Abstract
Most HIV-infected individuals develop antibodies to the gp120 and gp41 components of the viral spike; however, only a fraction of these individuals mount a broadly neutralizing serum response against HIV. We have cloned anti-HIV antibodies from the memory B-cell compartment of six individuals with variable viral loads and high titers of broadly neutralizing antibodies. Here, we report on the features of the anti-gp41 response in these patients. Competition experiments with previously characterized antibodies targeting defined epitopes on the gp41 ectodomain showed antibodies directed against the “immunodominant region” (cluster I), the carboxy-terminal heptad repeat (cluster II), and the membrane-proximal external region (cluster IV). On the other hand, antibodies directed against the amino-terminal part of the molecule, including the fusion peptide, polar region, and the N-terminal heptad repeat, were not detected. When all patients' data were combined, unique B-cell clones targeting cluster I, II, and IV accounted for 32%, 49%, and 53% of all anti-gp41-reactive B cells, respectively; therefore, no single region was truly immunodominant. Finally, although we found no new neutralizing epitopes or HIV-1-neutralizing activity by any of the gp41 antibodies at concentrations of up to 50 μg/ml, high concentrations of 7 out of 15 anti-cluster I antibodies neutralized tier 2 viruses.
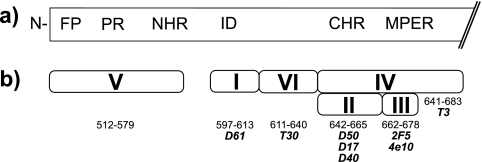
The trimeric envelope spike of the human immunodeficiency virus (HIV) consists of three heterodimers of the transmembrane glycoprotein (gp41) and the surface glycoprotein (gp120) (59). Whereas gp120 carries the CD4 and chemokine receptor binding sites, gp41 is crucial for fusion between the viral particle and the cell membrane (Fig. 1a). The glycine-rich fusion peptide, located at the amino-terminal region of gp41, is normally covered by gp120 but is transiently exposed for interaction with the target cell membrane when gp120 binds to its receptors (14). The fusion peptide is followed by a serine/threonine-rich polar region and heptad repeats, which form leucine zippers that mediate assembly of the coiled-coiled form of gp41 in response to gp120 engagement (8, 22, 24, 52). Finally, the membrane-proximal external region (MPER) also plays a role in virus-host membrane fusion (38); however, the mechanism by which it enhances fusion is not known.
FIG. 1.
HIV-1 gp41. (a) Diagrammatic representation of the gp41 ectodomain with the fusion peptide (FP), the polar region (PR), the amino-terminal and carboxy-terminal heptad repeat regions (NHR and CHR, respectively), the immunodominant region (ID), and the MPER (12). (b) The clusters I to VI are indicated. The amino acid residues (LAI sequence) are shown below the different clusters. Antibodies used in competition ELISAs are shown in italics.
Some regions of gp41 are accessible to antibodies on the native gp140 trimer; however, others are exposed to the immune system only after gp120 shedding (40). In addition, otherwise cryptic gp41 epitopes are uncovered during viral fusion with the cell membrane (13). Consistent with gp41 exposure to the immune system, serologic studies of infected individuals indicate that there is a strong humoral response to gp41 during HIV infection (35) which precedes the response against gp120 (26).
Antibodies to gp41 have been isolated from phage display libraries, as have Epstein-Barr virus (EBV) immortalized B cells from infected individuals (4, 53). Some of these anti-gp41 antibodies can neutralize HIV infection in vitro and interfere with the virus in vivo (4, 6, 50). However, there has yet to be a systematic study of the anti-gp41 memory B-cell response of individuals with high titers of broadly neutralizing anti-HIV antibodies.
In order to document the nature of the anti-gp41 antibody response in HIV-infected individuals with high titers of broadly neutralizing antibodies, we studied 131 such antibodies, accounting for 47 unique B-cell clones, which we obtained from the memory B-cell compartments of six patients with low-to-moderate HIV viral titers (43). Each unique clone was composed of up to 15 clonal members that were either identical or related by somatic mutation. The largest number of unique B-cell clones, 53%, was directed to a conformational epitope which neighbors the MPER (cluster IV), 49% were directed to the carboxy-terminal heptad repeat (cluster II), and 32% were directed to the previously identified “immunodominant region” (cluster I), of which 60% recognize a linear peptide (amino acids 579 to 604). Furthermore, B cells producing antibodies to this region comprise large expanded clones. In total, 57 out of the 131 anti-gp41 and 502 anti-gp140 antibodies cloned were directed to cluster I, some of which show tier 2 virus-neutralizing activity at high antibody concentrations.
MATERIALS AND METHODS
Plasma samples.
The HIV-1-infected patients were part of the Elite Controller Study of the Partners Aids Research Center (patients 2, 3, and 5) and clinical protocols at the Aaron Diamond Research Center (patient 1) and the National Institute of Allergy and Infectious Diseases (patients 4 and 6). The uninfected volunteer (healthy control [HC]) was recruited at the Rockefeller University. All work with human samples was performed in accordance with approved Institutional Review Board protocols (43).
Monoclonal antibodies.
Cloning of anti-human HIV-1 gp140 antibodies was performed as described previously (43, 46). All IgGs were expressed by cotransfection in HEK-293 cells (43, 46).
IgG adsorption and elution of gp41-positive IgG fractions.
Biotinylated HIV-1 gp41 (Prospec) contained the full-length extracellular domain of strain IIIB (amino acids [aa] 513 to 674). To coat streptavidin-magnetic beads (Dynal M-280 Streptavidin; Invitrogen) with HIV-1 gp41, 10 mg of beads was incubated with 100 μg of protein at room temperature for 45 min on a shaking platform. IgG was purified, dialyzed against Dulbecco's phosphate-buffered saline (DPBS; Gibco) and incubated with the coated and washed beads for 1 h at a ratio of 10 mg of coated beads per 10 mg of IgG. Following magnetic bead removal, the IgG was adsorbed five more times to ensure maximal adsorption. Beads containing the gp41-positive IgG fraction were washed with DPBS (Gibco) three times before specific antibody elution with 0.1 M glycine buffer, pH 3, into 1 M Tris-HCl buffer, pH 8.
Competition ELISA.
