Abstract
Respiratory syncytial virus (RSV) is the main cause of bronchiolitis, the major cause of hospitalization of infants. An ideal RSV vaccine would be effective for neonates, but the immune responses of infants differ markedly from those of adults, often showing a bias toward T-helper 2 (Th2) responses and reduced gamma interferon (IFN-γ) production. We previously developed recombinant RSV vectors expressing IFN-γ and interleukin-4 (IL-4) that allow us to explore the role of these key Th1 and Th2 cytokines during infection. The aim of the current study was to explore whether an immunomodulation of infant responses could enhance protection. The expression of IFN-γ by a recombinant RSV vector (RSV/IFN-γ) attenuated primary viral replication in newborn mice without affecting the development of specific antibody or T-cell responses. Upon challenge, RSV/IFN-γ mice were protected from the exacerbated disease observed for mice primed with wild-type RSV; however, antiviral immunity was not enhanced. Conversely, the expression of IL-4 by recombinant RSV did not affect virus replication in neonates but greatly enhanced Th2 immune responses upon challenge without affecting weight loss. These studies demonstrate that it is possible to manipulate infant immune responses by using cytokine-expressing recombinant viruses and that neonatal deficiency in IFN-γ responses may lead to enhanced disease during secondary infection.
Respiratory syncytial virus (RSV) is the most important cause of viral lung infection in infants (31). The hospitalization rate in the United States is 17 per 1,000 children, with much higher outpatient rates (15), and reinfection in infants is common (14). The mortality caused by RSV in developing countries and the high rates of hospitalization in industrialized countries mean that the development of an RSV vaccine is an urgent health priority. A major problem in the development of an RSV vaccine is the requirement for efficacy in very early life; this is problematic because of the immaturity of the immune system of infants (38). This immune immaturity decreases vaccine efficacy (29) and increases susceptibility to all respiratory viruses (34).
In general, protection against viral infection is mediated by a balance of neutralizing antibody responses and long-lasting virus-specific CD8 T-cell memory. Both of these components are severely impaired in infants. There are several aspects that contribute to the reduced immune response in early life. Total lymphocyte and dendritic cell numbers are significantly lower in neonates than in adults (3). Microarchitectural structures thought to be crucial for antibody development (i.e., lymphoid follicles, follicular dendritic cell networks, and germinal centers) are absent at birth and form only at between a few days and a few weeks of age (13). The full repertoire of antigen-presenting cells (APCs) is achieved only several weeks after birth (32). Finally, the responses primed by infantile exposure to pathogens, or other inflammatory stimuli, are significantly altered compared to adult responses, with memory responses being dominated by the T-helper 2 (Th2) arm of the immune system (2, 3).
Increased levels of interleukin-4 (IL-4), the archetypal Th2 cytokine, are associated with increased RSV disease severity in infants (1, 18, 25). In addition, severe RSV infection is linked with the development of wheeze and asthma in later life (30), diseases that are classically linked to aberrant Th2 responses. Infection of neonatal mice with RSV results in the development of Th2-skewed immunity and increased disease severity when mice are reinfected with RSV as adults (8). In comparison, RSV infection of adult mice results in a Th1 and CD8 T-cell response and the subsequent development of protective immunity (28, 35). It is therefore hypothesized that increased Th2 responses are detrimental to the outcome of neonatal RSV infection and that a reduction of Th2 responses and/or a promotion of Th1 responses would be beneficial.
We have previously demonstrated that recombinant RSV expressing gamma interferon (IFN-γ) (RSV/IFN-γ) can be used to promote Th1 immunity in adult mice, attenuating viral replication without affecting immunogenicity (5). In contrast, the coexpression of IL-4 by RSV (RSV/IL-4) causes defective T-cell responses but does not affect viral clearance (6). Upon challenge, RSV/IFN-γ-primed adult mice show enhanced CD8 T-cell recruitment, leading to immunopathology, while RSV/IL-4-primed mice showed enhanced eosinophilia (16). The aim of the current study was to investigate the effects of cytokine modulation on the neonatal immune response to RSV. We found that RSV/IFN-γ was protective against the disease-enhancing effects of neonatal RSV infection, while RSV/IL-4 significantly increased the Th2 skewing of the immune response without a worsening of disease.
MATERIALS AND METHODS
Virus stocks and mouse infection.
RSV (strain A2) was obtained from the ATCC, and recombinant RSV expressing mouse IFN-γ (RSV/IFN-γ) or IL-4 (RSV/IL-4) or wild-type recombinant virus (RSV/wt) was made as described previously (5, 6). Time-mated pregnant BALB/c mice (Harlan, Iscoed, United Kingdom) were purchased at <14 days of gestation, and pups were weaned at 3 weeks of age. Mice were infected intranasally (i.n.) with 4 × 104 focus-forming units (FFU) of virus/g of body weight at 4 days (neonatal, ∼105) under isoflurane anesthesia. Secondary RSV challenge was given i.n. at 8 weeks postpriming, with 106 FFU in 100 μl. Weight was measured daily to monitor disease severity. All work was approved and licensed by the United Kingdom Home Office. Experiments were performed at least twice with at least four mice per experiment.
Quantification of viral RNA.
RNA was extracted from the lung by use of RNA Stat-60 (AMS Biotech Ltd.), and cDNA was generated with random hexamers by use of Omniscript RT (Qiagen). Real-time PCR was carried out with a sequence in the RSV L gene by using 900 nM forward primer (5′-GAACTCAGTGTAGGTAGAATGTTTGCA-3′), 300 nM reverse primer (5′-TTCAGCTATCATTTTCTCTGCCAAT-3′), and 100 nM probe (5′-6-carboxyfluorescein (FAM)-TTTGAACCTGTCTGAACAT-6-carboxytetramethylrhodamine (TAMRA)-3′) on an ABI Prism 7000 sequence detection system. This detects viral genomic RNA, viral antigenomic RNA, and intracellular RSV L mRNA, referred to here as RSV L RNA.
