Abstract
Borna disease virus (BDV) frequently persists in the brain of infected animals. To analyze viral dissemination in the mouse nervous system, we generated a mouse-adapted virus that expresses green fluorescent protein (GFP). This viral vector supported GFP expression for up to 150 days and possessed an extraordinary staining capacity, visualizing complete dendritic arbors as well as individual axonal fibers of infected neurons. GFP-positive cells were first detected in cortical areas from where the virus disseminated through the entire central nervous system (CNS). Late in infection, GFP expression was found in the sciatic nerve, demonstrating viral spread from the central to the peripheral nervous system.
Borna disease virus (BDV) is a neurotropic, enveloped virus with a nonsegmented negative-strand RNA genome (2). It naturally infects the central nervous system (CNS) of a broad range of mammalian species (14), where it efficiently establishes persistence in neuronal and nonneuronal cells (4). We recently reported the recovery of a recombinant BDV from cDNA (7, 13) that expresses the green fluorescent protein (GFP) from an additional transcription unit integrated near the 5′ end of its genome (12). The first-generation GFP vector originating from tissue culture-adapted BDV strain He/80 (13) was severely attenuated in the CNS of adult rats (12) and unable to productively infect mice (our unpublished data). Although mice are resistant to infections with primary BDV isolates, the virus can be adapted to gain replication competence in the CNS of these animals (6, 9). After serial passage of recombinant BDV strain He/80 in the brains of MRL mice, we identified five point mutations that confer replication competence in mice (1). Three of these mutations cause amino acid changes, two in the polymerase L and one in the phosphoprotein P. The other two mutations are silent and located within putative regulatory sequences flanking the initiation codon of the X gene. In a previous study we showed that incorporation of the two adaptive mutations into the L gene (LRD) improves the growth properties of the GFP-expressing virus in the CNS of rats (12), but the virus remained severely growth retarded in mice (our unpublished data). The mutation in the P gene was previously shown to reduce the sensitivity of the viral polymerase complex for the inhibitory activity of X (1). A recombinant virus carrying this P mutation in combination with the two mutations in L (BDV-PKLRD) showed strongly enhanced replication speed in mice, whereas the silent mutations within the X gene were found to reduce replication speed and pathogenicity of BDV-PKLRD in MRL mice (1) and rats (our unpublished data). In the present study we incorporated all five adaptive mutations into the BDV genome in order to generate a fully mouse-adapted GFP-expressing BDV vector, designated mBDV-GFP. We found that mBDV-GFP productively infected the central and the peripheral nervous systems of C57BL/6 mice and expressed easily detectable amounts of GFP during the entire observation period of up to 150 days.
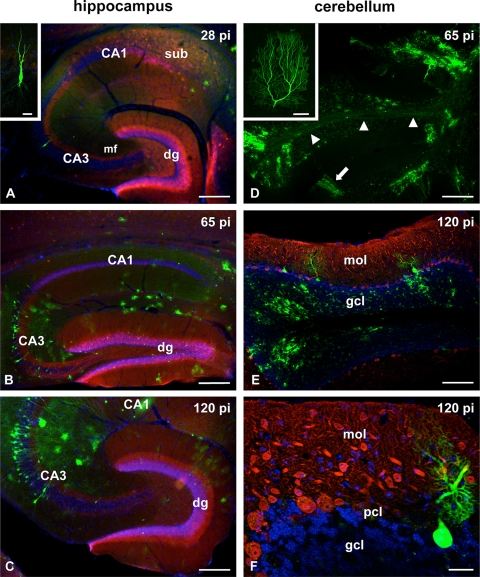
To analyze viral dissemination in the mouse nervous system and to gain insight into the phenotypic identity of BDV-infected cells, we infected C57BL/6 mice intracranially with 10,000 FFU of mBDV-GFP. At the indicated time points postinfection (p.i.), the animals were transcardially perfused with 4% paraformaldehyde, vibratome sectioned, and immunostained for anticalbindin and antiparvalbumin to identify dentate granule cells and cerebellar Purkinje neurons, respectively (3). As early as 28 days p.i. we detected cells strongly resembling astrocytes in the subiculum (sub) that expressed significant amounts of GFP (Fig. 1A). Only a few neurons appeared to be infected. Abundant GFP expression allowed the visualization of the dendritic processes of an individual pyramidal neuron (Fig. 1A). At later time points we observed infected neurons in all hippocampal subfields, and GFP-expressing somata were also present in the calbindin-stained dentate granule cell layer (Fig. 1B and C). In the cerebellum GFP expression was detected in Purkinje cells (PC) and in cells of the internal granule layer at 65 days p.i. (Fig. 1D). The endogenous GFP signal in infected PC was luminous enough to clearly visualize the characteristic dendritic arbor of individual PCs and their axonal processes protruding into the white matter (Fig. 1D to F). GFP-expressing PC and granule cells were still present in the cerebellum after extended periods of infection (Fig. 1E and F).