Specificity of HIV-1 gp41 binding antibodies was determined by competition enzyme-linked immunosorbent assays (ELISAs). Cloned anti-gp41 antibodies were biotinylated (EZ-Link Micro Sulfo-NHS-Biotinylation Kit; Pierce), and their specificities were initially determined by competition with previously characterized anti-gp41 cluster antibodies (Fig. 1b): D61 to cluster I amino acids 597 to 613 (12); D40, D17, and D50 to cluster II amino acids 642 to 665 (12); 4E10 and 2F5 to cluster III amino acids 662 to 678 (60); T3 to cluster IV amino acids 641 to 68 (12); and T30 to cluster VI amino acids 611 to 640 (12). The biotinylated antibodies 4E10 and 2F5 (cluster II), 2-378 and 2-55 (cluster I; the first number in designations of this type indicate the patient), 1-763 (cluster II and IV), and 3-255 (cluster IV) were then used in direct competition experiments to determine the properties of the remaining anti-gp41 antibodies.
ELISAs were performed with high-binding capacity ELISA plates (Costar) coated with 50 μl of gp41 (ectodomain aa 541 to 682; strain HxB2) (Acris, Herford) at 5 μg/ml in phosphate-buffered saline (PBS) overnight at room temperature. This protein preparation contains three major immunospecific bands migrating between 20 and 30 kDa, minor bands between 20 and 30 kDa, bands at 14 and 7 kDa, and an aggregation smear at 35 kDa and greater, as indicated by the manufacturer (Acris, Herford, Germany) (see Fig. 6a). Plates were washed three times with 200 μl of ultrapure water per well and incubated with 100 μl of blocking buffer (2 mM EDTA and 0.05% Tween-20 in PBS) for 30 min at room temperature and washed again. Biotinylated antibodies (0.8 μg/ml) were mixed with 4-fold serial dilutions of the competing antibodies starting at 10 μg/ml before they were applied to the coated ELISA plate. After 2 h of incubation at room temperature, plates were washed as described above. Bound biotinylated antibodies were detected with streptavidin-horseradish peroxidase (HRP) conjugate (AbD). The complex was detected with an HRP substrate kit (Bio-Rad) and measured at 405 nm to calculate the half-maximal (50%) inhibitory concentration (IC50) of the unbiotinylated antibodies.
FIG. 6.
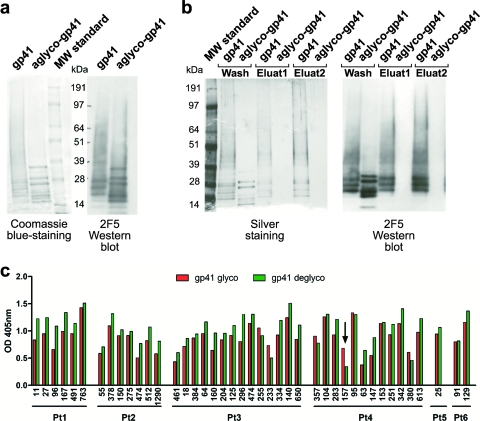
Rare glyco-dependent binding by anti-gp41 antibodies. (a) Coomassie blue-stained polyacrylamide gel and Western blot analysis with MAb 2F5 of control and deglycosylated gp41. As specified by the manufacturer (Acris), the protein shows three major immunospecific bands between 20 and 30 kDa, minor bands between 20 and 30 kDa, bands at 14 and 7 kDa, and an aggregation smear at 35 kDa and above. The aggregation smear condenses in major specific bands after deglycosylation. (b) Deglycosylation was further confirmed by Lens culinaris lectin precipitation, followed by elution with 0.5 M methyl α-d-mannopyranoside of glycosylated gp41 but not of the deglycosylated form. (c) Graph summarizes differences in binding of anti-gp41 antibodies to intact (red bars) and deglycosylated (green bars) gp41 as measured by ELISA (OD at 405 nm). A significant decrease in binding is indicated by a black arrow.
MPER peptide ELISA.
Peptide ELISAs were performed as previously described (32). Briefly, ELISA plates (Costar) were coated with 50 μl of the peptide recognized by 2F5 (SQNQQEKNEQELLALDKWAS; underlining refers to the minimal epitope) or 4E10 (LWNWFDITKWLWYIKIFIMI) at 5 μg/ml in PBS and incubated overnight at room temperature. After plates were washed three times with PBS-0.1% Tween 20 (PBST), wells were blocked with PBS-1% Tween 20-5% sucrose-3% milk powder for 1 h at room temperature. Serial dilutions of human IgG (starting at 100 μg/ml in PBST-1% bovine serum albumin [BSA]) were added, and samples were incubated for 1 h at room temperature and visualized with peroxidase-conjugated affinity-purified goat anti-human IgG (Jackson) using an HRP substrate kit (Bio-Rad). Controls were HC IgG, polyclonal HIV-1 immune globulin (HIV IG), 2F5, and 4E10 (Polymun Scientific, Austria), each of which was included in every experiment.
Deglycosylation.
To remove N-linked glycans, 50 μg of gp41 was treated with peptide N-glycosidase F (PNGase F; New England Biolabs) in 50 mM sodium phosphate at 37°C under nondenaturing conditions for 12 h. Deglycosylation was confirmed by band shift on an SDS-polyacrylamide (PA) gel with Coomassie blue staining and by lectin precipitation (agarose-bound Lens culinaris agglutinin; Vector Laboratories, Burlingame, CA), followed by elution with 0.5 M methyl α-d-mannopyranoside (Sigma) of the glycosylated, but not deglycosylated, gp41.
Neutralization assays.
Neutralization assays were performed as described previously (27, 29). Briefly, neutralization was detected as a reduction in firefly luciferase reporter gene expression after infection of TZM-bl cells with HIV-1 envelope glycoprotein (Env) pseudovirus variants. Murine leukemia virus was used as a negative control to rule out nonspecific toxicity by the antibodies.
RESULTS
Serologic neutralizing activity.
We cloned antibodies to gp140 from the memory B-cell compartment of six patients with variable viral loads and high titers of broadly neutralizing antibodies by staining cells with artificially trimerized gp140 (43). Although we did not find any monoclonal antibodies with broad neutralizing activity among the 502 cloned IgG antibodies to gp140, combinations of antibodies showed a breadth of neutralizing activity at high concentrations (43, 54). To determine whether antibodies to gp41 might contribute to the neutralizing activity in the serum of these patients, we adsorbed purified serum IgG on gp41. The depleted and gp41 binding fractions were tested for gp41 binding by ELISA, as well as for neutralizing activity on a previously described panel of tier 1 and tier 2 viruses (23). Anti-gp41 binding antibodies were efficiently depleted and recovered after adsorption on gp41 (Fig. 2a).
FIG. 2.
