Abstract
Adult T-cell leukemia (ATL) is caused by human T-cell leukemia virus type 1 (HTLV-1). Elevated expression of vascular endothelial growth factor (VEGF) in ATL patients is associated with leukemic cell invasion and infiltration in different organs. The regulatory protein Tax 1 encoded by HTLV-1 plays a pivotal role in T-cell transformation by deregulating the function and expression of several cellular factors. In the present study, we examined the effect of Tax 1 on VEGF expression at transcriptional and posttranscriptional levels in order to elucidate the regulatory mechanisms involved. Using functional assays, we demonstrate that Tax 1 downregulates the VEGF promoter through a cluster of Sp1 sites located close to the transcriptional start site. Using gel mobility shift assays, we show that Tax 1 reduced Sp1:DNA complex formation. We demonstrate that the level of secreted VEGF was significantly lower in Tax 1-transfected 293T cells compared to nontransfected cells, which is consistent with the observed downregulatory effect of Tax 1 at the transcription level. We showed that VEGF was secreted by HTLV-1-transformed and nontransformed cells, irrespective of Tax 1 expression. Overall our data indicate that, contrary to a previous report, Tax 1 downregulates VEGF expression and suggest there are Tax 1-independent mechanisms of VEGF activation in ATL.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) and a neurodegenerative disease termed tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (13, 26, 29, 41). The oncogenic potential of HTLV-1 is strongly associated with the viral regulatory protein Tax 1, which alters cellular signaling pathways by interacting with several cellular transcription factors. Tax stimulates transcription of the viral genome from the viral long terminal repeat (LTR) by interacting with activating transcription factor/cyclic AMP (cAMP) response element-binding protein (ATF/CREB) and PCAF (17, 35, 42). Activation of prosurvival signals such as the NF-κB and Akt pathways by Tax 1 plays a critical role in events leading to ATL development and leads to the abnormal expression of several cellular proteins normally involved in apoptosis, cell cycle control, and DNA damage repair (23, 34). The upregulation of genes encoding cytokines (1, 4, 12, 33) and growth factors (14, 15, 27) by Tax 1 is speculated to play a pivotal role in T-cell activation and proliferation and likely contributes to the pathogenesis of both ATL and TSP/HAM.
Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen involved in many physiological processes including embryonic development (11), wound healing (5), angiogenesis in tumors (9), and rheumatoid arthritis (18). The role of angiogenesis in solid tumors is well documented, but VEGF is also implicated in the pathogenesis of hematological malignancies, with increased levels being found in patients with acute lymphatic leukemia (ALL) (28) and multiple myeloma (30). Clinical studies have reported that ATL patients have high levels of plasma VEGF and that ATL cells may enhance their own chemotactic activity by an autocrine mechanism involving the secretion of VEGF and the expression of the VEGF receptor R1 on their cell surface (15). Tax 1 was reported to activate the VEGF promoter (8), suggesting a connection between VEGF levels, angiogenesis, and Tax 1 expression in the pathogenesis of ATL.
VEGF expression is regulated at both transcriptional and posttranscriptional levels (9). The 5′ region of the VEGF promoter contains a number of potential binding sites for different transcription factors, including AP-1, AP-2, Egr-1, nuclear factor-interleukin-6 (NF-IL-6), HIF-1, Stat-3, and Sp1, the more significant of these being the latter three (40). In addition external factors including cytokines and growth factors such as platelet-derived growth factor (PDGF) (10), IL-1β (19), IL-6 (7), basic fibroblast growth factor (bFGF) (22), and tumor necrosis factor alpha (TNF-α) (31) have all been reported to upregulate VEGF expression in different cell types. At a posttranscriptional level, VEGF mRNA is stabilized by the RNA-binding protein HuR, which results in increased levels of VEGF expression under hypoxic conditions (21).
In this study, we examined the effect of the HTLV Tax 1 protein on VEGF expression in order to elucidate the regulatory mechanisms involved. In contrast to a previous study, which showed that Tax 1 upregulates VEGF expression (8), we found that Tax 1 downregulated VEGF promoter activity through a cluster of Sp1 binding sites located close to the transcription start site. Having shown this, we investigated the possibility that Tax 1 may alter the ability of Sp1 to bind to the promoter. Electrophoretic mobility shift assays (EMSAs) using a Sp1 consensus sequence probe showed that Tax 1 reduced Sp1:DNA complex formation. Consistent with these findings, VEGF protein levels were reduced in Tax 1-expressing cells. However, in agreement with other studies, we show that VEGF was secreted by HTLV-infected and uninfected cell lines in vitro irrespective of Tax expression. Our results support the possibility that Tax 1-independent mechanisms of VEGF activation occur in ATL.
