donors (designated brain pool) had been orally inoculated with 10 g (2 g/day for 5 days) brain from naturally infected field isolates (TS-989-09147 or WDNR). The remaining two donors (designated blood pool) had received 250 ml of blood via i.v. infusion from an experimentally inoculated CWD+ deer (TS989-CSU112). Two deer from each cohort (1, 4, 5, or 6) were inoculated with whole blood or specific cell phenotypes from the brain pool donors, while the other two deer from each cohort were inoculated with similar components from the blood pool donors.
Blood donors for cohort 7.
One CWD− deer, housed at the Warnell School of Forestry and Natural Resources, University of Georgia, Athens (UGA)—a region where CWD has not been detected—served as the donor for two negative control donors that each received 250 ml whole blood via i.v. infusion.
Thus, the inocula used reflected both a conscious attempt to assess the universality of the results obtained given the constraints of a limited number of recipient animals and limited amounts of inoculum materials (cell fractions etc.) available.
Blood collections, harvests, and inoculations. (i) Cohorts 1 to 3.
One liter sodium citrate-treated whole blood was collected from each of two CWD+ donor deer (Table 2) for cohort 1 to 3 inoculations (Table 1). The blood was not pooled. Half (500 ml) of each whole blood collection was immediately administered i.v. to two recipient cohort 1 deer (250 ml/deer)/donor deer (four recipients). Plasma and blood mononuclear cells harvested from 250-ml nonpooled aliquots of whole blood were administered i.v. to cohorts 2 and 3 (Table 1).
(ii) Cohorts 1, 4, 5, and 6.
Similarly, a portion (250 ml) of the whole blood collected from the blood pool and brain pool donors (Table 2) was administered i.v. to two recipient cohort 1 deer/donor (Table 1) (four recipients). The remaining blood collected from the blood pool was pooled, as was the remaining blood from the brain pool, and each pool was further processed to harvest specific cell phenotypes by magnetic separation (as described below) that were then inoculated by i.v. infusion into cohort 4, 5, or 6 (Table 1).
Deer monitoring and sample collection.
All animals were monitored for evidence of CWD infection by serial tonsil biopsies taken at 3, 6, 12, and 15 months postinoculation (p.i.), and at study termination (19 months p.i.). Tonsil tissue was divided, and equal portions were either stored at −70°C or fixed in 10% formalin for 24 h before processing for immunohistochemistry (IHC) analysis. At the same sampling intervals, blood, saliva, feces, and urine were collected from each animal and stored at −70°C. At necropsy, the palatine tonsils, brainstem (medulla at the obex), and retropharyngeal lymph nodes, as well as other tissues, were collected for examination by IHC and Western blotting (WB) analyses to identify the presence of the protease-resistant prion protein associated with CWD (PrPCWD).
Cervid PrP transgenic mouse bioassay studies. (i) Cervid PrP transgenic mice.
Tg(CerPrP-E226)5037+/− mice (2), which express the elk PrP coding sequence, were generated in the Telling laboratory at the University of Kentucky. Mice were inoculated and maintained in accord with CSU IACUC guidelines.
(ii) Genotyping.
All mice were screened at weaning for the presence of the cervid/elk Prnp transgene by both conventional and real-time PCR. All inoculated mice that tested negative for cervid PrPRES at the completion of bioassay studies were rescreened to confirm the presence of the cervid Prnp transgene.
Biocontainment protocols.
The protocols for white-tailed deer described above also applied to cohorts of mice housed in filter-top isolation cages.
Mouse inoculation cohorts.
Groups of five to nine weanling mice (Table 3) were housed in separate cages throughout the study. Suite-dedicated protective clothing, utensils, and waste disposal were incorporated to exclude cross contamination by fomites, bedding, food, excretions, or contact. Cohorts 8 and 9 consisted of naïve Tg(CerPrP-E226)5037+/− mice that served as i.c. brain inoculate positive or negative controls each receiving 30 μl of a 1% brain homogenate prepared in phosphate-buffered saline (PBS) of either CWD+ deer D10 or CWD− deer UGA. Cohorts 10 to 25 consisted of naïve Tg(CerPrP-E226)5037+/− mice that were inoculated by the i.c., i.v., intraperitoneal (i.p.), or per os (p.o.) route using the same CWD+ blood components described for white-tailed deer inoculations above. Cohorts 10 to 13 received whole blood. Cohorts 14 to 17 received the blood mononuclear cell fraction (106 WBC plus platelets). Cohorts 18 to 21 received the cell-free plasma fraction. Cohorts 22 and 23 received B cells (106) harvested from the retropharyngeal lymph node or spleen. Cohort 24 received CD41/61+ platelets (109). Cohort 25 received CD14+ cells (105). Cohorts 26 to 41, consisting of five to nine naïve Tg(CerPrP-E226)5037+/− mice, served as the negative controls for this study and were inoculated i.c., i.v., i.p., or p.o. with blood components from the same negative control white-tailed deer donors (Table 2) as used for the negative control white-tailed deer inoculations (Table 1).
TABLE 3.
Tg(CerPrP) mouse cohorts inoculated with blood components from CWD+ donor deer
| Cohort(s) | No./cohort | Donor status | Inoculum | Volume/concn/no. of cells | Route of inoculation |
|---|---|---|---|---|---|
| 8 | 10 | CWD+ | Brain control | 30 μl/1% | i.c. |
| 9 | 10 | CWD− | Brain control | 30 μl/1% | i.c. |
| 10 | 9 | CWD+ | Whole blood | 30 μl | i.c. |
| 11 | 9 | CWD+ | Whole blood | 100 μl | i.v. |
| 12 | 9 | CWD+ | Whole blood | 150 μl | i.p. |
| 13 | 9 | CWD+ | Whole blood | 50 μl/day × 3 days | p.o. |
| 14 | 9 | CWD+ | BMCa | 106 + plateletsb | i.c. |
| 15 | 9 | CWD+ | BMC | 106 + platelets | i.v. |
| 16 | 9 | CWD+ | BMC | 106 + platelets | i.p. |
| 17 | 9 | CWD+ | BMC | 106 + platelets | p.o. |
| 18 | 9 | CWD+ | Cell-free plasma | 30 μl | i.c. |
| 19 | 9 | CWD+ | CWD+ | 100 μl | i.v. |
| 20 | 9 | CWD+ | CWD+ | 150 μl | i.p. |
| 21 | 9 | CWD+ | CWD+ | 50 μl × 3 days | p.o. |
| 22 | 9 | CWD+ | B cells 2-104+ (RLNc) | 106 | i.c. |
| 23 | 5 | CWD+ | B cells 2-104+ (spleen) | 106 | i.c. |
| 24 | 5 | CWD+ | Platelets CD41/61+ | 109 | i.c. |
| 25 | 5 | CWD+ | Monocytes CD14+ | 2 × 105 | i.c. |
| 26-41 | 5 or 9 | CWD− | Negative controls | 1 group each as for cohorts 10-25 using CWD− inoculum | i.c., i.v., i.p., or p.o. |
BMC, blood mononuclear cells.
106 BMC plus platelets.
RLN, retropharyngeal lymph node.
Mouse monitoring and sample collection.
All mice were monitored daily for evidence of CWD clinical disease. Upon detection of clinical disease, mice were euthanized and necropsied. Brain tissue was collected, divided into equal portions, and either stored at −70°C for WB or fixed in 10% formalin for 24 h before processing for IHC analysis to identify the presence of PrPCWD.
Blood cell and plasma harvests [white-tailed deer and Tg(CerPrP-E226)5037+/− mouse inocula].
Total blood cell populations were collected from 250 ml sodium citrate-treated whole blood by centrifugation at 1,200 rpm for 15 min at 4°C. The plasma fraction was collected and set aside on ice. The cell fraction from this initial centrifugation was diluted 1:1 in 1× PBS (Gibco, Inc.) and layered over Histopaque 1088 (Sigma) at a 1:1 ratio. These Histopaque gradients were centrifuged without a brake at 2,500 rpm for 30 min at room temperature. The discrete bands of WBC were collected, diluted in an equal volume of 1× PBS, and further centrifuged for 10 min at 2,500 rpm at 4°C (washed). The cell pellets were washed in wash buffer (1× PBS, 0.2% fetal bovine serum [FBS], 2 mM EDTA) three times. Platelets were collected from the plasma fraction by centrifugation at 3,000 rpm for 15 min. Cells and plasma recovered from Histopaque 1088 gradient separations and plasma centrifugations were either directly inoculated into deer and mouse bioassay studies or further processed to separate cell phenotypes.