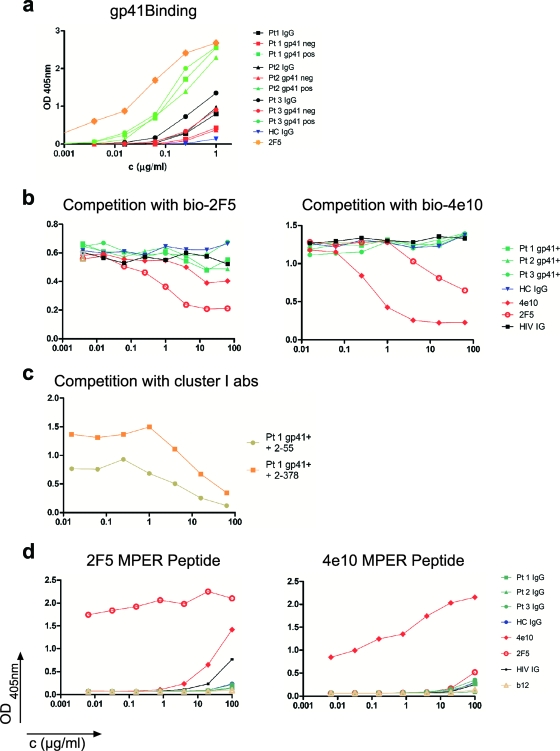
gp41 ELISA. (a) The result of the IgG gp41 adsorption. The affinity-purified gp41 binding IgG fractions (pos) and the anti-gp41-depleted (neg) IgG fractions are shown for each of three different patients (Pt1 to Pt3), according to the legend on the figure. HC, healthy control; OD, optical density. (b) Graphs show lack of competition between the affinity-purified anti-gp41 serum IgG fractions and biotinylated 2F5 or 4E10. Controls are unbiotinylated 2F5 and 4E10 as well as HC IgG and HIV IG. (c) Competition between affinity-purified anti-gp41 serum IgG and anti-cluster I antibodies (2-55 and 2-378). (d) Results of the ELISAs with the 2F5 and 4E10 MPER-specific peptides. Fractions are colored as indicated on the figure.
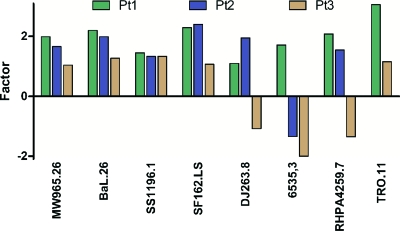
Unfractionated IgG neutralized HIV at concentrations ranging from 2 μg/ml up to 1,138 μg/ml (Table 1). For two of three patients studied, removal of the anti-gp41 binding antibodies resulted in a 2- to 3-fold increase in neutralizing potency; however, patient 3 showed a smaller increase and, for some viruses, a decrease in neutralizing activity after the removal of gp41 binding antibodies (Fig. 3). Consistent with the observation that anti-gp41 antibody depletion did little to alter the overall neutralizing activity, the purified anti-gp41 antibodies were significantly less active than unfractionated IgG in neutralization assays. We conclude that the anti-gp41 antibodies did not significantly contribute to the neutralizing activity of the patient sera studied. These findings are consistent with the observation that broad neutralizing activity in serum is not usually due to anti-gp41 antibodies (35, 48), with the exception of rare anti-MPER antibodies (18). However, since there are several distinct conformational states of gp41 during viral entry, including the prefusion, prehairpin intermediate, and postfusion conformations, we cannot rule out the possibility that not all anti-gp41 antibodies have been adsorbed and depleted by the soluble form of the protein used here.
TABLE 1.
TZMbl neutralization data of gp41-adsorbed IgG
| Patient and samplea | Neutralizing activity (IC50 [μg/ml])b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tier 1 virus (clade) |
Tier 2 virus (clade) |
|||||||
| MW965.26 (C) | BaL.26 (B) | SS1196.1 (B) | SF162.LS (B) | DJ263.8 (A) | 6535.3 (B) | RHPA4259.7 (B) | TRO.11 (B) | |
| Patient 1 | ||||||||
| IgG | 14.1 | 49.0 | 157.2 | 9.6 | 128.8 | 114.8 | 308.8 | 198.6 |
| gp41neg | 7.1 | 22.3 | 108.5 | 4.2 | 117.3 | 67.1 | 148.2 | 64.8 |
| gp41pos | 11.5 | >55 | >55 | >55 | >55 | NT | NT | >55 |
| Patient 2 | ||||||||
| IgG | 2.0 | 20.3 | 92.9 | 6.0 | 132.5 | 203.6 | 388.6 | 1.138 |
| gp41neg | 1.2 | 10.2 | 69.4 | 2.5 | 67.8 | 273.5 | 252.1 | >1.005 |
| gp41pos | 16.2 | >35 | >35 | >35* | >35 | NT | NT | >35 |
| Patient 3 | ||||||||
| IgG | 10.1 | 27.2 | 140.1 | 24.0 | 25.4 | 489.9 | 57.4 | 106.5 |
| gp41neg | 9.7 | 21.4 | 105.1 | 22.4 | 27.4 | 963.5 | 77.3 | 92.7 |
| gp41pos | 23.9 | >50 | >50 | >50 | >50 | NT | NT | >43 |
IgG refers to the neutralizing activity in patients' total IgG fraction, whereas gp41neg refers to the activity in the gp41-depleted fraction, and gp41pos refers to the activity in the eluate of anti-gp41 antibodies after gp41 binding.
Values in boldface represent the concentrations at which the tested serum samples showed 50% neutralization. *, values that almost reached the IC50 at the highest concentration tested; NT, not tested.
FIG. 3.
Neutralizing activity. Shown is the increase or decrease in the neutralizing activity of IgG against different pseudoviruses for patients 1 to 3 after depletion of the gp41 binding antibodies by gp41 coupled to magnetic beads.
Anti-gp41 binding epitopes.