Cell recovery.
The collection of bronchoalveolar lavage (BAL) fluids for cells and supernatants and the harvesting of lung tissues were carried out as previously described (7). For the preparation of lung mash supernatants, lungs were homogenized through 100-μm cell strainers (BD Pharmingen) and washed through with a 1-ml volume of RPMI medium five times; following centrifugation, this supernatant was retained for enzyme-linked immunosorbent assays (ELISAs). After the removal of the supernatants from all tissues, cells were treated with red blood cell lysis buffer for 5 min and then DNase I (40 μg/ml)-collagenase (50 μg/ml) in RPMI 1640 medium (10% fetal calf serum [FCS]) for 5 min at room temperature to remove clumps; finally, the cells were resuspended in RPMI medium. Cell viability was assessed by trypan blue exclusion, and total cell numbers were counted by use of a disposable multiwell hemocytometer (Immunesystems, United Kingdom). Airway cells were differentiated by hematoxylin and eosin (H&E) staining of BAL fluid cell samples.
Flow cytometry.
Prior to staining, cells were blocked with CD16/CD32 (Fc Block; BD). For surface staining, antibodies against the surface markers CD4, CD8, CD3, CD69, CD11b, CD11c, CD80, CD86, major histocompatibility complex (MHC) class II (MHCII), CD62L, CD44, and DX5 (BD) were added at a 1:100 dilution for 30 min on ice. RSV-specific CD8 cells were characterized by use of the RSV M2 MHCI pentamer (SYIGSINNI; Proimmune). Gating for lymphocytes was determined by back gating on CD3/CD8-double-positive cells. For the detection of intracellular cytokines, cells were incubated with 50 ng/ml phorbol myristate acetate (PMA), 500 ng/ml ionomycin, and 10 μg/ml brefeldin A for 4 h at 37°C. Samples were permeabilized with 0.5% saponin in phosphate-buffered saline (PBS) for 10 min. Anti-cytokine antibodies (anti-IFN-γ and anti-IL-4; BD) or isotype controls were added for a further 20 min at room temperature. Cells were analyzed with a CyAn ADP (Dako) flow cytometer, collecting data for at least 50,000 events.
Cytokine ELISA.
Cytokine levels in BAL fluid and lung mash supernatants were assessed. ELISA plates (Nunc) were coated with capture antibody (anti-IL-4, -IL-5, or -IFN-γ; BD) overnight at 4°C. Wells were washed and blocked with 1% bovine serum albumin (BSA) for 1 h at room temperature. One hundred microliters of sample or standard was added to blocked wells for 2 h. Bound cytokine was detected by using biotinylated anti-cytokine antibody, avidin horseradish peroxidase, and tetramethylbenzidine. Color development was terminated with 2 M H2SO4, and the optical density (OD) was read at 490 nm. The concentration of cytokine was determined from the standard curve.
RSV-specific antibody ELISA.
Serum antibody was assessed by ELISA. Antigen was prepared by infecting HEp-2 cells with RSV at 1 FFU/cell. Microtiter plates were coated overnight with 100 μl of a 1:500 dilution of either RSV or HEp-2 antigen. After blocking with 1% BSA for 1 h, dilutions of test samples were added for a further 1 h. Bound antibody was detected by using peroxidase-conjugated rabbit anti-mouse Ig (Dako) and o-phenylenediamine as a substrate. Color development was blocked with 2 M H2SO4, and the OD was read at 490 nm. RSV-specific antibody was determined by subtracting the RSV absorbance from the HEp-2 absorbance for the same sample. Specific isotypes were measured according to the same protocol, changing the primary antibody. Total, nonspecific IgE was measured according to the manufacturer's instructions (BD).
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM); statistical significance was calculated by analysis of variance (ANOVA) followed by Tukey tests when there were more than three groups and t tests for comparisons of two groups by using GraphPad Prism software.
RESULTS
Primary neonatal infection with recombinant RSV expressing either IFN-γ or IL-4.
We have previously described the construction and characterization of recombinant RSVs expressing either IFN-γ or IL-4 (16). These viruses grow in both human and mouse cell lines with kinetics similar to those of wild-type viruses (data not shown). In adult BALB/c mice, RSV/IL-4 grows at wild-type levels, while RSV/IFN-γ is attenuated approximately 10-fold (5, 6, 16). To assess the effect of these viruses on neonates, mice less than 1 week of age were infected with 8 × 104 FFU RSV/wt, RSV/IFN-γ, or RSV/IL-4 intranasally (i.n.). Weight was monitored daily as a measure of disease severity (Fig. 1A). Neonates gained weight rapidly from day 0 of infection, doubling their weight by day 8 postinfection (p.i.); there was no significant change in weight gain following infection.
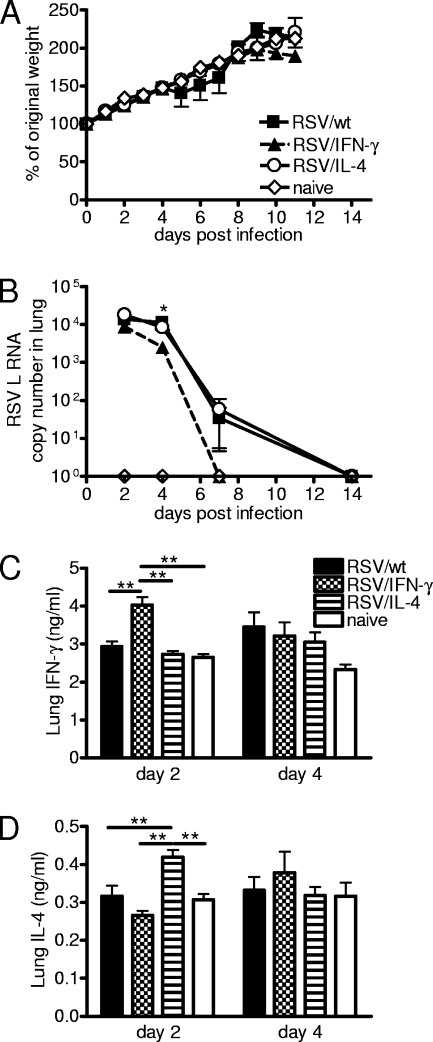
FIG. 1.