MATERIALS AND METHODS
Cell culture and transient tranfections.
293T, COS 7, and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal calf serum (Gibco) and antibiotics. The Jurkat, MT2, and CEM cell lines, as well as all human ATL cell lines were maintained in RPMI supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. All cultures were incubated at 37°C in 5% CO2. For enzyme-linked immunosorbent assay (ELISA) analysis, the fetal calf serum concentration was reduced to 1% in culture medium. All transfections were carried out using FuGENE (Roche) according to the manufacturer's instructions.
Construction of promoter-reporter plasmids.
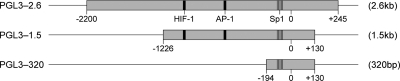
Promoter fragments of the VEGF promoter were amplified from 293T genomic DNA using nested PCR (Fig. 1). An upstream primer, VEGF F-2330 (5′ TCACTCGAGCAGGTTGTTGTAACACACCTTG 3′), designed with an XhoI restriction site (underlined), and a downstream primer, VEGF R + 298 (5′ CTGAAGCTTACAGTGATTTGGGGAAGTAG 3′), designed with a HindIII restriction site (underlined) (25), were used to amplify the VEGF promoter from +2330 to +298 in the first round of PCR amplification. The following PCR cycles were used: 94°C for 10 min; 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min 30 s (30 cycles); and 72°C for 10 min. VEGF 2.6, VEGF 1.5, and VEGF 320 were amplified from the first-round PCR product using primers VEGF F-2200 (5′ TCACTCGAGTTTACTAAGACCTGCTCTTTC 3′) and VEGF R + 245 (5′ CTGAAGCTTAATTAAAACGAGAAACAATACA 3′), VEGF F-1226 (5′T CACTCGAGTATGAGTCTGGGCTTGGGCTGATAGAAG3′) and VEGF R + 130, and VEGF F-194 (5′ TCACTCGAGGGTCGAGCTTCCCCTTCATTG 3′) and VEGF R + 130, respectively. The same PCR cycles were used as described above, except for VEGF 320 and VEGF 180, for which the extension time was reduced to 1 min. Four percent dimethyl sulfoxide (DMSO) was included in the PCRs to enhance DNA amplification. PCR products were subsequently cloned into PGL3 Basic luciferase vector (Promega) using the appropriate restriction enzymes. The nucleotide sequences of all constructs were determined using the BigDye Terminator sequencing kit (Applied Biosystems).
FIG. 1.
Schematic representation of VEGF full-length and deleted promoter PGL3 constructs showing the relative positions of binding sites for hypoxia-inducible factor 1 (HIF-1), AP-1, and Sp1.
Functional assays.
293T, COS 7, HeLa, and Jurkat cells were cotransfected with the indicated luciferase plasmids, expression plasmids, and/or the corresponding empty expression plasmids to normalize for DNA concentration using FuGENE 6 (Roche). The Renilla luciferase plasmid pRLTK (10 ng) was used as an internal control. Each transfection was carried out in duplicate, and luciferase activity was quantified using the Promega dual-luciferase reporter assay kit according to the manufacturer's instructions and as described previously (32). Normalized values are reported as the percent mean relative luciferase activity ± standard error of the mean (SEM) from three independent transfections.
Nuclear extraction and EMSA.
Nuclear extracts were prepared from pCA-Tax 1-transfected and untransfected 293T cells and MT2 and CEM cells using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology). EMSA, using a biotin-labeled Sp1 consensus oligonucleotide (Eurofins), was carried out on nuclear extracts using the Pierce LightShift Chemiluminescent EMSA kit.
VEGF ELISA.
A total of 1 × 106 293T cells were transfected with pCA-Tax and cultured in DMEM supplemented with 1% fetal calf serum (FCS) and antibiotics for 48 h. A total of 1 × 106 cells each of the MT2 and CEM cell lines, as well as the ATL cell lines (TE, TH, CR, and C91) and HTLV-2 cell line (H2) were cultured in RPMI supplemented as described above. Levels of secreted VEGF were quantified by ELISA kit (Calbiochem) according to the manufacturer's instructions.
RESULTS
Tax 1 downregulates VEGF promoter activity.