Retropharyngeal lymph node and spleen cell harvests [Tg(CerPrP-E226)5037+/− mouse inocula].
Retropharyngeal lymph node and spleen tissues were pressed through a fine wire mesh (0.45 μm), and WBC were collected as described above for the Histopaque gradient protocol.
Cell phenotype labeling and flow cytometry.
Cell phenotype MAbs were used to recover and determine the purity of 2-104+ B cells, CD14+ monocytes, and CD41/61+ platelets from the blood donor sources described above. Leukocyte and platelet blood cells were collected by centrifugation and Histopaque 1088 separation as described above and were then labeled with one of three antibodies (Table 4), i.e., anti-sheep pan-B-cell MAb 2-104 (equivalent to MAb 2-8 described by Young et al. [80]) (cell supernatant used undiluted), anti-sheep CD14 MAb clone VPM65 (cell supernatant used undiluted; Fitzgerald Industries Inc., Concord, MA), or anti-sheep CD41/61 MAb CAPPA 2A (1:100 dilution of a 1-mg/ml stock; VMRD, Pullman, WA). Cell aliquots were incubated with primary antibody for 20 min on ice and then washed three times in wash buffer. The secondary antibody, goat anti-mouse IgG or IgM fluorescein isothiocyanate (FITC), was diluted 1:100 in 1× PBS, 0.2% FBS and placed on the cells for 20 min on ice. The cells were again washed three times in wash buffer. The cells were then labeled with anti-FITC beads at 10 μl beads/107 cells, again incubated on ice for 20 min, and passed over LS or LD Dynatec magnetic bead separation columns (in accordance with the manufacturer's instructions). Cell populations of interest were eluted in 1× PBS containing 0.1% FBS. Eluted (FITC-labeled) cells were analyzed by flow cytometry (Dako-Becton Dickinson). To determine purity, the cells were gated by forward and side scatter to include primarily lymphocytes, which were counted, and volumes were adjusted to be equal to or greater than the total number of each cell-specific phenotype populating 1 × 107 peripheral blood mononuclear cells (as determined by prior flow cytometric analysis of specific cell phenotype populations in white-tailed deer; i.e., 2-104+ B cell populations were ∼10% and CD14+ cells ∼2% of the total leukocyte population). This was done to equate the total number of phenotype-specific cells (2-104+, CD14+) to that found in the total blood mononuclear cell fraction inoculum (cohort 2) that established infection (107 blood mononuclear cells) (Tables 1 and 5). The cells were either directly inoculated by i.v. inoculation into deer bioassays or frozen for future i.c. mouse bioassays.
TABLE 4.
MAbs used for cell-specific phenotype harvests
| MAb | Target | Source |
|---|---|---|
| 2-104a | CD72 B cells, lymphoid germinal center FDCs | Alan Young SDSU,b Brookings, SD |
| VPM65 | CD14 monocytes/ macrophages | Fitzgerald Ind., Concord, MA |
| CAPPA 2A | CD41/61 platelets | VMRD, Pullman, WAc |
MAb 2-104 is equivalent to MAb 2-880; see Table S1 in the supplemental material.
SDSU, South Dakota State University.
Veterinary Diagnostics, MAbs.
TABLE 5.
Bioassay results from naïve deer cohorts inoculated with CWD+ blood components
| Deer cohort no. | Inoculum | Tonsil PrPCWD result (no. positive/total) at postinoculation time of: |
Terminal necropsy PrPCWD resulta (no. positive/total) at 19 mo p.i. | % CWD+ | ||
|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | ||||
| 1 | Whole blood | 0/8 | 4/8 | 8/8 | 8/8 | 100 |
| 2 | Blood mononuclear cells | 0/4 | 1/4 | 2/4 | 4/4 | 100 |
| 3 | Cell-free plasma | 0/4 | 0/4 | 0/4 | 0/4 | 0 |
| 4 | 2-104+ B cells | 0/4 | 1/4 | 2/4 | 4/4 | 100 |
| 5 | CD41/61+ platelets | 0/4 | 0/4 | 1/4 | 3/4 | 75 |
| 6 | CD14+ monocytes | 0/4 | 0/4 | 0/4 | 0/4 | 0 |
| 7 | CWD− whole blood | 0/2 | 0/2 | 0/2 | 0/2 | 0 |
Retropharyngeal lymph node, tonsil, and medulla oblongata at obex.
Western blotting.
Tissue homogenates were prepared from the obex region of the medulla oblongata encompassing the dorsal motor vagal nucleus (medulla at the obex). Ten percent (wt/vol) homogenates were prepared in NP-40 buffer (10 mM Tris-HCl buffer [pH 7.5], 0.5% NP-40, 0.5% sodium deoxycholate) by Fastprep disruption at a setting of 6.5 for 45 s. Twenty-five microliters of each homogenate was mixed with 5 μl of proteinase K (PK; Invitrogen) to a final concentration of 20 μg/ml and incubated for 30 min at 37°C with shaking. PK activity was stopped with 4 μl 200 mM Pefablock SC, and an equivalent volume of each sample was mixed with 10 μl sample buffer (20% 10× reducing agent, 50% 4× LDS sample buffer; Invitrogen) and 5 μl NP-40 buffer (10 mM Tris-HCl [pH 7.5], 0.5% deoxycholic acid, 0.5% nonylphenoxylpolyethoxylethanol), heated to 95°C for 5 min, and separated by 12% Bis-Tris precast polyacrylamide gel electrophoresis (PAGE) (Invitrogen) at 150 V for 2.5 h in 1× morpholinepropanesulfonic acid (MOPS; Invitrogen). Proteins separated by PAGE were transferred to polyvinylidene fluoride (PVDF) membrane for 1 h at 100 V in transfer buffer (0.025 M Trizma base, 0.2 M glycine, 20% methanol, pH 8.3). After the PVDF membranes were blocked overnight at room temperature in Pierce Blocker, they were probed with PrP-specific antibody BAR224 (kindly supplied by J. Grassi), followed by horseradish peroxidase-conjugated goat anti-mouse IgG diluted in Pierce Blocker. Membranes were washed for 1 h after blocking and between antibodies with wash buffer (0.1 M Tris, 0.15 M NaCl, 0.2% Tween 20, pH 8.0). To visualize PrP bands, the PVDF membranes were developed with the Amersham ECL detection system and a digital GelDoc (Fuji Intelligent dark box) using LAS-3000 Lite ImageReader software.
Immunohistochemistry.
IHC analysis was performed by employing protocols described by Spraker et al. (69). Briefly, 3- to 5-mm sections of formalin-fixed, formic acid-treated tissues were deparaffinized at 60 to 70°C for 1 h, rehydrated via a series of xylene-ethanol baths, and treated in formic acid a second time (5 min) prior to a 20-min antigen retrieval (10× Dako target retrieval solution) cycle in a 2100 Retriever (PickCell Laboratories). Slides were further processed with the aid of a Ventana Discovery autostainer utilizing the Ventana Red Map stain kit, PrPCWD-specific primary antibody BAR224, and a biotinylated secondary goat anti-mouse antibody (Ventana). After autostaining, the slides were quickly rinsed in a warm water detergent solution, passed through a series of dehydration baths, and coverslipped.
RESULTS
White-tailed deer bioassays. (i) Cell versus non-cell-associated inoculates, cohorts 2 and 3.
Four of four deer (cohort 2) inoculated with blood mononuclear cells (leukocytes plus platelets) from 250 ml CWD+ deer blood (1 × 107 to 1.24 × 108) became tonsil biopsy PrPCWD positive (by IHC and WB analyses) between 6 and 19 months p.i. (Table 5). By contrast, PrPCWD was not detected in any tissue (tonsil, retropharyngeal lymph node, or medulla at obex) of any of four deer (cohort 3) that received the cell-free plasma portion (140 to 150 ml plasma) from this same 250 ml of CWD+ blood.