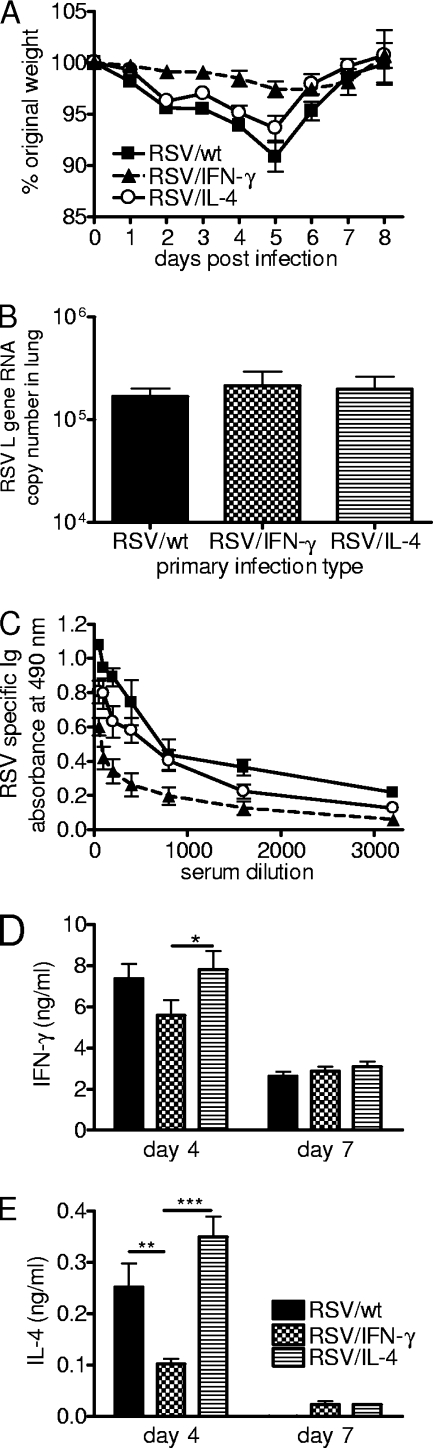
Recombinant virus replication and cytokine production in primary neonatal infection. Four-day-old BALB/c mice were infected with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4 i.n. or left naïve. (A) Weight was monitored daily, and the weight change compared to weight prior to infection was plotted. (B) TaqMan PCR for RSV L RNA was carried out with RNA from lungs collected on days 2, 4, 7, and 14 postinfection. (C and D) Lung supernatants were taken on days 2 and 4 postinfection, and the concentrations of IFN-γ (C) and IL-4 (D) were determined by ELISA. Data are representative of three experiments, and points represent data for five mice per group ± SEM. *, P < 0.05; **, P < 0.01.
RNA was extracted from the lungs on days 2, 4, 7, and 14 postinfection, and levels of RSV L RNA were assessed by real-time PCR (Fig. 1B). Following infection with RSV/wt or RSV/IL-4, there was a peak of viral RNA levels detected on days 2 and 4, which declined on day 7 and was cleared by day 14. There was no significant difference between RSV/wt and RSV/IL-4 infections. On day 2, the levels of total RSV L RNA from RSV/IFN-γ-infected mice were approximately half of those seen for the other groups, and on day 4 postinfection, the levels were 3-fold lower than those for RSV/wt infection (P < 0.05). There was no RSV L RNA detectable in the lungs of RSV/IFN-γ-infected mice from day 7 onwards, indicating that viral clearance was quicker than that after RSV/wt or RSV/IL-4 infection.
As was previously seen for adult mice, infection with RSV/IFN-γ caused a significant increase in the lung IFN-γ concentration on day 2 postinfection compared to infection with RSV/wt or RSV/IL-4 or that of naïve mice (P < 0.01) (Fig. 1C). Likewise, RSV/IL-4 caused a significant peak of IL-4 on day 2 postinfection compared to that of the other groups (P < 0.05) (Fig. 1D). Therefore, the recombinant RSVs are infectious in neonates, inducing local cytokine production, which alters the viral clearance kinetics.
Effect of the coexpression of IL-4 and IFN-γ on cellular recruitment during primary neonatal RSV infection.
We were interested in the effect of recombinant viral vectors on neonatal immune responses. Lung cells were collected from neonatal mice infected with RSV/wt, RSV/IFN-γ, or RSV/IL-4 or from mice left uninfected on days 4, 7, and 14 postinfection. The total number of cells in the lung increased with age, and RSV infection did not significantly alter this at any time point (Fig. 2A). Infection did not affect lymphocyte percentages in the lungs, which went from approximately 30% at day 4 postinfection to approximately 60% at day 14 postinfection in all cases (Fig. 2B).
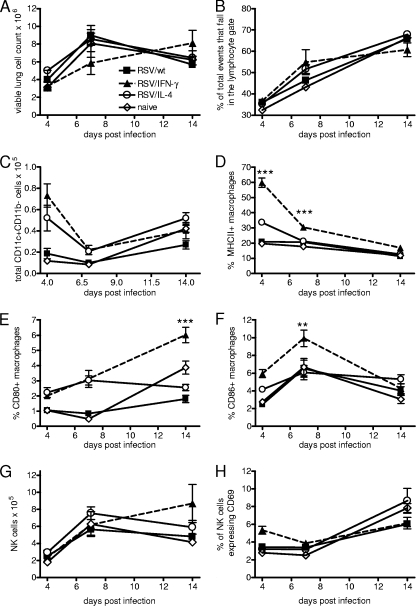
FIG. 2.