FIG. 1.
Distribution of mBDV-GFP-infected cells in the hippocampus and the cerebellum. C57BL/6 mice were infected with 10,000 FFU of mBDV-GFP. At the indicated time points (days) p.i., two animals were sacrificed for analysis. (A) Survey of an anticalbindin-immunostained hippocampal section showing the typical calbindin-immunoreactive labeling of granule cells, their mossy fiber projection (mf), and CA1 pyramidal neurons at 28 days p.i. Hoechst nuclear staining illustrates the cytoarchitectural organization of the hippocampus. In the subiculum (sub) GFP-containing cells, presumably astrocytes, are observed. In the CA3 region a single mBDV-GFP-infected pyramidal neuron can be identified. Strong GFP expression allows the visualization of the dendritic tree of this neuron (inset). dg, dentate gyrus. (B) At 65 days p.i. the infection had spread throughout the hippocampus. mBDV-GFP-infected neurons were found in the CA3 region and the granule cell layer (green dots) of the dentate gyrus. (C) At 120 days p.i. the intensity of GFP expression had increased and dendritic processes of infected neurons were easily detected in the CA3 region. The cytoarchitecture of the hippocampus and the calbindin staining of the dentate gyrus appeared unaltered. (D) Overview of cerebellar folia showing strong GFP labeling in the granular cell layer and of axons in the white matter (arrowheads) at 65 days p.i. The arrow points to a cluster of infected Purkinje cells (PC) extending their dendrites into the molecular layer. The inset shows the complete dendritic arbor of an infected PC at high magnification. (E) Micrograph of a cerebellar folium immunolabeled with an antibody against parvalbumin (PARV), a specific marker for PC, at 120 days p.i. Counterstaining with Hoechst stain reveals normal cytoarchitecture. Some BDV-infected PC were found between PARV-positive PC. In the granular layer (blue) many infected cells and their processes were visible. gcl, granular cell layer; mol, molecular layer. (F) An example of an mBDV-GFP-infected PC with its dendrites. The cell body is neighbored by PARV-immunoreactive somata of GFP-negative Purkinje neurons. pcl, Purkinje cell layer. Bars: 250 μm (A), 15 μm (inset of panel A), 250 μm (B and C), 600 μm (D), 60 μm (inset of panel D), 200 μm (E), and 30 μm (F).