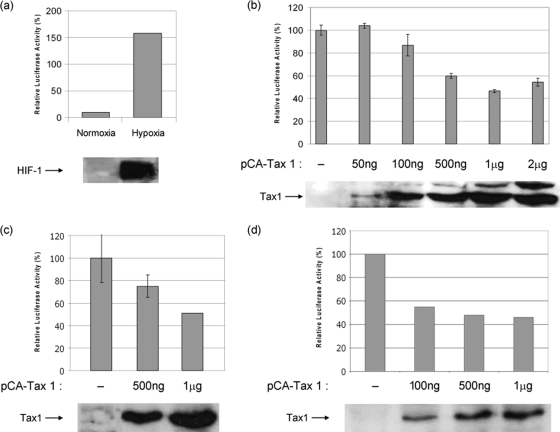
The activity of the full-length VEGF promoter construct, PGL3-2.6 (Fig. 1), was initially assessed in cells incubated under normoxic and hypoxic conditions. Hypoxia induces VEGF expression mainly through the activation and stabilization of hypoxia-inducible factor 1 (HIF-1), which is a well-documented transcriptional activator of the VEGF promoter (6). Incubation of cells transfected with the full-length VEGF promoter PGL3-2.6 under hypoxic conditions resulted in an approximately 15-fold increase in promoter activity compared to normoxic conditions (Fig. 2a). As expected, activation of the VEGF promoter under hypoxic conditions coincided with the induction of endogenous HIF-1α (Fig. 2a, lower panel). This clearly demonstrates the functionality of the VEGF promoter-reporter construct.
FIG. 2.
Tax 1 downregulates VEGF promoter activity. (a) Luciferase assays were carried out on lysates from 293T cells transfected with the full-length VEGF promoter construct PGL3-2.6 and incubated under normoxic and hypoxic conditions. The lower panel shows the expression level of HIF-1α in both conditions as determined by Western blotting. 293T (b), COS 7 (c), and HeLa (d) cells were cotransfected with PGL3-2.6 and the indicated concentrations of pCA-Tax1-HIS or empty pCA vector together with pRLTK, which was used as an internal control. Luciferase activity was quantified after 48 h and was normalized to thymidine kinase (TK) values. Basal activity was set at 100%. Differences in normalized luciferase activity between transfected and nontransfected cells were significant as determined by Student's t test. The lower panels in each figure show the levels of Tax 1 expression in lysates used for luciferase assays as determined by Western blotting.
To establish the effect of Tax 1 on VEGF promoter activity, 293T cells were cotransfected with the full-length VEGF promoter-reporter construct PGL3-2.6 and the Tax 1 expression plasmid pCA-Tax 1-HIS or the empty pCA plasmid. VEGF promoter activity was largely unaffected by low levels of Tax1 expression (Fig. 2b). However, the addition of higher concentrations of Tax 1 expression plasmid reduced basal promoter activity by 40%. This was not substantially reduced further by the addition of either 1 μg or 2 μg of pCA-Tax 1, which resulted in reductions in promoter activity of 53% and 43%, respectively. This suggests that the downregulatory effect of Tax 1 on the VEGF promoter may reach a plateau and may be limited by the availability of cellular factors that are required for this effect. In order to exclude the possibility that this effect may be cell-type specific, we carried out the same experiment using COS 7 and HeLa cells. In a similar manner to the effect seen in 293T cells, Tax 1 also significantly downregulated VEGF promoter activity in both these cell lines (Fig. 2c and d), which suggests that this effect is not cell type specific.
The Sp1 binding sites are involved in Tax 1-mediated downregulation of the VEGF promoter.
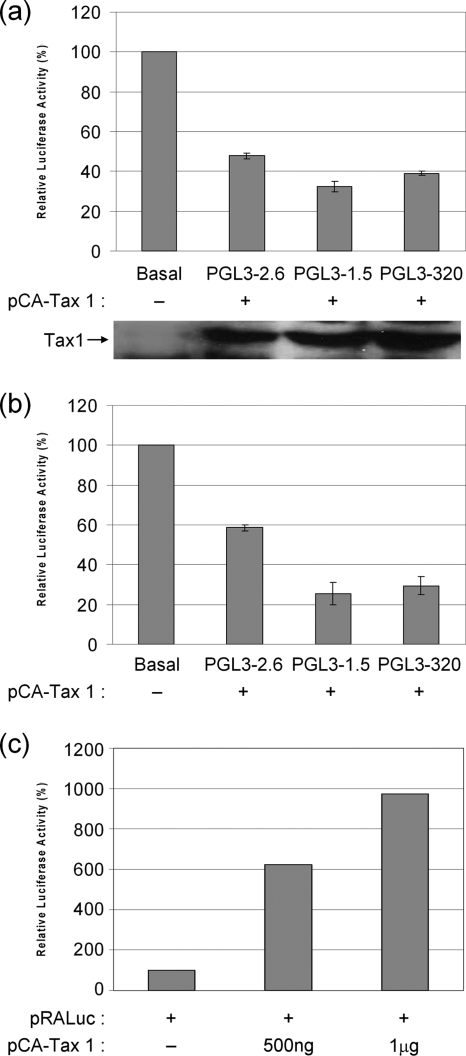
In order to determine the region of the VEGF promoter through which Tax 1 downregulates VEGF promoter activity, 293T and Jurkat cells were cotransfected with either the full-length VEGF promoter PGL3-2.6 or the promoter deletions PGL3-1.5 or PGL3-320 together with pCA-Tax 1-HIS or pCA empty vector. In 293T cells, Tax 1 induced decreases of 54%, 67%, and 61% in the promoter activities of PGL3-2.6, PGL3-1.5, and PGL3-320, respectively (Fig. 3a). In cotransfections of Jurkat cells, 2 μg Tax 1 induced similar decreases in the activity of the same promoter fragments (Fig. 3b). The ability of Tax 1 to repress the activity of PGL3-320 construct, which only contains the Sp1 sites, suggests that Tax 1 mediates its downregulation of VEGF promoter activity through the cluster of Sp1 sites in the promoter proximal region. Since Tax 1 was previously shown to activate the PDGF promoter via Sp1 (38), we sought to confirm that finding using our experimental conditions. We found that in contrast to its downregulatory effect on the VEGF promoter, Tax 1 strongly activated the PDGF promoter pRALuc as previously described (38) (Fig. 3c).