(ii) Specific blood cell phenotype (2-104+ B, CD14+, CD41/61+) inoculates, cohorts 4 to 6.
Four of four deer (cohort 4) inoculated with 2-104+ B cells (1 × 106 to 5 × 106) and three of four deer (cohort 5) receiving CD41/61+ platelets (6 × 109 to 35 × 109) became tonsil biopsy PrPCWD positive between 12 and 19 months p.i. All four deer (cohort 6) inoculated with CD14+ monocytes (4 × 105) remained CWD− through the observation period of 19 months.
(iii) Whole-blood control deer inoculates, cohorts 1 and 7.
PrPCWD was detected in tonsil biopsies of all eight deer inoculated with 250 ml whole blood from CWD+ deer (cohort 1) between 6 and 12 months p.i. All deer began to show signs of TSE disease between 15 and 26 months p.i., including wasting, hyperphagia, polydipsia, lowered head with wide leg stance, and lethargy. Both negative control deer (cohort 7) remained CWD− as determined by IHC and WB analyses.
(iv) White-tailed deer IHC and WB analyses.
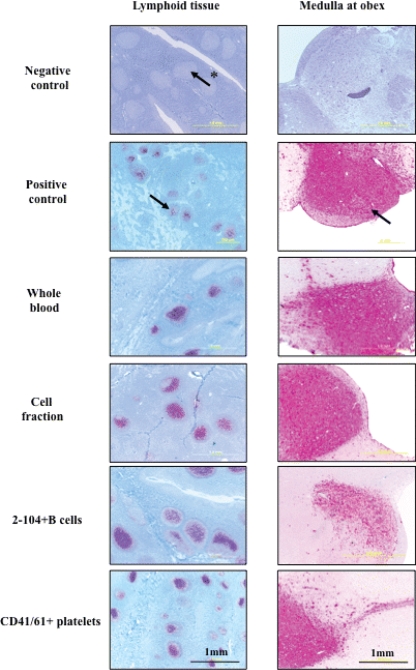
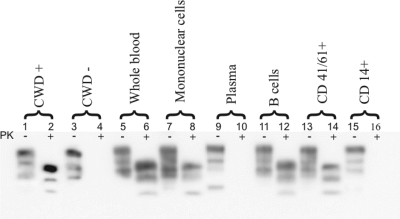
IHC analysis (Fig. 1) of terminal lymphoid tissue and medulla obex from deer inoculated with whole blood, blood mononuclear cells (leukocytes plus platelets), 2-104+ B cells, or CD41/61+ platelets demonstrated punctate PrPCWD staining within lymphoid follicles and brain tissue typical of that found associated with CWD-infected cervid controls. The cohort 4 (2-104+ B cell) deer brain IHC analysis in Fig. 1 showed overall less demonstrable PrPCWD in the medulla than the other CWD+ deer cohorts. Confirmatory WB analysis (Fig. 2) of brain tissue (medulla at obex) showing the presence of PK-resistant bands in lanes 6, 8, 12, and 14, corroborates CWD infection. Similar tissues from deer inoculated with cell-free plasma and CD14+ monocytes did not reveal PrPCWD staining by either conventional test (IHC or WB analysis) used to verify CWD infection.
FIG. 1.
Terminal lymphoid and brain (medulla at obex) IHC analysis results of naïve deer cohorts inoculated with CWD+ blood components. PrPCWD demonstrated by IHC analysis in tonsil, brain (medulla oblongata at obex), and retropharyngeal lymph node tissues of deer receiving whole blood, cell fraction, B cells, or CD41/61+ cells from CWD-infected donors. Arrows indicate PrPCWD staining (red) within the brain and lymphoid follicles. The arrow with the asterisk indicates a lymphoid follicle negative for PrPCWD.
FIG. 2.
WB analysis of cohorts of naïve deer inoculated with CWD+ blood components. WB demonstration of the typical PK digestion band shift (28 to 35 kDa) associated with prion infection (medulla at obex) of deer receiving whole blood, mononuclear cell fraction, B cells, or CD41/61+ cells. Deer receiving cell-free plasma or CD14+ cells from CWD-infected donors remained PrPCWD negative. Lanes 1 to 4 represent CWD+/CWD− deer controls (10% brain homogenate) without and with PK digestion at 25 μg/ml. Lanes 5 to 16, 10% brain homogenate of whole-blood-, mononuclear-cell-, plasma-, B-cell-, CD41/61+-, or CD14+-inoculated deer without and with PK digestion at 25 μg/ml.
Cervidized mouse [Tg(CerPrP-E226)5037+/−] bioassays. (i) Whole-blood inoculates (i.c., i.v., i.p., or p.o.), cohorts 10 to 13.
Seven of nine mice inoculated i.c. (cohort 10), one of nine mice inoculated i.v. (cohort 11), five of nine mice inoculated i.p. (cohort 12), and two of nine mice inoculated p.o. (cohort 13) with whole blood from a CWD+ deer began to show signs of TSE clinical disease, including weight loss, circling, rigid tail, hyperactivity, or inactivity, 270 to 490 days p.i. (dpi). Upon termination (2 to 4 weeks after the initial clinical signs appeared), PrPCWD was detected in the brains of all of the mice exhibiting clinical disease by IHC and WB analyses (range, 270 to 490 dpi) (i.c., 275 ± 5 dpi; i.v., 312 dpi; i.p., 340 ± 10 dpi; p.o., 482 ± 8 dpi).
(ii) Blood mononuclear cell (cell-associated) inoculates (leukocytes plus platelets) (i.c., i.v., i.p., or p.o.), cohorts 14 to 17.
PrPCWD was detected by IHC and WB analyses of the brains of six of nine mice inoculated i.c. (cohort 14), one of nine mice inoculated i.v. (cohort 15), one of nine mice inoculated i.p. (cohort 16), and zero of nine mice inoculated p.o. (cohort 17) with blood mononuclear cells (leukocytes plus platelets) from CWD+ deer. Clinical disease included weight loss and a rough hair coat (range, 275 to 494 dpi) (i.c., 290 ± 15 dpi; i.v., 303 dpi; i.p., 494 dpi; p.o., >600 dpi). Thus, consistent with results obtained with deer, Tg(cerPrP) mouse bioassays also indicated that CWD prion infectivity is associated with the leukocyte fraction of blood from CWD+ cervid donors.
(iii) Non-cell-associated (cell-free) plasma inoculates (i.c., i.v., i.p., or p.o.), cohorts 18 to 21.
As with bioassay results obtained with deer, PrPCWD was not detected in the brains of mice inoculated with cell-free plasma from CWD+ deer which were monitored for their natural life span (range, 623 to 862 dpi).
(iv) B-cell inoculates (i.c.), cohorts 22 and 23.
In that harvest of sufficient B cells from blood to permit bioassays in deer and mice was not possible, 2-104+ B cells harvested from retropharyngeal lymph nodes and spleens were analyzed. Five of five mice inoculated i.c. with retropharyngeal lymph node 2-104+ B cells from a terminal CWD+ deer (cohort 22) began to show clinical TSE disease, including hyperactivity and circling, at 282 ± 7 dpi. One of five mice inoculated i.c. with 2-104+ B cells from the spleen of this same CWD+ deer (cohort 23) developed signs of TSE disease at 180 dpi (weight loss, rough hair coat, rigid tail). All six mice demonstrating TSE clinical disease were PrPCWD positive by IHC and WB analyses of brain tissue (range, 275 to 289 dpi).
(v) Platelet inoculates (i.c.), cohort 24.
Three of five mice inoculated i.c. with CD41/61+ platelets were PrPCWD positive by IHC and WB analyses of brain tissue at 305 ± 10 dpi. All three of these mice exhibited TSE signs of wasting and rough hair coats (range, 295 to 315 dpi).
(vi) Monocyte blood cell inoculates (i.c.), cohort 25.
Neither TSE clinical disease nor PrPCWD could be detected in the five mice inoculated i.c. with CD14+ blood cells upon termination at 600 to 862 dpi (range, 600 to 862 dpi), thus paralleling the results of deer bioassays.
(vii) Blood component negative control inoculates, cohorts 26 to 41.