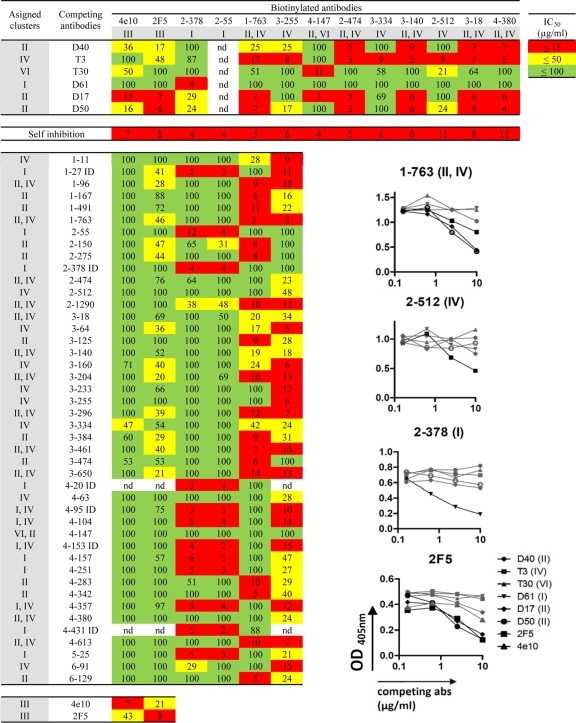
To characterize the epitopes recognized by the cloned anti-gp41 memory antibodies in the six patients with variable viral loads and high titers of broadly neutralizing serum antibodies, we performed competition ELISAs with well-characterized anti-gp41 antibodies (12, 43). We examined all of the previously reported unique anti-gp41 antibodies cloned from memory B cells (43). The following anti-gp41 antibodies were used as standards (Fig. 1b) (11, 12, 57): D61, which recognizes an amino-terminal determinant, corresponding to the immunodominant region, and spans amino acids 597 to 613 (cluster I; LAI strain) (12); D40, D17, and D50, which bind to a region (cluster II) that includes amino acids 642 to 665 located at the amino terminus of the MPER; 4E10 and 2F5, which bind to separate but adjacent peptides in the MPER that span amino acids 662 to 678 (cluster III); T3, which binds a conformational epitope neighboring the MPER and spans amino acids 641 to 683 (cluster IV); and T30, which binds the carboxy terminus of cluster I, amino acids 611 to 640 (cluster VI).
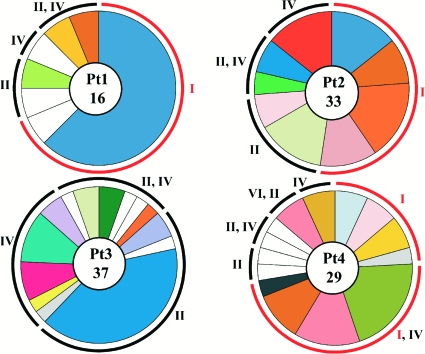
Despite some degradation in the gp41 protein preparation (see Fig. 6a), anti-cluster I, -cluster II, -cluster IV, and -cluster VI antibodies accounted for all of the anti-gp41 binding by the monoclonal antibodies isolated from patients with high titers of broadly neutralizing antibodies (Fig. 4 and 5 and Table 2). However, the distribution of unique, nonclonal antibodies varied between patients. For example, cluster I antibodies were most abundant in patient 2, and cluster II antibodies were most abundant in patient 3. When combined, the largest number of unique B-cell clones was directed to cluster IV (53%), followed by cluster II antibodies (49%) and cluster I antibodies (32%) (the sum shown in Table 2, 134%, results from antibodies that bind to two clusters). Although none of the antibodies tested interfered with the binding of the MPER-specific cluster III antibodies 4E10 and 2F5, antibodies to clusters II and IV, which flank this region, were common; therefore, this region of the molecule is accessible to the immune system in vivo. Indeed, each of the patients showed several antibodies that inhibited binding by both anti-cluster II and -cluster IV antibodies but not by cluster III antibodies, despite the proximity of these epitopes. The most likely explanation for the overlap between cluster II and cluster IV antibodies is the fact that cluster II antibodies recognize an epitope that is contained within the larger, conformationally dependent cluster IV epitope (12).
FIG. 4.
Clonal expansion and cluster distribution of anti-gp41 antibodies. Pie charts show the distribution of anti-gp41 antibodies to the different clusters (I, II, IV, and VI). Patients (Pt1 to Pt4) are indicated. The number in the center indicates the number of antibodies; slices are proportional to the size of the unique clones (43). The expanded cluster I antibodies are highlighted in red.
FIG. 5.
gp41 hot-spot inhibition map with IC50s (μg/ml). ID, the competing antibody reacts with the immunodominant peptide; ND, not done. Representative ELISA inhibition graphs are also shown (b). Black lines indicate inhibition. OD, optical density; abs, antibodies.
TABLE 2.
Number and percent distribution of anti-gp41 antibodies
| Patient no. | No. of unique B-cell clones (%) | No. of clonal members (%) | No. of clones (%) by cluster(s) |
|||||
|---|---|---|---|---|---|---|---|---|
| I | II | IV | II, IV | I, IV | VI, II | |||
| 1 | 7 | 2 | 2 | 1 | 2 | 0 | 0 | |
| 16 | 11 | 2 | 1 | 2 | 0 | 0 | ||
| 2 | 9 | 4 | 2 | 1 | 2 | 0 | 0 | |
| 42 | 22 | 9 | 6 | 5 | 0 | 0 | ||
| 3 | 14 | 0 | 3 | 5 | 6 | 0 | 0 | |
| 37 | 0 | 18 | 11 | 8 | 0 | 0 | ||
| 4 | 14 | 4 | 2 | 1 | 2 | 4 | 1 | |
| 29 | 7 | 2 | 2 | 2 | 14 | 2 | ||
| 5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 3 | 0 | 0 | 0 | 0 | 0 | ||
| 6 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | |
| 4 | 0 | 3 | 1 | 0 | 0 | 0 | ||
| Total B-cell clones (single cluster)a | 47 (100) | 11 (23) | 10 (21) | 9 (19) | 12 (26) | 4 (9) | 1 (2) | |
| Total B-cell clones (two clusters)a | (134) | 15 (32) | 23 (49) | 25 (53) | ||||
| Total clonal members (single cluster) | 131 (100) | 43 (33) | 34 (26) | 21 (16) | 17 (13) | 14 (11) | 2 (2) | |
| Total clonal members (two clusters) | (124) | 57 (44) | 53 (40) | 52 (40) | ||||
Totals reported refer to the sum of the individual clones or clonal members binding their cluster(s). Totals for a single cluster indicate the binding of the antibodies as mapped; totals for two clusters distribute the antibodies that bound to two clusters evenly to their single clusters.
Each of the unique antibodies tested was a member of a clone of antibodies ranging in size from 1 to 15 members. Together, 47 unique B-cell clones were expanded to account for a total of 131 independent antibodies. Within cluster I, antibody clones against linear (amino acids 579 to 604 [53]) and nonlinear epitopes were highly expanded in all of the patients except patient 3, who showed no antibodies to this region. Anti-cluster I antibodies comprised 15 different clonal families, with a total of 57 members. When the detected antibodies were combined, 32% of all unique anti-gp41 B-cell clones and 44% of all the anti-gp41 antibodies we obtained targeted this region. In contrast, antibodies to clusters II and IV comprised 49% and 53% of all unique anti-gp41 B-cell clones, respectively, and 40% each of all the anti-gp41 antibodies (the sum is greater than 100% because some antibodies appear to bind to two epitopes) (Table 2). Thus, antibodies to cluster I of gp41 are highly represented but not immunodominant in the memory B-cell compartment of the patients studied (53).