FIG. 3.
The Sp1 sites, located between positions −194 and −51, are involved in Tax 1-mediated downregulation of the VEGF promoter. 293T (a) and Jurkat (b) cells were cotransfected with PGL3-2.6, PGL3-1.5, or PGL3-320, the internal control pRLTK, and either the Tax expression plasmid pCA-Tax 1-HIS or empty pCA vector. Luciferase activity was quantified after 48 h and was normalized to TK activity. Basal activity was constant for all of the reporters and was set at 100%. Differences in normalized luciferase activity were significant as determined by Student's t test. (c) 293T cells were cotransfected with the indicated concentrations of pCA-Tax 1-HIS or empty pCA vector together with the PDGF luciferase reporter pRALuc (35) and the internal control pRLTK. Luciferase activity was quantified after 24 h and was normalized to TK activity. Basal activity was set at 100%.
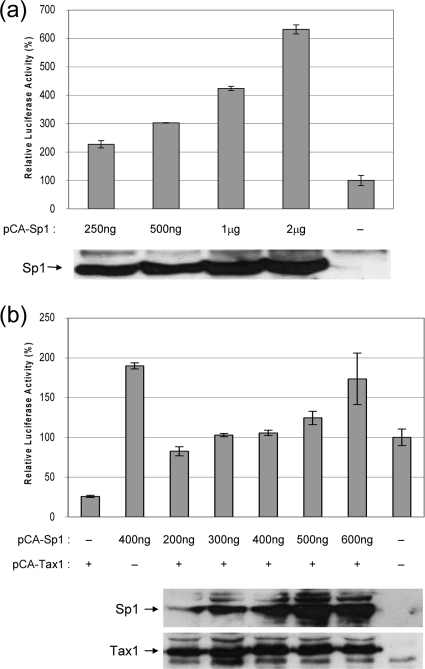
In order to further substantiate the role of Sp1 in the downregulation of the VEGF promoter by Tax 1, we sought to determine if the addition of exogenous Sp1 would alleviate the effect. As expected, increasing amounts of pCA-Sp1 vector activated PGL3-320 in a dose-dependent manner (Fig. 4a). Cotransfection of cells with pCA-Tax 1 and increasing amounts of pCA-Sp1 alleviated Tax 1-mediated downregulation of the VEGF promoter construct (Fig. 4b). This finding further supports our finding that Sp1 may be involved in the ability of Tax 1 to downregulate the VEGF promoter.
FIG. 4.
Sp1 relieves Tax 1-induced downregulation of the VEGF promoter. (a) 293T cells were cotransfected with PGL3-320 (Sp1 sites only) and increasing amounts of Sp1 expression vector or empty pCA vector. Transfections were carried out in triplicate. Luciferase activity was quantified 48 h after transfection and basal activity in the absence of exogenous Sp1 was set at 100%. The expression levels of Sp1 was detected in lysates by Western blotting using anti-Sp1 antibody (Calbiochem) and is shown in the lower panel. (b) 293T cells were cotransfected with PGL3-320, 600 ng pCA-Tax 1-HIS and increasing amounts of pCA-Sp1, or empty pCA plasmid. Transfections were carried out in triplicate. Forty-eight hours after transfection, luciferase activity was quantified and basal activity is set at 100% (last bar). The expression levels of Tax 1 and Sp1 in lysates from cells transfected with 600 ng of Tax 1 and 200 ng (lane 1), 300 ng (lane 2), 400 ng (lane 3), 500 ng (lane 4), 600 ng (lane 5) of Sp1, or the relevant empty plasmids (lane 6) was detected by Western blotting using anti-Tax 1 and anti-Sp1 antibody (Calbiochem), as shown in the lower panel.