The recruitment and activation of innate immune cells is altered by viral cytokine expression. Four-day-old BALB/c mice were infected with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4 i.n. or left naïve. (A and B) Cells were taken from lungs, and total lung cell numbers after homogenization (A) and percentages of lymphocytes (B) were determined on days 4, 7, and 14 postinfection. (C to F) Lung macrophages (CD11c+ CD11b− cells) were characterized by flow cytometry, measuring numbers (C) and MHCII (D), CD80 (E), and CD86 (F) expression levels. (G and H) Total numbers of NK cells (CD3− DX5+) (G) and activation of NK cells/CD69+ (H) were also measured. Data are representative of two experiments, and points represent data for five mice per group ± SEM.
The effect of cytokine on antigen-presenting cells in the neonatal lung was also assessed. There were no differences in the numbers of CD11c+ CD11b+ dendritic cells recruited to the lung following infection (data not shown). However, there were significantly more CD11c+ CD11b− macrophages in the lungs on day 4 after infection of RSV/IFN-γ-infected mice than in the lungs of RSV/wt- or RSV/IL-4-infected mice (P < 0.05) (Fig. 2C). The activation status of these cells was also significantly increased by RSV/IFN-γ infection, as measured by MHCII (Fig. 2D), CD80 (Fig. 2E), and CD86 (Fig. 2F) levels. NK cell numbers (CD3− DX5+) increased with age, and RSV infection had little effect on this (Fig. 2G). The cell surface protein CD69 is a marker of activation on both NK and T cells (39). On day 4 postinfection, only mice infected with RSV/IFN-γ had an increased percentage of CD69+ NK cells in their lungs (P < 0.05) (Fig. 2H). On subsequent days, there was no difference between RSV-infected animals and naïve animals regardless of the virus used.
The number of T cells in the lungs also increased from days 4 to 14 postinfection (day 18 of life). On day 4 postinfection, naïve mice had approximately 1.2 × 104 CD4 T cells (Fig. 3A) and approximately 4.8 × 103 CD8 T cells (Fig. 3B). By day 14 after infection (18 days of age), the number of CD4 T cells in the naïve lung had risen to approximately 5.3 × 104 cells per lung, while the number of CD8 T cells was 1 × 104 cells per lung. As with total cell and lymphocyte recruitment, infection with RSV/wt or either of the recombinant viruses seemed to have little effect on the pattern of T-cell recruitment.
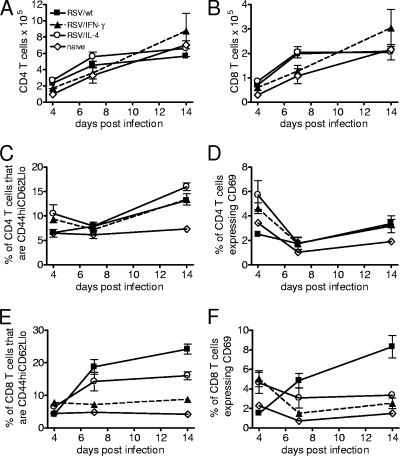
FIG. 3.
Neonatal T-cell responses are altered by viral cytokine expression. Four-day-old BALB/c mice were infected with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4 i.n. or left naïve. (A and B) Cells were taken from lungs, and total numbers of CD4 (A) and CD8 (B) T cells were measured on days 4, 7, and 14 postinfection by flow cytometry. (C to F) CD62l− CD44+ memory (C and E) and CD69+-activated (D and F) CD4 and CD8 cells, respectively, were also measured at the same time points. Data are representative of two experiments, and points represent data for five mice per group ± SEM.
In order to determine whether infection affected T-cell activity rather than affecting total recruitment, we analyzed the expression levels of a number of cell surface markers. CD62L and CD44 can be used to differentiate effector T cells (CD44hi CD62Llo) and memory T cells (CD44hi CD62Lhi) (37). Very few memory cells were seen in the lungs at any time point, but there were observable changes in the percentages of effector T cells with infection. The coexpression by RSV of either IFN-γ or IL-4 caused an increase in the proportion of CD4 effector cells on day 4 p.i. compared to RSV/wt-infected and naïve mice (Fig. 3C). By day 7 p.i. this difference was not observable, but on day 14 p.i., all infected mice showed a significant increase in the percentage of effector CD4 T cells compared to naïve mice, and at this time point, there was no difference between the different viral infections (P < 0.05). Expression of CD69 by CD4 T cells followed a pattern similar to that of CD44. On day 4 postinfection, RSV/IL-4- and RSV/IFN-γ-infected mice had higher percentages of CD69+ CD4 T cells than RSV/wt-infected mice (Fig. 3D). On day 14, there were slightly elevated percentages for infected mice compared to naïve mice.
There was no significant difference in CD44 expression by CD8 cells on day 4 postinfection. On day 7 and day 14 postinfection, mice infected with RSV/IFN-γ had significantly fewer CD8 effector cells than mice infected with RSV/wt or RSV/IL-4 (P < 0.05) (Fig. 3E). Infection with RSV/IFN-γ or RSV/IL-4 caused a significant upregulation of CD69 on CD8 T cells compared to infection with RSV/wt on day 4 postinfection (P < 0.05). On subsequent days, RSV/wt-infected neonates had increased numbers of CD69+ CD8 T cells; on day 14 postinfection, there were significantly more active CD8 T cells than for any other group (P < 0.05). At this time point, the percentages of activated CD8 T cells in mice infected with RSV/IFN-γ or RSV/IL-4 were similar to those found in naïve mice (Fig. 3F).