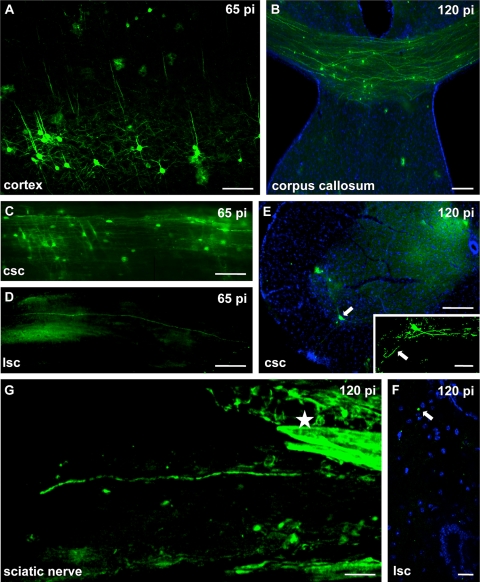
To check for viral dissemination throughout the CNS, we further examined cortical areas as well as cervical to lumbar segments of the spinal cord (SC). We detected abundant GFP expression in cortical neurons (Fig. 2A) and found GFP-positive cell bodies representing myelinating oligodendrocytes in the corpus callosum (Fig. 2B). From 65 days p.i. onwards we detected GFP expression in neurons and axons of the SC. GFP-positive neurons were mainly located in the cervical segment of the SC, and a single axon fiber was detected which extended into the lumbar part of the SC (Fig. 2C and D). At 120 days p.i., cross sections of the SC revealed a diffuse and punctate distribution of GFP signals. In the ventral horn of the cervical part we detected GFP expression in a typical motor neuron with dendritic processes and its axon directed toward the motor root (Fig. 2E). Small GFP-labeled dots were found in all parts of the white matter of the SC, presumably representing cross-sectioned axonal processes (Fig. 2E and F). Only at 120 days p.i. were we able to trace BDV infection in a fiber of the peripheral sciatic nerve. Strong GFP signals were also detected in the perineural sheets, suggesting abundant infection of this nonneuronal tissue (Fig. 2G). To the best of our knowledge, this is the first demonstration of BDV dissemination into the peripheral nervous system of mice, recapitulating similar findings in the nervous system of highly susceptible rats (5, 8, 15). Although persistently infected mice do not seem to transmit the virus, the slow progression of BDV infection from the central to the peripheral nervous system in mice is in accordance with reports showing that persistently infected rats secrete infectious BDV in the urine only after a prolonged period of infection (11). Our data thus support the idea that peripheral nerves provide a potential route for BDV to disseminate to secretory organs like the kidney and that the dissemination of BDV from the central to the peripheral nervous system is a critical step for a persistently infected rodent to become infectious.
FIG. 2.
mBDV-GFP infection of the cortex, the spinal cord, and the peripheral nervous system. C57BL/6 mice were infected with 10,000 FFU of mBDV-GFP. At the indicated time points p.i., two animals were sacrificed for analysis. (A) Intensely GFP-labeled cortical neurons with their typical dendritic pattern. (B) Coronal section of the corpus callosum reveals many GFP-positive axon profiles at 120 days p.i. (C) At 65 days p.i. numerous GFP-labeled cell bodies and axonal profiles were visible in this longitudinal section of the cervical (csc) part of the spinal cord. (D) At that time point, a single axon protruding into the lumbar (lsc) portion of the spinal cord could be traced. (E) Cross section through the cervical spinal cord exhibiting an infected motor neuron (arrow) in the ventral horn. For orientation the section was stained with Hoechst stain. The higher-magnification inset shows the GFP-positive motor neuron, illustrating the dendrites and the efferent axon (arrow) directed toward the anterior radix of the spinal cord. (F) Cross section of the lumbar spinal cord. At a very late time point (120 days p.i.) GFP-labeled circular structures (arrow), presumably representing axons, were found in the posterior part of the lumbar spinal cord. (G) GFP-positive nerve fiber of the peripheral sciatic nerve and the strongly GFP-positive perineural sheaths (asterisks). Bars: 100 μm (A), 50 μm (B), 200 μm (C and D), 250 μm (E), 50 μm (inset of panel E), 25 μm (F), 100 μm (G).