Tax 1 reduces Sp1 binding to the VEGF promoter.
Sp1 activates VEGF promoter activity by binding to its consensus sequences on the VEGF promoter and forming protein:DNA complexes. Having established that Tax 1 downregulates the promoter activity via these sites, we investigated the possibility that Tax 1 may alter the ability of Sp1 to bind to the promoter. EMSAs using an Sp1 consensus sequence probe were carried out on nuclear extract (NE) from Tax-transfected and nontransfected 293T cells (Fig. 5a). Reduced Sp1:DNA complex formation was consistently observed in extracts from Tax-transfected cells (lane 4) compared to nontransfected cells (lane 2). Similarly, EMSAs carried out on nuclear extracts from CEM and MT2 cells, using the same Sp1 consensus sequence probe, showed less Sp1:DNA complex formation in HTLV-1-transformed MT2 cells (Fig. 5b, lane 4) compared to nontransformed CEM cells (Fig. 5b, lane 2). The Sp1-DNA interaction was strongly inhibited by the addition of a 200-fold molar excess of unlabeled probe in each experiment demonstrating the specific nature of the interaction. These results suggest that Tax 1 may downregulate the VEGF promoter by reducing Sp1 binding to the promoter.
FIG. 5.
Tax 1 reduces Sp1 binding to the VEGF promoter. Electromobility shift assays were performed on nuclear extract (NE) obtained from pCA-Tax1-HIS-transfected and nontransfected 293T cells (a) and CEM and MT2 cells (b) using a biotin-labeled Sp1 consensus probe. Bound complexes were detected using chemiluminescence. Unlab, unlabeled. (c) The expression levels of Tax 1 in the nuclear extracts used in EMSAs were detected by Western blotting using anti-Tax 1. The levels of PARP (poly (ADP-ribose) polymerase 1) are shown as a loading control (lower panel).
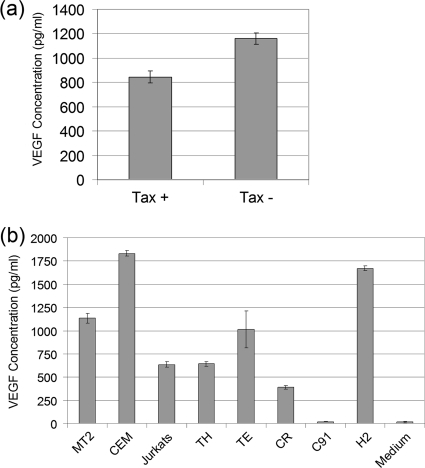
Levels of VEGF secreted by HTLV-positive and -negative cell lines.
Having established that Tax 1 downregulates VEGF expression at a transcriptional level, we sought to determine its effect at the protein level. ELISAs were carried out on cell culture supernatants from a number of cell lines, including Tax-transfected and nontransfected 293T cells, MT2, CEM, and a number of primary ATL cell lines to assay the level of secreted VEGF. Levels of VEGF were lower in Tax 1-transfected 293T cells compared to nontransfected cells, a significant difference as determined by Student's t test (P < 0.05) (Fig. 6a). Overall VEGF was detected in the culture supernatants from both HTLV-positive and -negative cell lines. Higher levels of VEGF was detected in CEM cells compared to MT2 cells with the 3 ATL patient cell lines, TH, TE, and CR, also secreting high levels of VEGF protein. In contrast to a previous report (8), the HTLV-1 cell line C91 showed little VEGF expression, with levels being comparable to background readings. Interestingly the HTLV-2 cell line H2 showed the second highest level of VEGF, with a concentration of 1,700 pg/ml. Overall these results are consistent with previous studies, which showed that ATL cells express VEGF (14, 15). However, our data suggest that Tax 1 alone may not determine VEGF expression.
FIG. 6.
Levels of VEGF secreted by Tax-positive and -negative cell lines. (a) 293T cells were transfected with 1 μg of pCA-Tax1-HIS or pCA empty. Cells were incubated for 48 h, and VEGF was quantified in cell culture supernatants by ELISA. (b) A total of 1 × 106 cells each of the MT2 and C91 (HTLV-1 transformed), H2 (HTLV-2 transformed), TH, TE, CR (ATL cell lines), and CEM and Jurkat cells lines was cultured for 48 h, and VEGF protein in cell culture supernatant was quantified by ELISA.