All mice inoculated with cell components or cell-free plasma from CWD− white-tailed deer donors remained free of TSE clinical disease for up to 862 dpi and were PrPCWD negative upon analysis of brain tissue by IHC and WB.
(viii) Positive and negative control brain inoculates, cohorts 8 and 9.
Clinical disease progression (wasting, circling, inability to right self, hyperactivity, or inactivity) and PrPCWD were detected by IHC and WB analyses of the brains of all 10 of 10 mice inoculated i.c. with CWD+ brain homogenate at 168 ± 4 dpi, while 0 of 10 negative control mice were PrPCWD positive (>600 dpi).
(ix) Cervidized mouse [Tg(CerPrP-E226)5037+/−] IHC analysis.
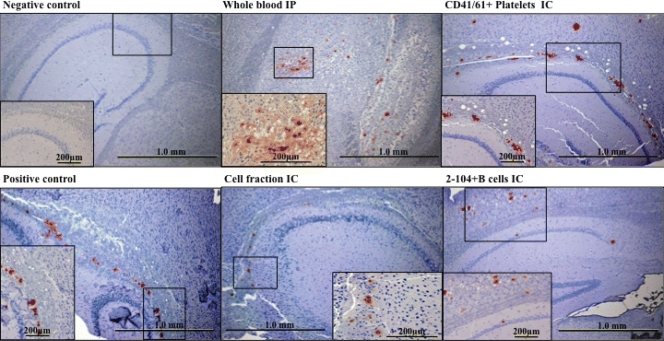
IHC analysis (Fig. 3) of sagittal brain tissue sections from terminal mice inoculated with whole blood, blood mononuclear cells (leukocytes plus platelets), 2-104+ B cells, or CD41/61+ platelets demonstrated punctate PrPCWD staining typically found associated with CWD infection (28). Similar PrPCWD staining was not detected in mice inoculated with cell-free plasma or CD14+ monocytes.
FIG. 3.
Brain IHC analysis results for Tg(CerPrP-E226)5037+/− mice inoculated with CWD+ blood components. PrPCWD demonstrated by IHC analysis in sagittal brain sections of Tg(CerPrP-E226)5037+/− mice receiving whole blood, cell fraction, CD41/61+, or B cells from CWD-infected donors. PrPCWD plaque deposits are typical of those previously described in CWD infection in white-tailed deer (69) and Tg(CerPrP-E226)5037+/− mice (28).
(x) Cervidized [Tg(CerPrP-E226)5037+/−] mice as a bioassay tool.
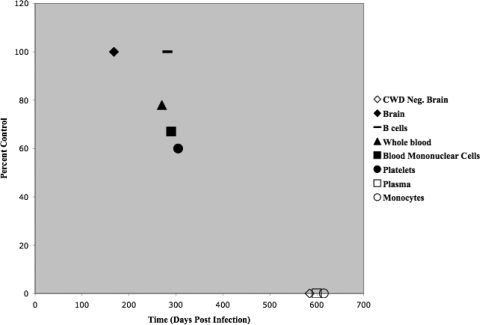
Multiple routes of inoculation (i.c., i.v., i.p., or p.o.) were utilized to assess the infectivity of CWD blood fractions in cervid PrP transgenic (TgCerPrP) mice. Clinical disease and PrPCWD were detected in TgCerPrP mice inoculated i.c. with (i) CWD+ brain (100% attack rate), (ii) whole blood (78%), (iii) blood mononuclear cells (67%), (iv) platelets (60%), (v) retropharyngeal lymph node 2-104+ B cells (100%), or (vi) splenic 2-104+ B cells (20%) (Fig. 4). CWD prion infection was also detected in mice inoculated with whole blood by the i.p. (56%) or p.o. (22%) route and mice inoculated i.v. or i.p. with blood mononuclear cells (11%). All mice inoculated with CWD− brain (i.c.), monocytes (i.c.), cell-free plasma (i.c., i.v., i.p., or p.o.), or blood mononuclear cells (p.o.) were maintained for greater than 600 dpi without evidence of clinical disease or PrPCWD detection by IHC and WB analyses (Table 6). Thus, the results from Tg(CerPrP-E226)5037+/− bioassay reinforced the infectious nature of whole blood, blood mononuclear cell, B-cell and platelet blood fractions, and did not detect prion infectivity in the monocyte or cell-free plasma blood fractions of blood from CWD-infected deer.
FIG. 4.
Attack rates in Tg(CerPrP-E226)5037+/− mice intracranially inoculated with CWD+ blood components. Shown are the percentages of Tg(CerPrP-E226)5037+/− mice that developed prion disease after i.c. inoculation with brain tissue (⧫), B cells (-), whole blood (▴), cell fraction (▪), platelets (•), cell-free plasma (□), or monocytes (○) from CWD-infected donor deer or brain tissue from a CWD− donor deer (⋄).
TABLE 6.
Bioassay of blood components from CWD+ deer in Tg(CerPrP) mice
| Cohort(s) | Donor status | Inoculum | Route of inoculation | Terminal PrPCWD resulta (no. positive/total) | Avg time (dpi) of observation for clinical disease ± SDb | % CWD+ |
|---|---|---|---|---|---|---|
| 8 | CWD+ | Brain | i.c. | 10/10 | 168 ± 4 | 100 |
| 9 | CWD− | Brain | i.c. | 0/10 | >600 | 0 |
| 10 | CWD+ | Whole blood | i.c. | 7/9 | 270 ± 5 | 78 |
| 11 | CWD+ | Whole blood | i.v. | 1/9 | 312 | 11 |
| 12 | CWD+ | Whole blood | i.p. | 5/9 | 340 ± 10 | 56 |
| 13 | CWD+ | Whole blood | p.o. | 2/9 | 482 ± 8 | 22 |
| 14 | CWD+ | BMCc | i.c. | 6/9 | 290 ± 15 | 67 |
| 15 | CWD+ | BMC | i.v. | 1/9 | 303 | 11 |
| 16 | CWD+ | BMC | i.p. | 1/9 | 494 | 11 |
| 17 | CWD+ | BMC | p.o. | 0/9 | >600 | 0 |
| 18 | CWD+ | Cell-free plasma | i.c. | 0/9 | >600 | 0 |
| 19 | CWD+ | Cell-free plasma | i.v. | 0/9 | >600 | 0 |
| 20 | CWD+ | Cell-free plasma | i.p. | 0/9 | >600 | 0 |
| 21 | CWD+ | Cell-free plasma | p.o. | 0/9 | >600 | 0 |
| 22 | CWD+ | RLNd B cells | i.c. | 5/5 | 282 ± 7 | 100 |
| 23 | CWD+ | Splenic B cells | i.c. | 1/5 | 180 | 20 |
| 24 | CWD+ | CD41/61+ cells | i.c. | 3/5 | 305 ± 10 | 60 |
| 25 | CWD+ | CD14+ cells | i.c. | 0/5 | >600 | 0 |
| 26-29 | CWD− | Whole blood | i.c., i.v., i.p., p.o. | 0/9 | >600 | 0 |
| 30-33 | CWD− | BMC | i.c., i.v., i.p., p.o. | 0/9 | >600 | 0 |
| 34-37 | CWD− | Cell-free plasma | i.c., i.v., i.p., p.o. | 0/9 | >600 | 0 |
| 38-41 | CWD− | RLNg B, splenic B, CD41/61+, or CD14+ cells | i.c. | 0/5 | >600 | 0 |
IHC/WB analyses.
The natural life spans of Tg(CerPrP-E226)5037+/− mice ranged from 601 to 862 dpi.
BMC, blood mononuclear cells.
RLN, retropharyngeal lymph node.
In summary, bioassay results from deer and Tg(CerPrP-E226)5037+/− mice were concordant. Namely, 79% of the deer and 35% of the cervidized mice developed CWD prion infection after inoculation with blood or blood mononuclear cell fractions [whole blood, 100% of the deer and 42% of the Tg(CerPrP) mice; blood mononuclear cells, 100% of the deer and 22% of the mice; 2-104+ B cells, 100% of the deer and 60% of the Tg(CerPrP) mice; CD41/61+ cells, 75% of the deer and 60% of the mice], while 0% of those inoculated with either CD14+ cells or cell-free plasma (0% of the deer and 0% of the mice) became PrPCWD positive (Table 7). Thus, CWD infectivity segregated with the mononuclear cell, platelet, and 2-104+ B-cell-enriched fractions and not with either the CD14+ monocyte or plasma compartments of blood from CWD+ deer.