Although GFP-positive cells were present throughout the observation period, we were surprised by the rather low abundance of these cells at late times of infection (Fig. 1B to F). To analyze whether downregulation of GFP expression was responsible for the low abundance of GFP-positive cells, we sacrificed two animals each at 6 weeks and 8 weeks p.i. and extracted protein from the CNS. Western blot analyses using antisera against BDV-N and GFP, respectively, showed an increase of the BDV-N signal between weeks 6 and 8, whereas the GFP signal was substantially weaker at the later time point (Fig. 3A). We further analyzed transcription of the authentic BDV mRNAs encoding the N, P, and X proteins and the GFP mRNA in the brains of two animals sacrificed 10 weeks p.i. (Fig. 3B). The authentic BDV mRNAs were readily detected on a blot loaded with 5 μg of total RNA per lane. In contrast, incubation of a blot containing 20 μg of total RNA per lane with a radiolabeled GFP probe resulted only in very weak GFP signals, indicating strong reduction of GFP transcription compared to that detected in infected Vero cells (12). Finally, immunofluorescent labeling of BDV-N in the dentate gyrus of a mouse sacrificed at 150 days p.i. revealed that the majority of BDV-infected dentate granular cells (red) did not contain detectable amounts of GFP (Fig. 3C). Only a minor population of cell somata showed a clear costaining for GFP and nucleoprotein N (yellow cell body and green dendrites), indicating the existence of a population of mBDV-GFP viruses with severely reduced GFP expression capacities. We recently demonstrated that BDV is able to downregulate transcription of an ectopic X gene by modification of the transcription termination/initiation signals directly upstream of the inserted gene (10). To analyze whether similar mechanisms were responsible for the downregulation of GFP expression in mice, we isolated RNA from the brains of mice sacrificed 4 and 10 weeks after infection with mBDV-GFP and amplified by reverse transcription-PCR (RT-PCR) the complete GFP gene, including the upstream sequences that regulate termination and reinitiation of transcription (Fig. 3D). Bulk and single clone analyses demonstrated that the termination signal upstream of the GFP gene was modified by insertion of one or two additional A residues (Fig. 3E), whereas the coding sequence of the GFP gene remained unaltered (data not shown). These findings clearly suggest that downregulation of GFP expression occurred at the level of transcription. Insertion of additional A nucleotides into the UA7 termination motif might interfere with efficient recognition of the termination signal as previously postulated (10). Northern blot analysis of GFP expression 10 weeks p.i. (Fig. 3B) provided no evidence for polymerase read-through at the termination signal (data not shown), which would result in the synthesis of a bicistronic L-GFP mRNA. We therefore favor the possibility that the additional A residues downregulate the efficiency of transcription reinitiation at the downstream initiation signal, thereby reducing the abundance of GFP mRNA. Further analyses will be required to distinguish between the two possibilities. It also remains unclear why it is apparently advantageous for the virus to downregulate GFP expression. We cannot exclude the possibility that the long-term expression of large quantities of GFP is toxic for infected neuronal cells, thereby inhibiting efficient virus spread. Alternatively, abundant transcription of the GFP mRNA by the viral RNA synthesis machinery might interfere with the balanced expression of viral proteins, slowing down BDV replication in the restrictive mouse system.
FIG. 3.
Loss of GFP expression at late time points after infection. C57BL/6 mice were infected with 10,000 FFU of mBDV-GFP. At the indicated time points p.i., the animals were sacrificed for analysis. (A) Analyses of virus load and GFP expression by Western blotting using a rabbit antiserum specific for BDV-N and a monoclonal antibody against GFP (Stratagene). Correct loading of the gel was verified by staining with a monoclonal antibody against β-tubulin (Sigma). (B) Northern blot analyses of 5 μg (NPX) and 20 μg (GFP) of total RNA isolated from the brains of two animals (lanes A and B) sacrificed 10 weeks p.i. The analyses were performed as previously described (12). The identities of viral transcripts are indicated. For a loading control, we show the ethidium bromide staining of the cellular 18S rRNA. (C) Staining of the dentate gyrus (DG) of an infected mouse at 150 days p.i. with a rabbit antiserum specific for BDV-N. Bound antibodies were detected with a Cy3-conjugated goat anti-rabbit IgG (red). The granule cell layer is intact as shown by Hoechst nuclear staining. Bar, 100 μm. (D) Schematic representation of the regulatory sequences that govern the transcription of the GFP gene. The position of the primer used for sequencing is indicated. (E) Sequence analyses of RT-PCR fragments amplified from viral RNA isolated from mice 4 and 10 weeks after infection with mBDV-GFP, respectively. The electropherograms represent the results of bulk sequencing of the PCR fragments. The selected windows show the transcription regulatory sequences indicated in panel D. Below are shown the sequencing results of individual PCR fragments subcloned into pTopo (Invitrogen). Nucleotides that diverge from the wild-type sequence are shown in red.