To confirm the absence of MPER-directed 2F5- and 4E10-like antibodies in the patients' sera, we performed competition ELISAs between affinity-purified anti-gp41 patient IgG and biotinylated 4E10 and 2F5. Controls included HIV IG, which did not inhibit 4E10 or 2F5 binding, as well as unbiotinylated 4E10 and 2F5 and IgG from noninfected individuals. Consistent with the results obtained with the monoclonal antibodies, the patients' affinity-purified anti-gp41 IgG did not block the binding of 4E10 or 2F5 to gp41 (Fig. 2b). However, as expected, the same affinity-purified fraction (shown for patient 1) blocked the binding of cluster I antibodies (2-55 and 2-378) (Fig. 2c). To increase the sensitivity of the assay, we performed ELISAs with anti-2F5 and -4E10 peptides (Fig. 2d). HIV IG bound to the 2F5 peptide at very high concentrations (>25 μg/ml), indicating that only rare or low-affinity antibodies to this peptide exist in HIV IG, and we found no binding to either peptide by our patients' IgG. We conclude that none of the patients studied produced significant titers of antibodies against the 2F5- and 4E10-like MPER epitopes.
To determine whether binding of any of the anti-gp41 antibodies was carbohydrate dependent, we deglycosylated gp41 with PNGase F to remove N-linked glycans and repeated the ELISAs. Deglycosylation was confirmed by a shift in mobility on denaturing polyacrylamide gels (Fig. 6a) and by lectin precipitation (Fig. 6b). Glycosylated gp41 bound to Lens culinaris lectin-Sepharose, whereas deglycosylated gp41 did not (Fig. 6b). We found one monoclonal anti-gp41 antibody (4-157) whose binding was carbohydrate dependent (Fig. 6c). This antibody was mapped to cluster I by competition experiments, but did not bind to the immunodominant peptide (amino acids 579 to 604).
Monoclonal neutralizing activity.
None of the anti-gp41 antibodies showed neutralizing activity at concentrations up to 50 μg/ml (43). To determine whether any of the 47 anti-gp41 antibodies showed neutralizing activity at higher concentrations, we performed in vitro neutralization assays with antibody concentrations of up to 2,380 μg/ml on two tier 2, clade B, Env-pseudotyped viruses from primary isolates (TRO.11 and RHPA4259.7). We found that 7 out of 15 cluster I antibodies were able to neutralize TRO.11 at high concentrations ranging from 433 to 1,712 μg/ml (IC50). Among all other antibodies, only one antibody against cluster IV showed neutralization at 1,276 μg/ml (Table 3). We conclude that, among anti-gp41 antibodies, those directed to cluster I show neutralizing activity but only at very high concentrations.
TABLE 3.
TZMbl neutralization data
| Patient no. | Antibody no. | Clustera | Neutralizing activity (IC50 [μg/ml])b |
|
|---|---|---|---|---|
| TRO.11 | RHPA4259.7 | |||
| 1 | 11 | IV | >500 | >500 |
| 27 | I-ID | 433 | >500 | |
| 96 | II, IV | >500 | >500 | |
| 167 | II | >500 | >500 | |
| 491 | II | >500 | >500 | |
| 696 | I-ID | 651 | >500 | |
| 763 | II, IV | >500 | >500 | |
| 2 | 55 | I | >1,030 | >1,030 |
| 149 | I-ID | >1,030* | >1,030 | |
| 150 | II | >1,500 | >1,500 | |
| 275 | II | >1,500 | >1,500 | |
| 378 | I-ID | >1,560 | >1,560 | |
| 474 | II, IV | >1,500 | >1,500 | |
| 512 | IV | 1,276 | >1,500 | |
| 1007 | I-ID | >1,000 | >1,000 | |
| 1290 | II, IV | >1,500 | >1,500 | |
| 3 | 18 | II, IV | >1,500 | >1,500 |
| 64 | IV | >1,500 | >1,500 | |
| 125 | II | >1,500 | >1,500 | |
| 140 | II, IV | >1,500 | >1,500 | |
| 160 | IV | >1,500 | >1,500 | |
| 204 | II, IV | >1,500 | >1,500 | |
| 233 | IV | >1,500 | >1,500 | |
| 255 | IV | >1,500 | >1,500 | |
| 296 | II, IV | >1,500 | >1,500 | |
| 334 | IV | >1,500 | >1,500 | |
| 384 | II | >1,500 | >1,500 | |
| 461 | II, IV | >1,500 | >1,500 | |
| 474 | II | >1,500 | >1,500 | |
| 650 | II, IV | >1,500 | >1,500 | |
| 4 | 20 | I-ID | 508 | >1,100 |
| 63 | IV | >1,500 | >1,500 | |
| 95 | I-ID, IV | >670 | >670 | |
| 104 | I, IV | 1,712 | >2,380 | |
| 147 | VI, II | >1,500 | >1,500 | |
| 153 | I-ID, IV | >1,500 | >1,500 | |
| 157 | I | >1,500 | >1,500 | |
| 251 | I | 841 | 1,234 | |
| 283 | II | >1,360 | >1,360 | |
| 342 | II | >1,500 | >1,500 | |
| 357 | I, IV | >665 | >665 | |
| 380 | II, IV | >1,500 | >1,500 | |
| 431 | I-ID | >690 | >690 | |
| 613 | II, IV | >1,500 | >1,500 | |
| 5 | 25 | I | 964 | >1,050 |
| 6 | 91 | IV | >1,500 | >1,500 |
| 129 | II | >1,500 | >1,500 | |
ID, binds to the immunodominant peptide.
Both TRO.11 and RHPA4259.7 are tier 2, clade B viruses. Values in boldface represent the concentration at which the tested monoclonal antibodies showed 50% neutralization. *, values that almost reached the IC50 at the highest antibody concentration tested.
DISCUSSION
gp41 is highly immunogenic and elicits antibodies in almost all HIV-infected individuals, with titers that can exceed 1:106 (2, 53). These titers are 25- to 625-fold higher than anti-gp120 titers (35). In addition, gp41 differs from gp120 in that all regions of the protein appear to be targeted by the human antibody response (35).