TABLE 7.
Summary of white-tailed deer and Tg(CerPrP) mouse blood component bioassay studies
| Inoculum | % Positive |
|
|---|---|---|
| White-tailed deer | Tg(CerPrP-E226) 5037+/− mice | |
| Whole blood | 100 | 42 |
| Blood mononuclear cells | 100 | 22 |
| B cells (2-104+) | 100 | 60 |
| Platelets (CD41/61+) | 75 | 60 |
| Monocytes (CD14+) | 0 | 0 |
| Cell-free plasma | 0 | 0 |
DISCUSSION
Factors influencing the number of cells bioassayed and routes of inoculation.
The number of mononuclear cells, B cells, monocytes, and platelets bioassayed was influenced by the total number of cells recoverable from whole blood and limitations in the volume of inoculum that could safely or feasibly be administered to mice. The total number of 2-104+ B cells or CD14+ monocytes used for inoculation was based on our previous flow cytometric analyses of white-tailed deer, which indicated that ∼10% of the mononuclear leukocytes were 2-104+ B cells and CD14+ cells accounted for ∼2% of the total mononuclear leukocyte population. Because CWD infection was generated by 107 total blood mononuclear cells (cohort 2), we surmised that a minimum of 106 2-104+ B cells (10%) or 2 × 104 CD14+ cells (2%) would be sufficient to determine whether this cell phenotype may carry blood-borne prion infectivity. Thus, these cell numbers were used for both deer and mouse cohorts. To mimic blood transfusion dynamics, all deer were inoculated by i.v. infusion. At the time this study was initiated, very little was known about peripheral trafficking of CWD in Tg(CerPrP-E226)5037+/− mice. We therefore used multiple routes of inoculation (i.c., i.v., i.p., or p.o.) to explore the ability of these mice to support CWD infection. While attack rates were incomplete, likely due to the limited volume of blood or blood cells we could administer or innate differences in sensitivity between deer and Tg(cerPrP) mice, we did see a similar pattern of CWD infectivity associated with cell versus cell-free blood components (Fig. 4). These results suggest that while infection may not be as robust as that incurred post i.c. inoculation, Tg(CerPrP-E226)5037+/− mice are capable of establishing and maintaining CWD infection via peripheral routes of inoculation. Due to logistical reasons and animal availability, it was not possible in this study to determine the minimum infectious dose for any of the inocula, although this information would surely be of interest and could be approached in subsequent more specific-inoculum-focused studies.
Interval to detection of CWD infection by tonsil biopsy.
Transmission of infectious prions by blood transfusion has now been established for scrapie, BSE, CJD, and CWD (3, 6, 30, 38, 39, 47, 54, 62). Identifying whether this infectivity is associated with the cellular, cell-free, or both fractions of blood has been a bit more challenging. Inherent limitations associated with the volume of inoculum that can be introduced by i.c. inoculation in rodent bioassay models impose constraints on assay sensitivity.
We were able to intravenously inoculate large volumes of CWD-infected whole blood and equivalent concentrations/volumes of blood constituents (leukocytes plus platelets, cell-free plasma, or cell phenotype fractions, i.e., 2-104+ B cells, CD14+ monocytes, or CD41/61+ platelets) harvested from the same donor pool into cohorts of naïve white-tailed deer. In recipient deer, the time to tonsil biopsy positivity after inoculation of blood, blood mononuclear cells plus platelets, 2-104+ B cells, or CD41/61+ platelets was variable—as early as 6 months to as late as 19 months p.i. We have observed similar PrPCWD detection kinetics in previous cervid bioassay studies employing several routes of inoculation and inocula from CWD+ deer (53, 54). While we cannot rule out horizontal transmission from the first positive animal in each cohort, the time frame for detection in the remaining deer (6 months) is half that which we have historically observed in deer inoculated with large quantities of saliva from CWD-infected deer (53, 54), suggesting much earlier exposure to infectious prions, i.e., more likely to relate to the experimental inoculum than to animal-to-animal transfer.
Infectious prions in cell-associated (leukocyte plus platelet) blood fraction.
Although identification of blood cell-associated TSE infectivity has been sought with disparate results, in CWD we detected infectivity in the cellular but not the cell-free plasma fraction of blood. While some bioassay studies have yielded negative findings (7, 72), likely due to the restricted sample volume assayable in rodent models, the transmission of infectious prions associated with buffy-coat WBC of TSE infected-donors has been well documented. Kuroda (44), Manuelidis (50), and Brown et al. (4-6) were the first to demonstrate this association in rodent models of CJD and Gertsmann-Straussler-Scheinker disease (GSS). Subsequent to these studies, scrapie prion infectivity in leukocyte populations has been detected by several researchers utilizing bioassays in rodent models (3, 10, 36, 50) and in sheep (39).
The replication of prions in the lymphoid tissues precedes CNS infection in several TSEs (CWD, scrapie, and variant CJD [vCJD]) (19, 27, 66, 67), raising the potential for hematogenous spread via recirculating lymphocytes. Consistent with this is the fact that leukodepletion reduces blood-borne prion transmission (24, 63).
Infectious prions in MAb 2-104+ B cells.
Immunohistological detection of PrPRES/Sc in lymphoid tissues of scrapie-infected sheep provided some of the first evidence for lymphoid system involvement in TSE diseases (19, 20, 61, 70). Subsequent studies utilizing confocal microscopy confirmed an association between PrPRES and immune cells (FDCs, tingible body macrophages, and B cells) and extended the repertoire of prion diseases with lymphoid involvement to include CWD and vCJD (42, 55, 60, 66, 67). B cells have been associated with PrPRES transport and/or deposition within the lymphoid system (9, 10, 18, 22, 23, 32, 43, 56, 64, 66, 81). The present study supports this contention in demonstrating that MAb 2-104+ primarily B cells harvested from peripheral blood or retropharyngeal lymph nodes contain sufficient infectious prions to transmit CWD to native or transgenic hosts.
B cells harvested for the deer bioassay studies were collected from peripheral whole blood. However, as noted earlier, due to cell loss associated with Ficoll and magnetic bead separation, we were not able to harvest sufficient 2-104+ B cells from peripheral blood to adequately analyze the infectivity of these cells in both deer and Tg(CerPrP-E226)5037+/− mice. We therefore harvested B cells from the spleen and the retropharyngeal lymph nodes from the terminal harvest of one of the donor deer for bioassays of mice. The B cells harvested from these tissues (by mechanical disruption/filtration) (for mouse bioassay) and those collected from whole-blood Ficoll separations (for deer bioassay) were sorted using MAb 2-104. MAb 2-104 is known to be specific for all peripheral blood B cells in sheep and has been found to cross-react in a similar fashion in cervid species (see Fig. S1 in the supplemental material). Molecular studies suggest that the target antigen is the sheep homologue of CD72 (80), with more recent findings verifying that MAb 2-104 may identify FDCs but does not recognize CD21, T cells, monocytes, or granulocytes (see Fig. S1 in the supplemental material). It is therefore feasible that MAb 2-104 could label germinal-center FDCs in addition to B cells. It would be expected that most FDCs would remain within the connective tissue stroma, be rendered nonviable during mechanical disruption, or be removed by the gradient separation (17). The morphology of the cells harvested for these studies by these methods supported their identity as B lymphocytes. We cannot, however, exclude the possibility that CWD infectivity was sheared from dendritic processes of FDCs by mechanical disruption and therefore could be a constituent of the B-cell harvests from retropharyngeal lymph nodes used for the mouse bioassay study. As FDCs are not found in peripheral blood, this is not a concern for the B-cell harvests from whole blood used for deer bioassays.