On day 14 postinfection, after viral clearance from the lungs, CD4 T cells were stimulated with PMA and ionomycin and stained for intracellular IL-4 and IFN-γ. The number of IFN-γ+ CD4 T cells was low in all groups; however, both RSV/wt and RSV/IFN-γ had significantly more IFN-γ+ CD4 T cells than naïve mice (P < 0.05) (Fig. 4A). RSV/IL-4 infection did not result in an increased number of IFN-γ+ CD4 T cells. RSV/wt and RSV/IL-4 infections resulted in significantly increased numbers of IL-4+ CD4 T cells in the lungs compared to RSV/IFN-γ infection (P < 0.05) (Fig. 4B).
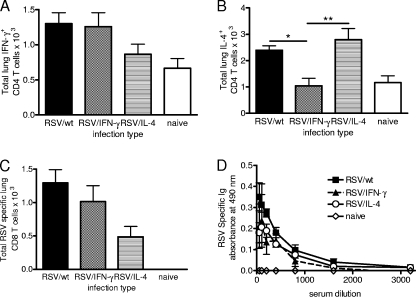
FIG. 4.
T-cell cytokine production and RSV-specific antibody responses following recombinant RSV infection. Four-day-old BALB/c mice were infected with 8 × 104 FFU of RSV/wt (black bars), RSV/IFN-γ (hatched bars), or RSV/IL-4 (lined bars) i.n. or left naïve (open bars). (A and B) On day 14 postinfection, PMA-ionomycin-stimulated CD4 T cells were stained for the intracellular presence of IFN-γ (A) and IL-4 (B). (C) At the same time point, CD8 T cells were stained with an RSV M2-specific pentamer. (D) Serum was taken at 14 days postinfection, and the level of RSV-specific Ig was determined by ELISA. Data are representative of two experiments, and points represent data for five mice per group ± SEM. *, P < 0.05; **, P < 0.01.
An MHC class I tetramer containing the immunodominant RSV-specific CD8 T-cell epitope (SYIGSINNI) located in the M2-1 protein (23) was also used on day 14 to determine the number of RSV-specific CD8 T cells. RSV/wt and RSV/IFN-γ infections resulted in the recruitment of more RSV-specific CD8 T cells than did RSV/IL-4 infection (Fig. 4C). In contrast to the cellular responses, similar serum levels of RSV-specific antibody were detected in mice infected with all three viruses at day 14 postinfection (Fig. 4D). From this, we observe that cytokine coexpression subtly alters the profile of cells recruited during neonatal infection.
IFN-γ coexpression during primary infection prevents the development of enhanced disease upon challenge as adults.
As both recombinant viruses altered the immune response to neonatal RSV infection, we hypothesized that secondary immune responses and disease outcome would also be affected. Neonatal mice were primed and then rechallenged 8 weeks after priming with 106 FFU of wild-type RSV. As we have previously reported (8), neonatal infection with RSV/wt resulted in weight loss upon secondary infection, peaking on day 4 and with recovery at around day 7 (Fig. 5A). Mice primed with RSV/IL-4 also lost weight upon challenge, with a profile similar to that of RSV/wt-primed mice, but RSV/IFN-γ-primed mice lost significantly less weight than the RSV/IL-4-primed mice on days 2 to 5 postinfection (P < 0.05).
FIG. 5.
IFN-γ expression in the neonatal period protects against exacerbated weight loss. Four-day-old BALB/c mice were infected i.n. with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4. The mice were challenged with 106 FFU of wild-type RSV A2 i.n. at 8 weeks of age. (A) After challenge, weight was monitored daily and plotted against the starting weight. (B) On day 4 postchallenge, RNA was extracted from the lungs, and the amount of RSV L RNA was determined by real-time PCR. (C) Total RSV-specific Ig was measured in serum at day 7. (D and E) BAL fluid supernatants were taken on days 4 and 7 to determine the levels of IFN-γ (D) and IL-4 (E) by ELISA. Data are representative of two repeats, and points represent data for five mice per group ± SEM. **, P < 0.01; ***, P < 0.001.
In spite of the change in weight loss, viral loads were similar for all three groups on day 4 postinfection, and no viral RNA was detectable on day 7 or 14 postchallenge (Fig. 5B). RSV/IFN-γ-primed animals had significantly reduced anti-RSV antibody titers (P < 0.05) (Fig. 5C). Interestingly, neonatal priming with RSV/IFN-γ resulted in reduced concentrations of both IFN-γ (Fig. 5D) and IL-4 (Fig. 5E) in the BAL fluid on day 4 postinfection compared to RSV/IL-4 (P < 0.05).
Cellular responses during secondary infection.
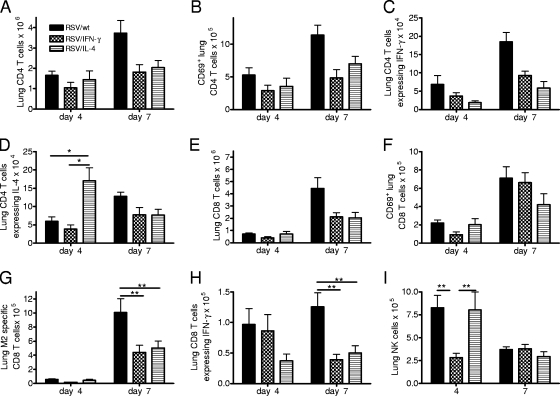
The numbers of CD4, CD8, and NK cells in the lungs were assessed on days 4 and 7. There were significantly more CD4 T cells in the lungs of RSV/wt-primed mice on day 7 postchallenge than in the lungs of mice primed with RSV/IFN-γ or RSV/IL-4 (P < 0.01) (Fig. 6A). The CD4 cells were significantly more activated (CD69+; P < 0.05) (Fig. 6B) and produced more IFN-γ (P < 0.01) (Fig. 6C). There were no differences in the numbers, activation statuses, or levels of IFN-γ production of the CD4 T cells from RSV/IL-4- or RSV/IFN-γ-primed mice. However, there were significantly more IL-4+ CD4 T cells in the lungs of mice primed with RSV/IL-4 on day 4 postchallenge than in the lungs of either RSV/IFN-γ- or RSV/wt-primed mice (P < 0.01) (Fig. 6D).