Serologic examination of 23 randomly selected HIV-1-positive individuals showed that the fusion peptide and the polar region induce low-to-medium serum antibody titers (35). In contrast, high serum antibody titers (up to 1:7 × 105) were documented against the N- and C-terminal heptad repeats (35), the membrane-proximal region (35), and the cluster I region (53). The latter was identified as immunodominant by the observation that 53 out of 53 HIV-positive patients' sera tested showed reactivity to a synthetic 12-mer peptide from this region (LGLWGCSGKLIC) (15, 16). Consistent with this peptide's immunogenicity, injection of the peptide coupled to keyhole limpet hemocyanin into rabbits resulted in serum antibody titers of 1:1 × 106 (15). Although antibodies to the antigens that are membrane proximal are also present in most patients' sera, these do not appear to resemble the broadly neutralizing 2F5- and 4E10-like antibodies (18, 56). Instead, the anti-MPER antibodies that were commonly found among patients appear to be directed to a peptide partially overlapping the 2F5 and 4E10 epitope (amino acids 665 to 683) (35). Similarly, gp41-immunized rabbits produced antibodies to cluster I but failed to produce anti-MPER antibodies (58).
In contrast to the serologic studies described above, which were performed on immunized rabbits and randomly selected HIV-1-infected individuals, our patients were selected for broad neutralizing antibody responses, relatively low viral titers, and moderate CD4+ T-cell counts (43). In addition, the B cells from which our antibodies were cloned were selected for their binding to soluble trimeric gp140 (54), which is recognized by most anti-gp41 antibodies, including those binding to the MPER, the C-terminal heptad repeat, cluster I, and the fusion peptide polar region (antibody 5F3) (6). However, antibody D3 mapped to cluster V, which includes the fusion peptide, the polar region, and the N-terminal heptad repeat, does not recognize the synthetic trimer (data not shown); therefore, antibodies with this type of reactivity to gp41 would not be detected in our analysis.
We found that 47 unique clones of anti-gp41 antibodies were variably expanded, with each containing 1 to 15 members, for a total of 131 independently cloned antibodies. All of these antibodies could be assigned to clusters I, II, IV, and VI, but none recognized the fusion peptide, the polar region, or the N-terminal heptad repeat or the MPER peptide. This finding is consistent with both the absence of antibodies to the MPER peptide in the patient serum and the inability of a control anti-cluster V antibody to bind to the artificial gp140 trimer. Unique antibodies reacting with cluster I, the immunodominant region, were not predominant in the collection; they represented only 32% of all the unique B-cell clones. Instead, a conformational epitope neighboring the MPER (cluster IV) was the dominant immunogen. However, anti-cluster I clones were disproportionately expanded, accounting for 44% of all anti-gp41 antibodies when all of the distinct clonal family members were considered. Among the 15 unique clones of anti-cluster I antibodies, 9 recognized the linear peptide, and 6 were against conformational determinants in this region. Similarly, 5 out of 10 antibodies produced from seven seropositive patients by EBV immortalization were directed to cluster I, and of these, 4 were directed to the linear peptide (53). In contrast, phage display antibody libraries that were constructed from patients and that were selected on recombinant gp41 showed a much lower frequency of cluster I binders (5 out of 25) (4). However, antibody cloning by phage display is biased by multiple rounds of selection and amplification; in addition, the process may not reflect the native antibody repertoire because heavy and light chains are paired randomly, potentially creating novel combining sites. Thus, the frequency of specific antibodies estimated by this method may not be an accurate reflection of the antibodies found in a patient's B-cell repertoire. We conclude that in patients with high titers of broadly neutralizing HIV antibodies and variable viral loads, anti-cluster I antibodies account for 32% of all unique B-cell clones; these are preferentially expanded to account for 44% of all anti-gp41 antibodies, a significant fraction of which are directed to the linear peptide comprised of amino acids 579 to 604. The gp41 antibodies found in the patients studied might result from B-cell stimulation by the intact virion or by nonfunctional membrane-associated gp41 on the surface of infected cells or even damaged virions (7, 19, 31).
Our studies reveal several previously unappreciated epitopes on gp41. For example, four antibodies cloned from the memory B cells were inhibited by monoclonal antibodies directed to both cluster I and IV but not by antibodies to cluster VI, which is between the two. Inhibition by anti-cluster I and -cluster IV but not anti-cluster VI suggests that the former two are closely opposed to each other in the trimer, either in cis or in trans between two different gp41 molecules in the trimer.
In addition to conformational epitopes within cluster I, we found a novel carbohydrate-dependent epitope in the same region of gp41. This region contains three well-conserved, predicted glycosylation sites (21). Like 2G12, which recognizes a carbohydrate-dependent epitope on gp120 (42), gp41 binding by antibody 4-157 is sensitive to deglycosylation, which thereby establishes that the cluster I region of gp41 is indeed glycosylated (21).
Among the 345 amino acid residues in gp41, one-third show up to 90% conservation among HIV-1 group M isolates (20). These include highly conserved residues critical for the formation of the coiled-coil gp41 pocket (28), for gp120-gp41 interactions (55), and for envelope biosynthesis (45). These critical residues are found primarily in the heptad repeats and the cluster I region. Indeed, mouse anti-cluster I antibodies exhibit extensive cross-reactivity with primary envelope proteins from divergent HIV-1 clades, indicating the presence of a highly conserved secondary antigenic structure in the cluster I loop region (12). Given the importance of these regions in the function of the viral spike and their relatively high level of conservation, these might be important targets for neutralizing antibodies if they were accessible either on the virion or as membrane-associated gp41 postfusion on the surface of infected cells. Consistent with this idea, the IgG fraction of serum from rabbits immunized with gp41 neutralized 52% of 21 HIV primary isolates in a peripheral blood mononuclear cell (PBMC)-based assay, with IC50s lower than 50 μg/ml (58). Finally, several groups of investigators have recently shown that a small fraction of HIV-infected patients develops anti-gp41 neutralizing antibody responses, mainly due to anti-MPER antibodies (3, 39, 47).
Despite the serologic data noted above, there are only a few neutralizing monoclonal anti-gp41 antibodies. D5 is a phage-derived antibody obtained from a naïve single-chain variable-fragment (scFv) library that inhibits the assembly of fusion intermediates in vitro by binding to the N-terminal heptad repeat (27). Antibody m44, another phage-derived antibody, recognizes a conformational epitope implicated in the binding of the C-terminal heptad repeat (57). Less is known about how this antibody inhibits infection, but the mechanism may be related to the one used by D5 since both target the heptad repeats that form six-helix coiled-coiled fusion intermediates in vitro. Clone 3 is specific for the immunodominant region and shows neutralizing activity against a diverse group of laboratory-adapted HIV-1 strains (10, 51). This antibody was produced by EBV-transformed PBMCs from an asymptomatic HIV-1-positive donor (10). Other antibodies against this region show complement-mediated infection-enhancing activity in vitro (36, 37).