Differences exist between lymphatic recirculating and nonrecirculating lymphocyte populations found in peripheral blood and lymphoid tissues. The reassortment of lymphocytes into these two subsets correlates with lymphocyte lineage and the expression of maturation and/or adhesion markers. Not all of the cells in peripheral blood have equal access to the lymphatic recirculation pathway (80). Recirculating lymphocytes constitute 60% of the peripheral blood lymphocytes (PBLs) in the blood, while nonrecirculating PBLs (40%) are, by definition, excluded from lymph and confined to blood and the spleen. CD21 expression correlates with the recirculation competency of these subsets. B cells expressing the CD21 molecule (CD21+) preferentially migrate across the high endothelial venules (HEV) and are able to recirculate between the peripheral blood and lymphatics, while those not expressing CD21 (CD21−) do not cross the HEV and therefore remain in peripheral blood (nonrecirculating). Based on previous data, the spleen would therefore contain representative populations of both recirculating and nonrecirculating B cells, whereas lymph nodes would only be expected to contain recirculating B cells and therefore represent 60% of the total peripheral blood B cells (25, 80). Given that blood, lymph nodes, and splenic B cells were able to induce infection in recipient Tg(CerPrP) mice and deer, it is likely that the recirculating B-cell population (i.e., CD21+) was responsible. However, the possibility cannot be excluded that a unique population of germinal-center resident B cells was present in samples harvested from the spleen and lymph nodes, which would not be present in the peripheral blood. As well, although platelet contamination was not observed in this cell fraction, it cannot be completely ruled out as a possible contributor to infectivity.
Infectious prions in CD41/61+ platelets.
Evidence associating prion infectivity with platelets has been variable—from no detection in sporadic-CJD platelets (11) to reported infectivity in hamster scrapie (36), GSS, and vCJD (10). Here we report the transmission of infectious CWD via CD41/61+ platelets in both naïve white-tailed deer and cervid transgenic mouse bioassays.
PrPC is produced endogenously by cells of the platelet lineage, which could implicate platelets in PrPRES propagation or trafficking within the body or transmission in contaminated blood products (11, 34, 35, 37, 48). Forty-three to 53%, 63 to 95%, and 69 to 93% of bovine (3), ovine (4), and white-tailed deer (unpublished findings) platelets, respectively, express PrPC, and given the number of circulating platelets versus the number of leukocytes, the majority of blood-borne PrPC expression is platelet associated (11, 48, 71). This blood component could be largely responsible for the transmission of vCJD by transfusion (45, 46).
Absence of infectious prions in plasma from CWD+ deer.
The documentation of TSE infectivity (5, 6, 12, 21, 24, 74), or lack thereof (7, 19, 26, 51, 52), associated with cell-free serum or plasma is proportionate historically. These discrepancies may be explained by the inherent limits on the volume of fluid that can be inoculated in rodent bioassays, the presence/absence of contaminating cells, or variation in the biology of prion diseases. Here we report absence of infectious CWD prions in cell/platelet-free plasma collected from an equivalent volume of whole blood (250 ml) shown to be capable of infecting naïve white-tailed deer (cohort 1). The virtual elimination of vCJD transmission by leukoreduction argues strongly that, as for CWD, prion infectivity is strongly leukocyte and/or platelet associated in vCJD (14, 15, 45, 46, 75).
Absence of infectious prions in CD14+ cells from CWD+ deer.
The presence of PrPRES in lymph node tingible body macrophages of scrapie-infected sheep (1, 31, 40, 56) and CWD-infected deer (66) led us to investigate the possibility that circulating CD14+ monocytes may contain infectious prions capable of transmitting disease. Somewhat to our surprise, the results indicated that in CWD this is not the case. We were not able to detect PrPCWD in the brain or lymphoid tissues of white-tailed deer or cervidized mice inoculated with up to 4 × 105 CD14+ macrophages (twice the number of cells present in the blood mononuclear cell inoculum that produced CWD).
In vitro experiments have determined that bone marrow-derived macrophages can acquire and degrade PrPBSE (65), which could lead to decreased rates of infection in an in vivo setting (8). Maignien et al. (49) found that depletion of macrophage numbers at the gut/follicle interface prior to TSE infection leads to an increase in the infection rate. While macrophages are capable of receptor-mediated uptake of infectious particles, in particular, infectious prions, it appears that their role may be associated with lysosomal degradation versus sites of prion amplification or trafficking.
Summary.
We have detected infectious prions in the cellular fraction (mononuclear leukocytes plus platelets) and not in the cell-free plasma fraction of blood from CWD+ deer. B cells from blood or retropharyngeal lymph nodes and platelets, but not CD14+ monocytes or plasma, contained infectious prions capable of transmitting CWD. These results (i) support the identity of a hematogenous route of CWD infection and reinforce the notion that all tissues are exposed to infection, (ii) help in understanding the pathogenesis and trafficking of CWD prions, and (iii) highlight the utility of CWD as a model in the development of antemortem assays to detect prion infections.
Supplementary Material
Acknowledgments
We thank A. Avery and M. Zabel for technological conversations and input into this work, K. O'Rourke and L. Hamburg for performing the Prnp genotype analysis, J. Grassi and J. Langeveld for their generous gifts of antibodies, N. Albarado and E. McNulty for sample collection and tireless dedication to animal welfare, Amy Nalls for insightful editing, and fellow graduate students D. Seelig, N. Denkers, and N. Haley for necropsy banter, support, and study critique.
This work was supported by the National Institute of Allergy and Infectious Diseases, NIH (contract N01-AI-25491).
Footnotes
Published ahead of print on 10 March 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Andréoletti, O., P. Berthon, E. Levavasseur, D. Marc, F. Lantier, E. Monks, J. M. Elsen, and F. Schelcher. 2002. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J. Histochem. Cytochem. 50:1357-1370. [DOI] [PubMed] [Google Scholar]
- 2.Angers, R., T. Seward, D. Napier, M. Green, E. Hoover, T. Spraker, K. O'Rourke, A. Balachandran, and G. Telling. 2009. Chronic wasting disease prions in elk antler velvet. Emerg. Infect. Dis. 15:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bons, N., S. Lehmann, N. Mestre-Frances, D. Dormant, and P. Brown. 2002. Brain and buffy coat transmission of bovine spongiform encephalopathy to the primate Microcebus murinus. Transfusion 42:513-516. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P. 1995. Can Creutzfeldt-Jakob disease be transmitted by transfusion? Curr. Opin. Hematol. 2:472-477. [DOI] [PubMed] [Google Scholar]
- 5.Brown, P., L. Cervenakova, P. McShane, B. R. Rubenstein, and W. N. Drohan. 1999. Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit Creutzfeldt-Jakob disease in humans. Transfusion 39:1169-1178. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P., R. G. Rohwer, B. C. Dunstan, C. MacAuley, D. C. Gajdusek, and W. N. Drohan. 1998. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38:810-816. [DOI] [PubMed] [Google Scholar]
- 7.Bruce, M. E., I. McConnell, R. G. Will, and J. W. Ironside. 2001. Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 358:208-209. [DOI] [PubMed] [Google Scholar]
- 8.Carp, R. I., and S. M. Callahan. 1981. In vitro interaction of scrapie agent and mouse peritoneal macrophages. Intervirology 16:8-13. [DOI] [PubMed] [Google Scholar]
- 9.Casaccia, P., A. Ladogana, Y. G. Xi, and M. Pocchiari. 1989. Levels of infectivity in the blood throughout the incubation period of hamsters peripherally injected with scrapie. Arch. Virol. 108:145-149. [DOI] [PubMed] [Google Scholar]
- 10.Cervenakova, L., O. Yakovleva, C. McKenzie, S. Kolchinsky, L. McShane, W. N. Drohan, and P. Brown. 2003. Similar levels of infectivity in the blood of mice infected with human-derived vCJD and GSS strains of transmissible spongiform encephalopathy. Transfusion 43:1687-1694. [DOI] [PubMed] [Google Scholar]
- 11.Choi, E. M., M. D. Geschwind, C. Deering, K. Pomeroy, A. Kuo, B. L. Miller, J. G. Safar, and S. B. Prusiner. 2009. Prion proteins in subpopulations of white blood cells from patients with sporadic Creutzfeldt-Jakob disease. Lab. Invest. 89:624-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, M. C., and D. A. Haig. 1967. Presence of the transmissible agent of scrapie in the serum of affected mice and rats. Vet. Rec. 80:504. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, P., and A. C. Ghani. 2005. Projections of the future course of the primary vCJD epidemic in the UK: inclusion of subclinical infection and the possibility of wider genetic susceptibility. J. R. Soc. Interface 2:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleemput, I., M. Leys, D. Ramaekers, and L. Bonneux. 2006. Balancing evidence and public opinion in health technology assessments: the case of leukoreduction. Int. J. Technol. Assess. Health Care 22:403-407. [DOI] [PubMed] [Google Scholar]
- 15.Coste, J., C. Prowse, R. Eglin, and C. Fang. 2009. A report on transmissible spongiform encephalopathies and transfusion safety. Vox Sang. 96:284-291. [DOI] [PubMed] [Google Scholar]
- 16.Deslys, J., C. Lasmezas, and D. Dormont. 1994. Selection of specific strains in iatrogenic Creutzfeldt-Jakob disease. Lancet 343:848-849. [DOI] [PubMed] [Google Scholar]
- 17.Eaton, S., M.-J. Anderson, S. Hamilton, L. Gonzalez, J. Sales, M. Feffrey, H. W. Reid, M. S. Rocchi, and F. Chianini. 2009. CD21 B cell populations are altered following subcutaneous scrapie inoculation in sheep. Vet. Immunol. Immunopathol. 131:105-109. [DOI] [PubMed] [Google Scholar]
- 18.Eklund, C. M., W. Hadlow, and R. C. Kennedy. 1963. Some properties of the scrapie agent and its behavior in mice. Proc. Soc. Exp. Biol. Med. 112:974-979. [Google Scholar]
- 19.Eklund, C. M., R. C. Kennedy, and W. J. Hadlow. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15-22. [DOI] [PubMed] [Google Scholar]
- 20.Fraser, H., and A. G. Dickinson. 1970. Pathogenesis of scrapie in the mouse: the role of the spleen. Nature 226:462-463. [DOI] [PubMed] [Google Scholar]
- 21.Gajdusek, D. C., C. J. Gibbs, Jr., and M. Alpers (ed.). 1965. Slow, latent, and temperate virus infections. NINDB monograph no. 2. U.S. Department of Health, Education, and Welfare, Washington, DC.