FIG. 6.
T-cell responses in lungs upon challenge. Four-day-old BALB/c mice were infected i.n. with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4. The mice were challenged with 106 FFU of wild-type RSV A2 i.n. at 8 weeks of age. (A to D) Lung CD4 T-cell numbers (A) and activation (B) and the intracellular expression levels of IFN-γ (C) and IL-4 (D) were measured on days 4 and 7 postinfection. (E to I) Lung CD8 T-cell numbers (E), activation (F), M2 specificity (G), and intracellular IFN-γ expression following stimulation (H) and NK cell numbers (I) were measured at the same time points. Data are representative of two repeats, and points represent data for five mice per group ± SEM. *, P < 0.05; **, P < 0.01.
CD8 T-cell recruitment into the lungs peaked on day 7 postchallenge for all groups, with recruitment being the greatest after priming with RSV/wt compared to priming with RSV/IFN-γ or RSV/IL-4 (P < 0.01) (Fig. 6E). There was no significant difference in the activation of these cells, as measured by using CD69 (Fig. 6F), but there were significantly more RSV-specific (P < 0.01) (Fig. 6G) and IFN-γ-producing (P < 0.01) (Fig. 6H) CD8 T cells following RSV/wt priming on day 7 postchallenge. On day 4 postchallenge, RSV/IL-4- and RSV/wt-primed mice had significantly more lung NK cells than RSV/IFN-γ-primed mice (P < 0.01) (Fig. 6I). On day 7 postinfection, NK cell numbers were greatly reduced and were similar for all three primed groups.
IL-4 priming results in significant enhancement of Th2 responses upon challenge.
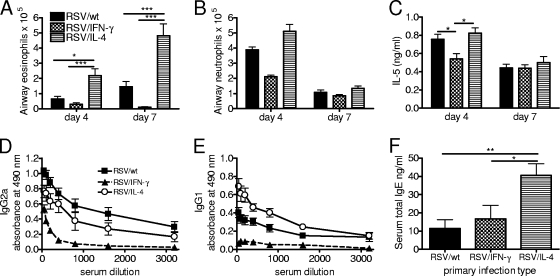
The disease seen upon the rechallenge of mice primed with RSV in the neonatal period was previously described to be the result of a Th2-skewed immune response (8). Eosinophil (Fig. 7A) and neutrophil (Fig. 7B) recruitment was observed mainly for mice primed neonatally with RSV/wt or RSV/IL-4 but not RSV/IFN-γ. The coexpression of IL-4 during neonatal infection caused an approximately 4-fold increase in airway eosinophilia on day 4 and a 2.5-fold increase in airway eosinophilia on day 7 postchallenge compared to RSV/wt infection (P < 0.01). IL-5 is a key cytokine in the recruitment and survival of eosinophils, and on day 4 postchallenge, levels of IL-5 were significantly lower for RSV/IFN-γ-treated animals than for the other two groups (P < 0.05) (Fig. 7C).
FIG. 7.
Th1/Th2 balance of the recall response. Four-day-old BALB/c mice were infected i.n. with 8 × 104 FFU of RSV/wt, RSV/IFN-γ, or RSV/IL-4. The mice were challenged with 106 FFU of wild-type RSV A2 i.n. at 8 weeks of age. (A and B) BAL was carried out on days 4 and 7 postinfection, and the numbers of airway eosinophils (A) and neutrophils (B) recruited to the lung were determined by H&E staining. (C) IL-5 concentrations in BAL fluid supernatants were determined by ELISA. (D to F) Serum was taken on day 7 postchallenge, and the levels of RSV-specific IgG2a (D) and IgG1 (E) and total serum IgE (F) were quantified by ELISA. Data are representative of two repeats, and points represent data for five mice per group ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As an additional readout of the Th1/Th2 balance of rechallenged mice, RSV-specific antibody subtypes were determined on day 7 postchallenge. IgG2a responses were slightly, but not significantly, higher in RSV/wt-primed mice than in RSV/IL-4-primed animals (Fig. 7D). RSV-specific IgG1 (Th2-associated subtype) responses were significantly higher in mice primed with RSV/IL-4 than in mice primed with either RSV/wt (P < 0.05) or RSV/IFN-γ (P < 0.001) (Fig. 7E). Serum was also tested for the presence of total IgE on day 7 postchallenge. Neonatal priming with RSV/IL-4 resulted in significantly elevated levels of total IgE compared to priming with RSV/wt or RSV/IFN-γ (P < 0.05) (Fig. 7F). Interestingly, all three neonatally primed groups had significantly elevated levels of serum IgE compared to those of naïve mice or mice primed as adults, for which the development of serum IgE is not normally observed (data not depicted). Therefore, the coexpression of IL-4 by RSV during primary neonatal priming enhances the Th2 nature of the secondary response.
DISCUSSION
The aim of the current study was to investigate the effects of cytokine modulation on the neonatal immune response to RSV in the context of improving neonatal vaccine efficacy. As described previously (5, 16), reduced viral titers were observed following infection with the RSV/IFN-γ virus compared to RSV/wt and RSV/IL-4. This finding could be due to the insertion of the IFN-γ cDNA within the RSV genome affecting replication. However, it was shown previously that RSV can accept inserted genes with little effect on genome transcription or replication (5). A similar phenotype of reduced viral load was observed with a recombinant RSV that expresses IL-18; in this case, replication occurs normally in vitro but is reduced in vivo (17). It is possible that both RSV recombinants have impaired growth in vivo but that IL-4 expression leads to an impaired antiviral defense and enhanced viral replication that matches the impaired growth caused by gene insertion (6). However, the simplest explanation is that IFN-γ expression decreased the viral load because it augments antiviral immunity.