DISCUSSION
ATL is characterized by the development of leukemic cells with multilobulated nuclei (flower cells) that infiltrate the skin, liver, lung, and gastrointestinal tract (36). Clinical studies have shown that the migration and infiltration of ATL cells is linked to elevated levels of VEGF expression in ATL patients (14, 15). In addition, Tax 1 has been reported to activate the VEGF promoter (8), even though the mechanisms involved were not elucidated. In the present study, we investigated the role played by Tax 1 in the regulation of VEGF expression. Unexpectedly, our studies demonstrate that Tax 1 either had no effect on basal VEGF promoter activity or caused a significant downregulation (on average 50%) in basal activity, depending on the expression level of Tax 1.
The VEGF promoter contains a number of potential binding sites for different transcription factors, including AP-1, AP-2, Egr-1, NF-IL-6, HIF-1, Stat-3, and Sp1, the more significant of these being the latter three (40). In agreement with previous data (6), we found that hypoxic conditions and the induction of HIF-1α strongly activated our VEGF promoter construct and thus confirmed its functionality. Previous studies have shown that the four Sp1 sites, located between positions −194 and −51 relative to the transcription start site, were sufficient not only for basal activity of the VEGF promoter but also its responsiveness to TNF-α (31) and PDGF (10). Deletion analysis of the full-length VEGF promoter in the present study showed that the Sp1 sites were also sufficient for the downregulation of VEGF by Tax 1. In order to determine the effect of Tax 1 on the ability of Sp1 to bind its responsive elements, we performed EMSAs using a probe containing the Sp1 consensus sites. Reduced levels of Sp1 protein:DNA complex was detected in nuclear extracts from Tax 1-transfected cells compared to nontransfected cells. The modest reduction in the amount of Sp1 bound to the probe in the presence of Tax 1 may be related to the transfection efficiency of the cells and the fact that only a proportion of cells express Tax. Similar results were obtained with nuclear extracts from the HTLV-1-infected MT2 cells compared to noninfected CEM cells. Overall these data suggest that Tax 1 may repress VEGF promoter activity by reducing Sp1 binding. This conclusion is supported by the findings that the addition of exogenous Sp1 alleviated the effect of Tax 1 on the VEGF promoter in a dose-dependent manner, which further substantiates the involvement of the Sp1 sites in the downregulation of VEGF promoter activity by Tax 1. Sp1 is ubiquitously expressed in many cell types and has been shown to bind numerous different promoters to regulate transcription (37). The binding of Sp1 to responsive elements within the HTLV-1 LTR has been shown to contribute to Tax-independent activation of the viral promoter (20). Another study showed that Tax activates the PDGF promoter by interacting with Sp1 (38). We confirmed the ability of Tax 1 to activate the PDGF promoter in the present study, which suggests that the repression of the VEGF promoter via Sp1 by Tax may be promoter specific. It is possible that in contrast to the study mentioned above, the interaction between Tax 1 and Sp1 in the case of the VEGF promoter leads to a downregulation of promoter activity by reducing the ability or availability of Sp1 to bind the promoter.
VEGF is a predominantly secreted protein, with the exception of VEGF isoforms 189 and 206, which are cell associated (40). In the present study, the level of secreted VEGF165 protein was determined in Tax-transfected and nontransfected 293T, CEM, and Jurkat cell lines, HTLV-1-transformed cells (MT2), three ATL patient cell lines, and an HTLV-2-transformed cell line (H2). Levels of VEGF were lower in Tax-transfected 293T cells compared to nontransfected cells, which is consistent with the downregulatory effect of Tax 1 observed at the transcriptional level. Our data show that the HTLV-1-negative cell lines CEM and Jurkat produce elevated levels of VEGF. The three ATL patient cell lines, TH, TE, and CR, all expressed high levels of VEGF protein, with the HTLV-2 cell line H2 producing VEGF at a level second only to CEM cells. These results are consistent with previous studies, which show that ATL cells express high levels of VEGF, but our data suggest that this may occur in a Tax-independent manner. This idea is supported by studies which show that Tax 1 expression is usually silenced in ATL (16, 24) and raises the possibility that factors other than Tax 1 may be responsible for VEGF activation in some ATL cases. Our results are at variance with data from a previous study which suggested that the elevated levels of VEGF produced by ATL cells and HTLV-1-infected cell lines may be linked to the ability of Tax 1 to potently activate the VEGF promoter (8). Instead our data clearly show that Tax 1 either has no effect or has a downregulatory effect on VEGF promoter activity. The reasons for the differences between the findings of our study and El-Sabban's study (8) are not clear but may relate to a number of factors, including the cell lines used in both studies. For instance, in contrast to the study by El Sabban et al., our data show that VEGF was produced by CEM and Jurkat cells. Several previous studies have shown that CEM cells produce VEGF (2, 3, 39), but expression of VEGF by Jurkat cells may depend on the origins of the cell line being used (2, 3). Another possibility is the nature of the VEGF promoter used in both studies. We confirmed the functionality of our VEGF promoter using hypoxic conditions and exogenous Sp1, which are both recognized activators of VEGF expression, but without details of the VEGF promoter construct used in the study by El Sabban et al. (8), we unfortunately cannot make a direct comparison between the two studies.