Finally, there are three broadly neutralizing antibodies directed to the membrane-proximal external region: 2F5 (33, 49), 4E10, and Z13 (60). Such antibodies are rare (3) and were detected only in the sera of 3 out of 156 chronically HIV-1-infected individuals (17). The poor immunogenicity of this region may be attributed to its lack of exposure on the surface of the native virus (9, 34, 41, 44; reviewed in reference 30). The neutralizing activity of these antibodies appears to be dependent on binding to both the MPER and to the viral membrane (25). It has been proposed that antibody binding to the viral membrane serves to concentrate the antibody in the region of the MPER, thereby favoring its interaction with this poorly exposed antigenic epitope (1). Consistent with the idea that lipid binding may be important in the function of the anti-MPER antibodies, liposomes containing MPER peptide induced multispecific antibodies that simultaneously recognize the lipid and the MPER antigen; they also neutralized HIV SF162 in a PBMC-based neutralization assay (25).
We found that none of the gp41 antibodies cloned from the memory B cells of the six individuals studied were able to neutralize HIV at concentrations of up to 50 μg/ml (43). Surprisingly, 7 out of 15 antibodies to cluster I neutralize one of two tested tier 2 viruses at high concentrations. Although it is unlikely that an individual anti-cluster I antibody would have a biological effect, large numbers of gp41 antibodies might enhance neutralizing activity due to additive or even synergistic neutralizing effects (43). Antibody binding to the cluster I hinge region between the heptad repeats might interfere with the formation of the prehairpin fusion intermediate by steric hindrance (27). Why such high concentrations of cluster I antibodies are necessary for neutralization remains unclear (5); one possibility is that this region is poorly accessible to antibodies binding the intact virion.
Acknowledgments
We thank Henry Zebrosky, Rockefeller Proteomics Resource Center, for the production of gp41 peptides. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV IG from NABI and NHLBI and HIV-1 gp41 monoclonal antibody (5F3) from Hermann Katinger.
The work was supported by NIH grant 1 P01 AI08677-01 and a grant from the International AIDS Vaccine Initiative. M.C.N. is a Howard Hughes Medical Institute investigator.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Alam, S. M., M. Morelli, S. M. Dennison, H. X. Liao, R. Zhang, S. M. Xia, S. Rits-Volloch, L. Sun, S. C. Harrison, B. F. Haynes, and B. Chen. 2009. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 106:20234-20239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barin, F., M. F. McLane, J. S. Allan, T. H. Lee, J. E. Groopman, and M. Essex. 1985. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science 228:1094-1096. [DOI] [PubMed] [Google Scholar]
- 3.Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J. M., H. J. Ditzel, C. F. Barbas, N. Sullivan, J. Sodroski, P. W. Parren, and D. R. Burton. 1996. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res. Hum. Retroviruses 12:911-924. [DOI] [PubMed] [Google Scholar]
- 5.Broder, C. C., P. L. Earl, D. Long, S. T. Abedon, B. Moss, and R. W. Doms. 1994. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc. Natl. Acad. Sci. U. S. A. 91:11699-11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359-369. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 8.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 9.Coëffier, E., J. M. Clément, V. Cussac, N. Khodaei-Boorane, M. Jehanno, M. Rojas, A. Dridi, M. Latour, R. El Habib, F. Barré-Sinoussi, M. Hofnung, and C. Leclerc. 2000. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine 19:684-693. [DOI] [PubMed] [Google Scholar]
- 10.Cotropia, J., K. E. Ugen, S. Kliks, K. Broliden, P. A. Broliden, J. A. Hoxie, V. Srikantan, W. V. Williams, and D. B. Weiner. 1996. A human monoclonal antibody to HIV-1 gp41 with neutralizing activity against diverse laboratory isolates. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:221-232. [DOI] [PubMed] [Google Scholar]
- 11.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finnegan, C. M., W. Berg, G. K. Lewis, and A. L. DeVico. 2002. Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion. J. Virol. 76:12123-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallaher, W. R. 1987. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell 50:327-328. [DOI] [PubMed] [Google Scholar]
- 15.Gnann, J. W., Jr., P. L. Schwimmbeck, J. A. Nelson, A. B. Truax, and M. B. Oldstone. 1987. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J. Infect. Dis. 156:261-267. [DOI] [PubMed] [Google Scholar]
- 16.Gnann, J. W., Jr., J. A. Nelson, and M. B. Oldstone. 1987. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J. Virol. 61:2639-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray, E. S., M. C. Madiga, M. L. Moore, K. Mlisana, S. S. A. Karim, J. M. Binley, G. M. Shaw, J. R. Mascola, and L. Morris. 2009. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J. Virol. 83:11265-11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, L. Morris, and the CAPRISA 002 Study Team. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5:579-595. [DOI] [PubMed] [Google Scholar]
- 20.Holguín, A., E. R. De Arellano, and V. Soriano. 2007. Amino acid conservation in the gp41 transmembrane protein and natural polymorphisms associated with enfuvirtide resistance across HIV-1 variants. AIDS Res. Hum. Retroviruses 23:1067-1074. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, W. E., J. M. Sauvron, and R. C. Desrosiers. 2001. Conserved, N-linked carbohydrates of human immunodeficiency virus type 1 gp41 are largely dispensable for viral replication. J. Virol. 75:11426-11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 23.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 25.Matyas, G. R., L. Wieczorek, Z. Beck, C. Ochsenbauer-Jambor, J. C. Kappes, N. L. Michael, V. R. Polonis, and C. R. Alving. 2009. Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4e10 epitope and lipid epitopes. AIDS 23:2069-2077. [DOI] [PubMed] [Google Scholar]
- 26.McMichael, A. J., P. Borrow, G. D. Tomaras, N. Goonetilleke, and B. F. Haynes. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10:11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, M. D., R. Geleziunas, E. Bianchi, S. Lennard, R. Hrin, H. Zhang, M. Lu, Z. An, P. Ingallinella, M. Finotto, M. Mattu, A. C. Finnefrock, D. Bramhill, J. Cook, D. M. Eckert, R. Hampton, M. Patel, S. Jarantow, J. Joyce, G. Ciliberto, R. Cortese, P. Lu, W. Strohl, W. Schleif, M. McElhaugh, S. Lane, C. Lloyd, D. Lowe, J. Osbourn, T. Vaughan, E. Emini, G. Barbato, P. S. Kim, D. J. Hazuda, J. W. Shiver, and A. Pessi. 2005. A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc. Natl. Acad. Sci. U. S. A. 102:14759-14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo, H., A. K. Konstantinidis, K. D. Stewart, T. Dekhtyar, T. Ng, K. Swift, E. D. Matayoshi, W. Kati, W. Kohlbrenner, and A. Molla. 2004. Conserved residues in the coiled-coil pocket of human immunodeficiency virus type 1 gp41 are essential for viral replication and interhelical interaction. Virology 329:319-327. [DOI] [PubMed] [Google Scholar]