Our study demonstrates that foreign genes can be expressed efficiently and permanently in the mouse nervous system using a mouse-adapted BDV vector. Such vectors may represent a good alternative to classical transgenic approaches to study the effect of therapeutic genes in the mouse nervous system, especially in situations where transgene expression in only a fraction of neurons and glial cells is desired. Our data further demonstrate that rodent-adapted BDV-GFP vectors represent a valuable tool to further analyze viral dissemination from the central nervous system to the periphery during persistent infection. Detailed analysis of these processes will be instrumental to further elucidate the mechanisms underlying BDV transmission and epidemiology.
Acknowledgments
We thank Heidi Banse and Rosita Frank for excellent technical assistance and Otto Haller and Sandra Wille for helpful comments on the manuscript.
This work was supported by grants SCHN 765/1-5 and STA 338/8-1 to U.S. and P.S. and grant HE 1520 to B.H. from the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Ackermann, A., P. Staeheli, and U. Schneider. 2007. Adaptation of Borna disease virus to new host species attributed to altered regulation of viral polymerase activity. J. Virol. 81:7933-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briese, T., A. Schneemann, A. J. Lewis, Y. S. Park, S. Kim, H. Ludwig, and W. I. Lipkin. 1994. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. U. S. A. 91:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celio, M. R. 1990. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35:375-475. [DOI] [PubMed] [Google Scholar]
- 4.de la Torre, J. C. 2002. Bornavirus and the brain. J. Infect. Dis. 186(Suppl. 2):S241-S247. [DOI] [PubMed] [Google Scholar]
- 5.Herzog, S., C. Kompter, K. Frese, and R. Rott. 1984. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med. Microbiol. Immunol. 173:171-177. [DOI] [PubMed] [Google Scholar]
- 6.Kao, M., H. Ludwig, and G. Gosztonyi. 1984. Adaptation of Borna disease virus to the mouse. J. Gen. Virol. 65:1845-1849. [DOI] [PubMed] [Google Scholar]
- 7.Martin, A., P. Staeheli, and U. Schneider. 2006. RNA polymerase II-controlled expression of antigenomic RNA enhances the rescue efficacies of two different members of the Mononegavirales independently of the site of viral genome replication. J. Virol. 80:5708-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales, J. A., S. Herzog, C. Kompter, K. Frese, and R. Rott. 1988. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med. Microbiol. Immunol. 177:51-68. [DOI] [PubMed] [Google Scholar]
- 9.Nishino, Y., D. Kobasa, S. A. Rubin, M. V. Pletnikov, and K. M. Carbone. 2002. Enhanced neurovirulence of Borna disease virus variants associated with nucleotide changes in the glycoprotein and L polymerase genes. J. Virol. 76:8650-8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poenisch, M., S. Wille, U. Schneider, and P. Staeheli. 2009. Second-site mutations in Borna disease virus overexpressing viral accessory protein X. J. Gen. Virol. 90:1932-1936. [DOI] [PubMed] [Google Scholar]
- 11.Sauder, C., and P. Staeheli. 2003. Rat model of Borna disease virus transmission: epidemiological implications. J. Virol. 77:12886-12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider, U., A. Ackermann, and P. Staeheli. 2007. A Borna disease virus vector for expression of foreign genes in neurons of rodents. J. Virol. 81:7293-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider, U., M. Schwemmle, and P. Staeheli. 2005. Genome trimming: a unique strategy for replication control employed by Borna disease virus. Proc. Natl. Acad. Sci. U. S. A. 102:3441-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 15.Stitz, L., K. Noske, O. Planz, E. Furrer, W. I. Lipkin, and T. Bilzer. 1998. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J. Virol. 72:8884-8892. [DOI] [PMC free article] [PubMed] [Google Scholar]