- 22.Glatzel, M., E. Abela, M. Maissen, and A. Aguzzi. 2003. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N. Engl. J. Med. 349:1812-1820. [DOI] [PubMed] [Google Scholar]
- 23.Gordon, W. 1957. Discussion to Palmer, A. C. Studies in scrapie. Vet. Rec. 69:1324-1327. [Google Scholar]
- 24.Gregori, L., N. McCombie, D. Palmer, P. Birch, S. O. Sowemimo-Coker, A. Giulivi, and R. G. Rohwer. 2004. Effectiveness of leucoreduction for removal of infectivity of transmissible spongiform encephalopathies from blood. Lancet 364:529-531. [DOI] [PubMed] [Google Scholar]
- 25.Gupta, V., I. McConnell, R. G. Dalziel, and J. Hopkins. 1998. Two B cell subpopulations have distinct recirculation characteristics. Eur. J. Immunol. 28:1597-1603. [DOI] [PubMed] [Google Scholar]
- 26.Hadlow, W. J., C. M. Eklund, and R. C. Kennedy. 1974. Course of experimental scrapie virus infection in the goat. J. Infect. Dis. 129:559-567. [DOI] [PubMed] [Google Scholar]
- 27.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 28.Haley, N., D. Seelig, M. Zabel, G. Telling, and E. Hoover. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health Protection Agency. 18 January 2007, posting date. Fourth case of transfusion-associated vCJD infection in the United Kingdom. http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733711457?p=1171991026241. [DOI] [PubMed]
- 30.Health Protection Agency. 17 February 2009, posting date. vCJD abnormal prion protein found in a patient with haemophilia at post mortem. http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1234859690542?p=1231252394302.
- 31.Herrmann, L. M., W. P. Cheevers, W. C. Davis, D. P. Knowles, and K. I. O'Rourde. 2003. CD21-positive follicular dendritic cells: a possible source of PrPsc in lymph node macrophages in scrapie infected sheep. Am. J. Pathol. 162:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog, C., J. Riviere, N. Lescoutra-Etchegaray, A. Charbonnier, V. Leblanc, N. Sales, J. P. Deslys, and C. I. Lasmezas. 2005. PrPTSE distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt-Jakob disease. J. Virol. 79:14339-14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilton, D. A., A. C. Ghani, L. Conyers, P. Edwards, L. McCardle, D. Ritchie, M. Penney, D. Hegazy, and J. W. Ironside. 2004. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J. Pathol. 203:733-739. [DOI] [PubMed] [Google Scholar]
- 34.Holada, K., T. H. Mondoro, J. Juller, and J. G. Vostal. 1998. Increased expression of phosphatidylinositol-specific phospholipase C resistant prion proteins on the surface of activated platelets. Br. J. Haematol. 103:276-282. [DOI] [PubMed] [Google Scholar]
- 35.Holada, K., J. Simak, P. Brown, and J. G. Vostal. 2007. Divergent expression of cellular prion protein on blood cells of human and nonhuman primates. Transfusion 47:2223-2232. [DOI] [PubMed] [Google Scholar]
- 36.Holada, K., J. G. Vostal, P. W. Theisen, C. MacAuley, L. Gregori, and R. G. Rohwer. 2002. Scrapie infectivity in hamster blood is not associated with platelets. J. Virol. 76:4649-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holada, K., and J. G. Vostal. 2000. Different levels of prion protein (PrPc) expression on hamster, mouse and human blood cells. Br. J. Haematol. 110:472-480. [DOI] [PubMed] [Google Scholar]
- 38.Houston, F., J. D. Foster, A. Chong, N. Hunter, and C. J. Bostock. 2000. Transmission of BSE by blood transfusion in sheep. Lancet 356:999-1000. [DOI] [PubMed] [Google Scholar]
- 39.Hunter, N., J. Foster, A. Chong, S. McCutcheon, D. Parnham, S. Eaton, C. MacKenzie, and F. Houston. 2002. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 83:2897-2905. [DOI] [PubMed] [Google Scholar]
- 40.Jeffrey, M., G. McGovern, S. Martin, C. M. Goodsir, and K. L. Brown. 2000. Cellular and sub-cellular localisation of PrP in the lymphoreticular system of mice and sheep. Arch. Virol. Suppl. 16:23-38. [DOI] [PubMed] [Google Scholar]
- 41.Kim, T. Y., H. J. Shon, Y. S. Joo, U. K. Mun, K. S. Kang, and Y. S. Lee. 2005. Additional cases of chronic wasting disease in imported deer in Korea. J. Vet. Med. Sci. 67:753-759. [DOI] [PubMed] [Google Scholar]
- 42.Kitamoto, T., T. Muramoto, S. Mohri, K. Doh-Ura, and J. Tateishi. 1991. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J. Virol. 65:6292-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-690. [DOI] [PubMed] [Google Scholar]
- 44.Kuroda, Y., C. J. Gibbs, Jr., L. A. Herbert, and D. C. Gajdusek. 1983. Creutzfeldt-Jakob disease in mice: persistent viremia and preferential replication of virus in low-density lymphocytes. Infect. Immun. 41:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefrère, J., and P. Hewit. 2009. From mad cows to sensible blood transfusion: the risk of prion transmission by labile blood components in the United Kingdom and in France. Transfusion 49:797-812. [DOI] [PubMed] [Google Scholar]
- 46.Lefrère, J. J., and B. Danic. 2009. Pictorial representation of transfusion over the years. Transfusion 49:1007-1017. [DOI] [PubMed] [Google Scholar]
- 47.Llewelyn, C. A., P. E. Hewitt, R. S. Knight, K. Amar, S. Cousens, J. Mackenzie, and R. G. Will. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417-421. [DOI] [PubMed] [Google Scholar]
- 48.MacGregor, I., J. Hope, G. Barnard, L. Kirby, O. Drummond, D. Pepper, V. Hornsey, R. Barclay, H. Bessos, M. Turner, and C. Prowse. 1999. Application of a time-resolved fluoroimmunoassay for the analysis of normal prion protein in human blood and its components. Vox Sang. 77:88-96. [DOI] [PubMed] [Google Scholar]
- 49.Maignien, T., M. Shakweh, P. Calvo, D. Marce, N. Sales, E. Fattal, J. P. Deslys, P. Couvreur, and C. I. Lasmezas. 2005. Role of gut macrophages in mice orally contaminated with scrapie or BSE. Int. J. Pharm. 298:293-304. [DOI] [PubMed] [Google Scholar]
- 50.Manuelidis, E. E., J. H. Kim, J. R. Mericangas, and L. Manuelidis. 1985. Transmission to animals of Creutzfeldt-Jakob disease from human blood. Lancet 2:896-897. [DOI] [PubMed] [Google Scholar]
- 51.Marsh, R. F., and R. P. Hanson. 1969. Transmissible mink encephalopathy: neuroglial response. Am. J. Vet. Res. 30:1637-1642. [PubMed] [Google Scholar]