In summary, we conclude from our data that factors other than Tax 1 may be involved the upregulation of VEGF expression in ATL. Additional studies will be required to determine the identity of such factors and what role they play in the regulation of VEGF expression in ATL.
Acknowledgments
This work was supported by the National Virus Reference Laboratory, Ireland.
We thank L. Ratner for providing the PDGF luciferase reporter pRALuc and J. Nyborg for the Sp1 expression plasmid CMV-Sp1.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Ahuja, J., V. Lepoutre, B. Wigdahl, Z. K. Khan, and P. Jain. 2007. Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed. Pharmacother. 61:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avramis, I. A., G. Christodoulopoulos, A. Suzuki, W. E. Laug, I. Gonzalez-Gomez, G. McNamara, E. A. Sausville, and V. I. Avramis. 2002. In vitro and in vivo evaluations of the tyrosine kinase inhibitor NSC 680410 against human leukemia and glioblastoma cell lines. Cancer Chemother. Pharmacol. 50:479-489. [DOI] [PubMed] [Google Scholar]
- 3.Avramis, I. A., R. Kwock, and V. I. Avramis. 2001. Taxotere and vincristine inhibit the secretion of the angiogenesis inducing vascular endothelial growth factor (VEGF) by wild-type and drug-resistant human leukemia T-cell lines. Anticancer Res. 21:2281-2286. [PubMed] [Google Scholar]
- 4.Banerjee, P., R. Rochford, J. Antel, G. Canute, S. Wrzesinski, M. Sieburg, and G. Feuer. 2007. Proinflammatory cytokine gene induction by human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 Tax in primary human glial cells. J. Virol. 81:1690-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, L. F., K. T. Yeo, B. Berse, T. K. Yeo, D. R. Senger, H. F. Dvorak, and L. van de Water. 1992. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 176:1375-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, and E. Keshert. 1998. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, T., D. Nahari, L. W. Cerem, G. Neufeld, and B. Z. Levi. 1996. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 271:736-741. [DOI] [PubMed] [Google Scholar]
- 8.El-Sabban, M. E., R. A. Merhi, H. A. Haidar, B. Arnulf, H. Khoury, J. Basbous, J. Nijmeh, H. de Thé, O. Hermine, and A. Bazarbachi. 2002. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood 99:3383-3389. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara, N. 2000. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog. Horm. Res. 55:15-35. [PubMed] [Google Scholar]
- 10.Finkenzeller, G., A. Sparacio, A. Technau, D. Marmé, and G. Siemeister. 1997. Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene 15:669-676. [DOI] [PubMed] [Google Scholar]
- 11.Fong, G. H., J. Rossant, M. Gertsenstein, and M. L. Breitman. 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376:66-70. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa, Y., M. Saito, W. Matsumoto, K. Usuku, Y. Tanaka, S. Izumo, and M. Osame. 2003. Different cytokine production in tax-expressing cells between patients with human T cell lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis and asymptomatic HTLV-I carriers. J. Infect. Dis. 187:1116-1125. [DOI] [PubMed] [Google Scholar]
- 13.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 14.Hayashibara, T., T. Fujimoto, T. Miyanishi, Y. Yamada, T. Maita, S. Kamihira, and M. Tomonaga. 1999. Vascular endothelial growth factor at high plasma levels is associated with extranodal involvement in adult T cell leukemia patients. Leukemia 13:1634-1645. [DOI] [PubMed] [Google Scholar]
- 15.Hayashibara, T., Y. Yamada, T. Miyanishi, H. Mori, T. Joh, T. Maeda, N. Mori, T. Maita, S. Kamihira, and M. Tomonaga. 2001. Vascular endothelial growth factor and cellular chemotaxis: a possible autocrine pathway in adult T-cell leukemia cell invasion. Clin. Cancer Res. 7:2719-2726. [PubMed] [Google Scholar]
- 16.Hironaka, N., K. Mochida, N. Mori, M. Maeda, N. Yamamoto, and S. Yamaoka. 2004. Tax-independent constitutive IkappaB kinase activation in adult T-cell leukemia cells. Neoplasia 6:266-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch, A. E., L. A. Harlow, G. K. Haines, E. P. Amento, E. N. Unemori, W. L. Wong, R. M. Pope, and N. Ferrara. 1994. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J. Immunol. 152:4149-4156. [PubMed] [Google Scholar]