It was reported previously that the enhanced disease seen after the rechallenge of RSV-primed neonates is characterized as Th2 skewed, with increased levels of IL-4-secreting CD4 T cells, eosinophilia, and IL-13 production (8, 10). Many of the features of the immune response that were increased by RSV/IL-4 priming, for example, IgE and eosinophilia, have been postulated to be important in severe RSV disease. In infants, IgE levels correlate with the severity of disease (36), and IgE is required for enhanced airway hyperresponsiveness in neonatally primed mice (9). In a similar fashion, eosinophilia has been associated with enhanced lung disease both following RSV infection (20) and during asthma and allergy (19).
While the overexpression of IL-4 during neonatal priming resulted in increased levels of IgE and eosinophilia upon challenge, it did not result in increased disease. The role of eosinophils in allergic airway disease is currently being reevaluated, with the demonstration that they have a beneficial role in innate antiviral immunity and are dispensable for the development of asthma (12, 22, 27). Furthermore, a depletion of CD4 cells during neonatal murine RSV infection only marginally reduces weight loss despite eliminating eosinophilia (35). We have also observed that CD8 T cells are a critical determinant of disease, but there may be a balance between protective and pathogenic CD8 T cells (33). These data indicate that while Th2 responses are often seen during enhanced RSV disease, their presence does not correlate directly with severity.
Despite a lack of disease enhancement with increased IL-4 expression, the priming of neonates with RSV/IFN-γ abrogated weight loss after rechallenge. Recently, Lee et al. demonstrated that the administration of recombinant IFN-γ during neonatal infection prevented the development of airway hyperresponsiveness or eosinophilia upon rechallenge (24). The proposed mechanism for this was an increase in Th1 memory responses and a reduction in Th2 memory responses. RSV/IFN-γ, however, did not increase the recruitment of IFN-γ-secreting CD4 cells or the production of IFN-γ during rechallenge, suggesting that it did not enhance Th1 cell development. Therefore, the data presented here suggest that there may be an alternative mechanism of protection.
Viral infection of adults is known to cause long-term changes in lung-resident APCs, including macrophages (11). Interestingly, infection of neonates with RSV/wt did not result in a significant maturation and activation of these cells in vivo. However, priming with IFN-γ caused a rapid and significant maturation of macrophages in the neonatal lung. Therefore, one possible mechanism of protection for RSV/IFN-γ could be through the maturation of the innate components of lung immunity.
Upon rechallenge, RSV/IFN-γ priming resulted in significantly reduced neutrophil and NK cell recruitment at the peak of weight loss. This suggests that, along with T cells, cells of the innate immune system contribute to weight loss. For infantile bronchiolitis, neutrophils were observed to be the major cell type in the airways of RSV-infected infants (26). The activation of NK cells by the overexpression of IL-18 enhances disease and weight loss during primary RSV infection (17), and NK cells are the major source of early IFN-γ production during RSV infection (21). Both NK cells and neutrophils are activated and recruited to the lungs by a similar range of mediators, produced primarily by resident lung macrophages in RSV infection, supporting the idea that macrophages may be involved in neonatally primed disease (4, 28).
We conclude that exogenous cytokine expression can modulate the neonatal immune response and alter the immunological memory generated during early childhood. Importantly, the ability of IFN-γ to protect in this neonatal model contrasts with the results for adult mice, where RSV/IFN-γ enhances disease during rechallenge (16). It is therefore necessary to consider the age of first priming in the development of vaccines, since the age at which the vaccine is administered is critical for determining the pattern of immune response that results.
Acknowledgments
This work was supported by Wellcome Trust Programme grant 071381/Z/03/Z (United Kingdom) and a Medical Research Council (United Kingdom) studentship. A.B. and P.L.C. are funded by an NIAID intramural program.
Footnotes
Published ahead of print on 3 March 2010.