- 29.Montefiori, D. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. Unit 12.11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed]
- 30.Montero, M., N. E. van Houten, X. Wang, and J. K. Scott. 2008. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 72:54-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 32.Mouquet, H., S. Farci, P. Joly, B. Maillère, J. Leblond, L. Drouot, J. Leprince, M. C. Tonon, P. Loiseau, D. Charron, F. Tron, and D. Gilbert. 2006. A truncated alternative spliced isoform of human desmoglein 1 contains a specific T-cell epitope binding to the pemphigus foliaceus-associated HLA class II DR1*0102 molecule. J. Immunol. 177:6517-6526. [DOI] [PubMed] [Google Scholar]
- 33.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Rüker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyambi, P. N., H. A. Mbah, S. Burda, C. Williams, M. K. Gorny, A. Nádas, and S. Zolla-Pazner. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 74:7096-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opalka, D., A. Pessi, E. Bianchi, G. Ciliberto, W. Schleif, M. McElhaugh, R. Danzeisen, R. Geleziunas, M. Miller, D. M. Eckert, D. Bramhill, J. Joyce, J. Cook, W. Magilton, J. Shiver, E. Emini, and M. T. Esser. 2004. Analysis of the HIV-1 gp41 specific immune response using a multiplexed antibody detection assay. J. Immunol. Methods 287:49-65. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, W. E. J., M. K. Gorny, J. Y. Xu, W. M. Mitchell, and S. Zolla-Pazner. 1991. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J. Virol. 65:4169-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson, W. E. J., T. Kawamura, M. K. Gorny, D. Lake, J. Y. Xu, Y. Matsumoto, T. Sugano, Y. Masuho, W. M. Mitchell, E. Hersh, et al. 1990. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp41 enhance HIV-1 infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 87:3185-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzwedel, K., J. T. West, and E. Hunter. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sather, D. N., J. Armann, L. K. Ching, A. Mavrantoni, G. Sellhorn, Z. Caldwell, X. Yu, B. Wood, S. Self, S. Kalams, and L. Stamatatos. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 42.Scanlan, C. N., P. R., M. R. Wormald, E. O. Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheid, J. F., H. Mouquet, N. Feldhahn, M. S. Seaman, K. Velinzon, J. Pietzsch, R. G. Ott, R. M. Anthony, H. Zebroski, A. Hurley, A. Phogat, B. Chakrabarti, Y. Li, M. Connors, F. Pereyra, B. D. Walker, H. Wardemann, D. Ho, R. T. Wyatt, J. R. Mascola, J. V. Ravetch, and M. C. Nussenzweig. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636-640. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Z. Y., K. J. Oh, M. Kim, J. Yu, V. Brusic, L. Song, Z. Qiao, J. H. Wang, G. Wagner, and E. L. Reinherz. 2008. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28:52-63. [DOI] [PubMed] [Google Scholar]
- 45.Syu, W. J., W. R. Lee, B. Du, Q. C. Yu, M. Essex, and T. H. Lee. 1991. Role of conserved gp41 cysteine residues in the processing of human immunodeficiency virus envelope precursor and viral infectivity. J. Virol. 65:6349-6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiller, T., E. Meffre, S. Yurasov, M. Tsuiji, M. C. Nussenzweig, and H. Wardemann. 2008. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 329:112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomaras, G. D., and B. F. Haynes. 2009. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr. Opin. HIV AIDS 4:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomaras, G. D., N. L. Yates, P. Liu, L. Qin, G. G. Fouda, L. L. Chavez, A. C. Decamp, R. J. Parks, V. C. Ashley, J. T. Lucas, M. Cohen, J. Eron, C. B. Hicks, H. X. Liao, S. G. Self, G. Landucci, D. N. Forthal, K. J. Weinhold, B. F. Keele, B. H. Hahn, M. L. Greenberg, L. Morris, S. S. Karim, W. A. Blattner, D. C. Montefiori, G. M. Shaw, A. S. Perelson, and B. F. Haynes. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trkola, A., A. B. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinge, C. F. Barbas, D. R Burton, D. D. Ho, et al. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trkola, A., H. Kuster, P. Rusert, B. Joos, M. Fischer, C. Leemann, A. Manrique, M. Huber, M. Rehr, A. Oxenius, R. Weber, G. Stiegler, B. Vcelar, H. Katinger, L. Aceto, and H. F. Günthard. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615-622. [DOI] [PubMed] [Google Scholar]
- 51.Viveros, M., C. Dickey, J. P. Cotropia, G. Gevorkian, C. Larralde, K. Broliden, M. Levi, A. Burgess, C. Cao, D. B. Weiner, M. G. Agadjanyan, and K. E. Ugen. 2000. Characterization of a novel human immunodeficiency virus type 1 neutralizable epitope within the immunodominant region of gp41. Virology 270:135-145. [DOI] [PubMed] [Google Scholar]
- 52.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 53.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 65:4832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, X., M. Farzan, R. Wyatt, and J. Sodroski. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 74:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.York, J., and J. H. Nunberg. 2004. Role of hydrophobic residues in the central ectodomain of gp41 in maintaining the association between human immunodeficiency virus type 1 envelope glycoprotein subunits gp120 and gp41. J. Virol. 78:4921-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuste, E., H. B. Sanford, J. Carmody, J. Bixby, S. Little, M. B. Zwick, T. Greenough, D. R. Burton, D. D. Richman, R. C. Desrosiers, and W. E. Johnson. 2006. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV-1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 80:3030-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, M. Y., B. K. Vu, A. Choudhary, H. Lu, M. Humbert, H. Ong, M. Alam, R. M. Ruprecht, G. Quinnan, S. Jiang, D. C. Montefiori, J. R. Mascola, C. C. Broder, B. F. Haynes, and D. S. Dimitrov. 2008. Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens. J. Virol. 82:6869-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, M. Y., Y. Wang, M. K. Mankowski, R. G. Ptak, and D. S. Dimitrov. 2009. Cross-reactive HIV-1-neutralizing activity of serum IgG from a rabbit immunized with gp41 fused to IgG1 Fc: possible role of the prolonged half-life of the immunogen. Vaccine 27:857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu, P., J. Liu, J. Bess Jr., E. Chertova, J. D. Lifson, H. Grisé, G. A. Ofek, R. A. Taylor, and K. H. Roux. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847-852. [DOI] [PubMed] [Google Scholar]
- 60.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]