- 19.Li, J., M. A. Perrella, J. C. Tsai, S. F. Yet, C. M. Hsieh, M. Yoshizumi, C. Patterson, W. O. Endege, F. Zhou, and M. E. Lee. 1995. Induction of vascular endothelial growth factor gene expression by interleukin-1 beta in rat aortic smooth muscle cells. J. Biol. Chem. 270:308-312. [DOI] [PubMed] [Google Scholar]
- 20.Livengood, J. A., and J. K. Nyborg. 2004. The high-affinity Sp1 binding site in the HTLV-1 promoter contributes to Tax-independent basal expression. Nucleic Acids Res. 32:2829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López de Silanes, I., A. Lal, and M. Gorospe. 2005. HuR: post-transcriptional paths to malignancy. RNA Biol. 2:11-13. [DOI] [PubMed] [Google Scholar]
- 22.Mason, J. C., E. A. Lidington, S. R. Ahmad, and D. O. Haskard. 2002. bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction. Am. J. Physiol. Cell Physiol. 282:C578-C587. [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270-280. [DOI] [PubMed] [Google Scholar]
- 24.Mori, N., M. Fujii, S. Ikeda, Y. Yamada, M. Tomonaga, D. W. Ballard, and N. Yamamoto. 1999. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 93:2360-2368. [PubMed] [Google Scholar]
- 25.Mukhopadhyay, D., B. Knebelmann, H. T. Cohen, S. Ananth, and V. P. Sukhatme. 1997. The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Mol. Cell. Biol. 17:5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-1 associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 27.Pantazis, P., E. Sariban, C. A. Bohan, H. N. Antoniades, and V. S. Kalyanaraman. 1987. Synthesis of PDGF by cultured human T cells transformed with HTLV-I and II. Oncogene 1:285-289. [PubMed] [Google Scholar]
- 28.Perez-Atayde, A. R., S. E. Sallan, U. Tedrow, S. Connors, E. Allred, and J. Folkman. 1997. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am. J. Pathol. 150:815-821. [PMC free article] [PubMed] [Google Scholar]
- 29.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajkumar, S. V., and R. A. Kyle. 2001. Angiogenesis in multiple myeloma. Semin. Oncol. 28:560-564. [DOI] [PubMed] [Google Scholar]
- 31.Ryuto, M., M. Ono, H. Izumi, S. Yoshida, H. A. Weich, K. Kohno, and M. Kuwano. 1996. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J. Biol. Chem. 271:28220-28228. [DOI] [PubMed] [Google Scholar]
- 32.Sheehy, N., L. Lillis, K. Watters, M. Lewis, V. Gautier, and W. Hall. 2006. Functional analysis of human T lymphotropic virus type 2 Tax proteins. Retrovirology 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siekevitz, M., M. B. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. U. S. A. 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun, S. C., and S. Yamaoka. 2005. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene 24:5952-5964. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, T., J. I. Fujisawa, M. Toita, and M. Yoshida. 1993. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. U. S. A. 90:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takatsuki, K., K. Yamaguchi, F. Kawano, T. Hattori, H. Nishimura, H. Tsuda, I. Sanada, K. Nakada, and Y. Itai. 1985. Clinical diversity in adult T-cell leukemia-lymphoma. Cancer Res. 45:4644s-4645s. [PubMed] [Google Scholar]
- 37.Tan, N. Y., and L. M. Khachigian. 2009. Sp1 phosphorylation and its regulation of gene transcription. Mol. Cell. Biol. 29:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trejo, S. R., W. E. Fahl, and L. Ratner. 1997. The tax protein of human T-cell leukemia virus type 1 mediates the transactivation of the c-sis/platelet-derived growth factor-B promoter through interactions with the zinc finger transcription factors Sp1 and NGFI-A/Egr-1. J. Biol. Chem. 272:27411-27421. [DOI] [PubMed] [Google Scholar]
- 39.Vacca, A., D. Ribatti, M. Iurlaro, A. Albini, M. Minischetti, F. Bussolino, A. Pellegrino, R. Ria, M. Rusnati, M. Presta, V. Vincenti, M. G. Persico, and F. Dammacco. 1998. Human lymphoblastoid cells produce extracellular matrix-degrading enzymes and induce endothelial cell proliferation, migration, morphogenesis, and angiogenesis. Int. J. Clin. Lab. Res. 28:55-68. [DOI] [PubMed] [Google Scholar]
- 40.Xie, K., D. Wei, Q. Shi, and S. Huang. 2004. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 15:297-324. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. U. S. A. 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao, L. J., and C. Z. Giam. 1991. Interaction of the human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc. Natl. Acad. Sci. U. S. A. 88:11445-11449. [DOI] [PMC free article] [PubMed] [Google Scholar]