- 52.Marsh, R. F., J. M. Miller, and R. P. Hanson. 1973. Transmissible mink encephalopathy: studies on the peripheral lymphocyte. Infect. Immun. 7:352-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathiason, C. K., S. A. Hays, J. Powers, J. Hayes-Klug, J. Langenberg, S. J. Dahmes, D. A. Osborn, K. V. Miller, R. J. Warren, G. L. Mason, and E. A. Hoover. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathiason, C. K., J. G. Powers, S. J. Dahmes, D. A. Osborn, K. V. Miller, R. J. Warren, G. L. Mason, S. A. Hays, J. Hayes-Klug, D. M. Seelig, M. A. Wild, L. L. Wolfe, T. R. Spraker, M. W. Miller, C. J. Sigurdson, G. C. Telling, and E. A. Hoover. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133-136. [DOI] [PubMed] [Google Scholar]
- 55.McBride, P. A., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168:413-418. [DOI] [PubMed] [Google Scholar]
- 56.McGovern, G., and M. Jeffrey. 2007. Scrapie-specific pathology of sheep lymphoid tissues. PLoS One 2:e1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller, M., E. Williams, N. Hobbs, and L. L. Wolfe. 2004. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10:1003-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35-36. [DOI] [PubMed] [Google Scholar]
- 59.Miller, M. W., M. A. Wild, and E. S. Williams. 1998. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J. Wildl. Dis. 34:532-538. [DOI] [PubMed] [Google Scholar]
- 60.Muramoto, T., T. Kitamoto, J. Tateishi, and I. Goto. 1992. The sequential development of abnormal prion protein accumulation in mice with Creutzfeldt-Jakob disease. Am. J. Pathol. 140:1411-1420. [PMC free article] [PubMed] [Google Scholar]
- 61.Pattison, I. H., and K. M. Jones. 1968. Detection of the scrapie agent in tissues of normal mice and in tumours of tumour-bearing but otherwise normal mice. Nature 218:102-104. [DOI] [PubMed] [Google Scholar]
- 62.Peden, A. H., M. W. Head, D. L. Ritchie, J. E. Bell, and J. W. Ironside. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527-529. [DOI] [PubMed] [Google Scholar]
- 63.Prowse, C. V., and A. Bailey. 2000. Validation of prion removal by leukocyte-depleting filters: a cautionary tale. Vox Sang. 79:248. [DOI] [PubMed] [Google Scholar]
- 64.Raeber, A. J., M. A. Klein, R. Frigg, E. Flechsig, A. Aguzzi, and C. Weissmann. 1999. PrP-dependent association of prions with splenic but not circulating lymphocytes of scrapie-infected mice. EMBO J. 18:2702-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rybner-Barnier, C., C. Jacquemot, C. Cuche, G. Dorre, L. Majlessi, M. M. Gabellec, A. Moris, O. Schwartz, J. Di Santo, A. Cumano, C. Leclerc, and F. Lazarini. 2006. Processing of the bovine spongiform encephalopathy-specific prion protein by dendritic cells. J. Virol. 80:4656-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sigurdson, C. J., C. Barillas-Mury, M. W. Miller, B. Oesch, L. J. van Keulen, J. P. Langeveld, and E. A. Hoover. 2002. PrP(CWD) lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J. Gen. Virol. 83:2617-2628. [DOI] [PubMed] [Google Scholar]
- 67.Sigurdson, C. J., E. S. Williams, M. W. Miller, T. R. Spraker, K. I. O'Rourke, and E. A. Hoover. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80(Pt. 10):2757-2764. [DOI] [PubMed] [Google Scholar]
- 68.Sohn, H. J., J. H. Kim, K. S. Choi, J. J. Nah, Y. S. Joo, Y. H. Jean, S. W. Ahn, O. K. Kim, and A. Balachandran. 2002. A case of chronic wasting disease in an elk imported to Korea from Canada. J. Vet. Med. Sci. 64:855-858. [DOI] [PubMed] [Google Scholar]
- 69.Spraker, T. R., R. R. Zink, B. A. Cummings, M. A. Wild, M. W. Miller, and K. I. O'Rourke. 2002. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet. Pathol. 39:110-119. [DOI] [PubMed] [Google Scholar]
- 70.Stamp, J. T., J. G. Brotherston, I. Zlotnik, J. M. Mackay, and W. Smith. 1959. Further studies on scrapie. J. Comp. Pathol. 69:268-280. [DOI] [PubMed] [Google Scholar]
- 71.Starke, R., P. Harrison, I. Mackie, G. Wang, J. D. Erusalimsky, R. Gale, J. M. Masse, E. Cramer, A. Pizzey, J. Biggerstaff, and S. Machin. 2005. The expression of prion protein (PrP(C)) in the megakaryocyte lineage. J. Thromb. Haemost. 3:1266-1273. [DOI] [PubMed] [Google Scholar]
- 72.Tamai, Y., H. Kojima, R. Kitajima, F. Taguchi, Y. Ohtani, T. Kawaguchi, S. Miura, M. Sato, and Y. Ishihara. 1992. Demonstration of the transmissible agent in tissue from a pregnant woman with Creutzfeldt-Jakob disease. N. Engl. J. Med. 327:649. [DOI] [PubMed] [Google Scholar]
- 73.Tateishi, J., T. Kitamato, and H. Hiratani. 1985. Creutzfeldt-Jakob disease pathogen in growth hormone preparations is eliminatable. Lancet 2:1299-1300. [DOI] [PubMed] [Google Scholar]
- 74.Taylor, D. M., I. McConnell, and C. E. Ferguson. 2000. Closely similar values obtained when the ME7 strain of scrapie agent was titrated in parallel by two individuals in separate laboratories using two sublines of C57BL mice. J. Virol. Methods 86:35-40. [DOI] [PubMed] [Google Scholar]
- 75.Turner, M. L., and C. A. Ludlam. 2009. An update on the assessment and management of the risk of transmission of variant Creutzfeldt-Jakob disease by blood and plasma products. Br. J. Haematol. 144:14-23. [DOI] [PubMed] [Google Scholar]
- 76.U.S. Geological Survey. 23 March 2009, posting date. Map of chronic wasting disease in North America. http://www.nwhc.usgs.gov/disease_information/chronic_wasting_disease/index.jsp.
- 77.Williams, E. 2005. Chronic wasting disease. Vet. Pathol. 42:530-549. [DOI] [PubMed] [Google Scholar]
- 78.Williams, E. S., and M. W. Miller. 2002. Chronic wasting disease in deer and elk in North America. Rev. Sci. Tech. 21:305-316. [DOI] [PubMed] [Google Scholar]
- 79.Williams, E. S., and S. Young. 1992. Spongiform encephalopathies in Cervidae. Rev. Sci. Tech 11:551-567. [DOI] [PubMed] [Google Scholar]
- 80.Young, A. J., W. L. Marston, M. Dessing, L. Dudler, and W. R. Hein. 1997. Distinct recirculating and non-recirculating B-lymphocyte pools in the peripheral blood are defined by coordinated expression of CD21 and l-selectin. Blood 90:4865-4875. [PubMed] [Google Scholar]
- 81.Zabel, M. D., M. Heikanwalder, M. Prinz, I. Arrighi, P. Schwarz, J. Kranich, A. von Teichman, K. M. Haas, N. Zeller, T. F. Tedder, J. H. Weis, and A. Aguzzi. 2007. Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J. Immunol. 179:6144-6152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.