REFERENCES
- 1.Aberle, J. H., S. W. Aberle, W. Rebhandl, E. Pracher, M. Kundi, and T. Popow-Kraupp. 2004. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin. Exp. Immunol. 137:146-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, B., and R. Q. Du. 1998. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J. Immunol. 160:4217-4224. [PubMed] [Google Scholar]
- 3.Adkins, B., C. LeClerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 4.Benoit, A., Y. Huang, J. Proctor, G. Rowden, and R. Anderson. 2006. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 145:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukreyev, A., S. S. Whitehead, N. Bukreyeva, B. Murphy, and P. L. Collins. 1999. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc. Natl. Acad. Sci. U. S. A. 96:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev, A., I. M. Belyakov, G. A. Prince, K. C. Yim, K. K. Harris, J. A. Berzofsky, and P. L. Collins. 2005. Expression of interleukin-4 by recombinant respiratory syncytial virus is associated with accelerated inflammation and a nonfunctional cytotoxic T-lymphocyte response following primary infection but not following challenge with wild-type virus. J. Virol. 79:9515-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culley, F. J., A. M. Pennycook, J. S. Tregoning, T. Hussell, and P. J. Openshaw. 2006. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J. Virol. 80:4521-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakhama, A., Y. M. Lee, H. Ohnishi, X. Jing, A. Balhorn, K. Takeda, and E. W. Gelfand. 2009. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J. Allergy Clin. Immunol. 123:138.e5-145.e5 [DOI] [PubMed] [Google Scholar]
- 10.Dakhama, A., J. W. Park, C. Taube, A. Joetham, A. Balhorn, N. Miyahara, K. Takeda, and E. W. Gelfand. 2005. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 175:1876-1883. [DOI] [PubMed] [Google Scholar]
- 11.Didierlaurent, A., J. Goulding, S. Patel, R. Snelgrove, L. Low, M. Bebien, T. Lawrence, L. S. van Rijt, B. N. Lambrecht, J. C. Sirard, and T. Hussell. 2008. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 205:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flood-Page, P. T., A. N. Menzies-Gow, A. B. Kay, and D. S. Robinson. 2003. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 167:199-204. [DOI] [PubMed] [Google Scholar]
- 13.Fu, Y. X., and D. D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399-433. [DOI] [PubMed] [Google Scholar]
- 14.Glezen, W. P., L. H. Taber, A. L. Frank, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543-546. [DOI] [PubMed] [Google Scholar]
- 15.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 16.Harker, J., A. Bukreyev, P. L. Collins, B. Wang, P. J. Openshaw, and J. S. Tregoning. 2007. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 81:13105-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harker, J. A., A. Godlee, J. L. Wahlsten, D. C. Lee, L. G. Thorne, D. Sawant, J. S. Tregoning, R. R. Caspi, A. Bukreyev, P. L. Collins, and P. J. Openshaw. 2010. Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells. J. Virol. 84: 4073-4082. [DOI] [PMC free article] [PubMed]
- 18.Hoebee, B., E. Rietveld, L. Bont, M. Oosten, H. M. Hodemaekers, N. J. Nagelkerke, H. J. Neijens, J. L. Kimpen, and T. G. Kimman. 2003. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J. Infect. Dis. 187:2-11. [DOI] [PubMed] [Google Scholar]
- 19.Humbles, A. A., C. M. Lloyd, S. J. McMillan, D. S. Friend, G. Xanthou, E. E. McKenna, S. Ghiran, N. P. Gerard, C. Yu, S. H. Orkin, and C. Gerard. 2004. A critical role for eosinophils in allergic airways remodeling. Science 305:1776-1779. [DOI] [PubMed] [Google Scholar]
- 20.Hussell, T., A. Georgiou, T. E. Sparer, S. Matthews, P. Pala, and P. J. M. Openshaw. 1998. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J. Immunol. 161:6215-6222. [PubMed] [Google Scholar]
- 21.Hussell, T., and P. J. M. Openshaw. 1998. Intracellular interferon-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 79:2593-2601. [DOI] [PubMed] [Google Scholar]
- 22.Kariyawasam, H. H., and D. S. Robinson. 2007. The role of eosinophils in airway tissue remodelling in asthma. Curr. Opin. Immunol. 19:681-686. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. E. Crowe, Jr., and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, Y. M., N. Miyahara, K. Takeda, J. Prpich, A. Oh, A. Balhorn, A. Joetham, E. W. Gelfand, and A. Dakhama. 2008. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 177:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legg, J. P., I. R. Hussain, J. A. Warner, S. L. Johnston, and J. O. Warner. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 168:633-639. [DOI] [PubMed] [Google Scholar]
- 26.McNamara, P. S., P. Ritson, A. Selby, C. A. Hart, and R. L. Smyth. 2003. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 88:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phipps, S., C. E. Lam, S. Mahalingam, M. Newhouse, R. Ramirez, H. F. Rosenberg, P. S. Foster, and K. I. Matthaei. 2007. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578-1586. [DOI] [PubMed] [Google Scholar]
- 28.Pribul, P. K., J. Harker, B. Wang, H. Wang, J. S. Tregoning, J. Schwarze, and P. J. Openshaw. 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 82:4441-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegrist, C. A., and R. Aspinall. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185-194. [DOI] [PubMed] [Google Scholar]
- 30.Sigurs, N., P. M. Gustafsson, R. Bjarnason, F. Lundberg, S. Schmidt, F. Sigurbergsson, and B. Kjellman. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171:137-141. [DOI] [PubMed] [Google Scholar]
- 31.Smyth, R. L. and P. J. Openshaw. 2006. Bronchiolitis. Lancet 368:312-322. [DOI] [PubMed] [Google Scholar]
- 32.Sun, C. M., L. Fiette, M. Tanguy, C. Leclerc, and R. Lo-Man. 2003. Ontogeny and innate properties of neonatal dendritic cells. Blood 102:585-591. [DOI] [PubMed] [Google Scholar]
- 33.Tregoning, J. S., P. K. Pribul, A. M. Pennycook, T. Hussell, B. Wang, N. W. Lukacs, J. Schwarze, F. J. Culley, and P. J. M. Openshaw. 2010. The chemokine MIP1 alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PLoS One 5:e9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tregoning, J. S., and J. Schwarze. 2010. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 23:74-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tregoning, J. S., Y. Yamaguchi, J. Harker, B. Wang, and P. J. Openshaw. 2008. The role of T cells in the enhancement of RSV infection severity during adult re-infection of neonatally sensitized mice. J. Virol. 82:4115-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welliver, R. C., T. N. Kaul, and P. L. Ogra. 1980. The appearance of cell-bound IgE in respiratory-tract epithelium after respiratory syncytial virus infection. N. Engl. J. Med. 303:1198-1202. [DOI] [PubMed] [Google Scholar]
- 37.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 38.Wright, P. F., R. A. Karron, R. B. Belshe, J. Thompson, J. E. Crowe, Jr., T. G. Boyce, L. L. Halburnt, G. W. Reed, S. S. Whitehead, E. L. Anderson, A. E. Wittek, R. Casey, M. Eichelberger, B. Thumar, V. B. Randolph, S. A. Udem, R. M. Chanock, and B. R. Murphy. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 182:1331-1342. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler, S. F., F. Ramsdell, and M. R. Alderson. 1994. The activation antigen CD69. Stem Cells 12:456-465. [DOI] [PubMed] [Google Scholar]