Abstract
Human T-cell leukemia virus type 1 (HTLV-1) Tax affects cellular genomic stability and senescence. As yet, the mechanism(s) for these events caused by Tax is incompletely understood. Here, we show that Tax expression in primary human cells induces reactive oxygen species (ROS), which elicits DNA damage and the expression of senescence marker. Treatment with a ROS scavenger or knockdown of Tax expression by small interfering RNA (siRNA) abrogated Tax-induced DNA damage and the expression of senescence marker. Our data suggest that ROS induction explains Tax-induced cellular DNA damage and cellular senescence.
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T cell leukemia/lymphoma (ATLL) (2, 17, 26, 34). The viral Tax oncoprotein is implicated in the initiation of cellular transformation (5, 26). Tax has been reported to cause pleiotropic effects, including induction of cellular DNA damage and chromosomal instability (CIN) (1, 16, 23, 29). Tax-induced DNA damage and CIN could trigger cellular senescence as a reactive response for protection against oncogenesis (19, 28). To date, how Tax causes DNA damage and CIN remains incompletely explained.
Oncoproteins can generate DNA damage in two ways. First, the oncoprotein can neutralize cellular checkpoints and repair processes, thereby allowing damage caused by ambient events (ultraviolet [UV] light, carcinogens, etc.) to manifest. Second, the oncoprotein can directly create DNA lesions. To date, while many studies have shown that Tax can obstruct the cell's checkpoints and DNA damage repair mechanism(s) (6, 9, 12, 14, 21, 22), it has not been demonstrated that Tax can directly create DNA damage. Oncoproteins, such as Ras, have been reported to modulate intracellular reactive oxygen species (ROS) (20). ROS is a DNA-damaging agent (7, 10, 27). We thus explored if Tax, like Ras, increases cellular ROS in cells.
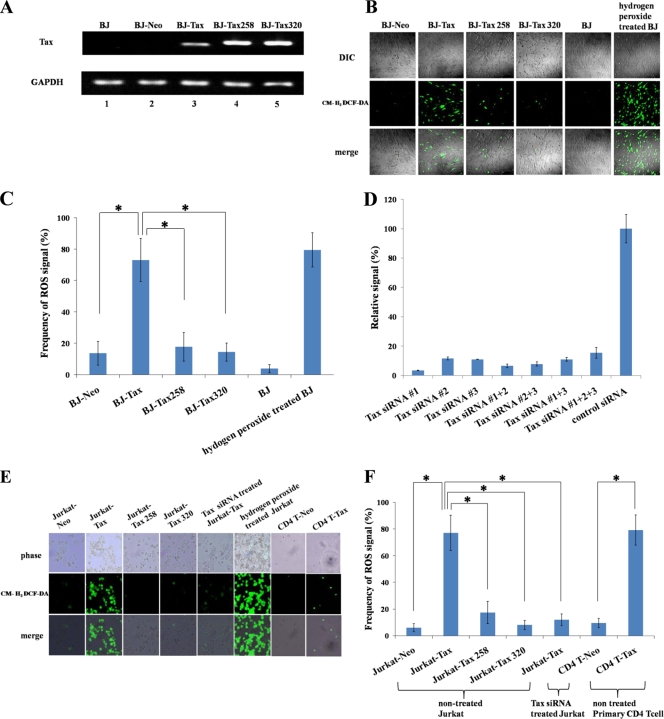
For our assays, we employed normal human foreskin fibroblasts (BJ cells; ATCC, Manassas, VA) at fewer than 10 passages, primary human cord blood CD4+ T cells (Lonza, Walkersville, MD), and the HTLV-1-negative human acute lymphocytic leukemia T-Jurkat cell line (kindly provided by Yuetsu Tanaka of the University of the Ryukyus). To examine ROS induction, we tested wild-type Tax, a Tax point mutant (the S258A mutant, which is intact for CREB but defective for NF-κB activity), and a second Tax point mutant (the L320G mutant, which is intact for NF-κB but defective for CREB activity) (32). To construct Tax-expressing retroviral vectors, we used the pMSCV-neo vector (Clontech, Mountain View, CA), which expresses genes of interest at a moderate level. We employed this vector-promoter because we have found that high overexpression of Tax would rapidly induce apoptosis in BJ cells (data not shown). Wild-type Tax and the two Tax mutants were cloned into the pMSCV-neo vector, and retroviral particles that express Tax were produced using the packaging cell line PT-67 in accordance with the manufacturer's instructions (Clontech, Mountain View, CA). Normal human foreskin BJ fibroblasts, Jurkat cells, and primary human CD4+ T cells were then transduced with viral particles that express wild-type Tax or either of the two Tax mutants. Transduced cells were then selected for 7 days with G418 (Invitrogen, Carlsbad, CA), and Tax expression was verified by reverse transcription-PCR (RT-PCR) (Fig. 1A). BJ-Tax, BJ-Tax 258, and BJ-Tax 320 (Fig. 1A, lanes 3, 4, and 5) cells showed distinct evidence for Tax expression, whereas the negative-control cells (BJ and BJ-Neo) (Fig. 1A, lanes 1 and 2) did not.
FIG. 1.
ROS production in Tax-transduced normal human BJ fibroblasts, Jurkat cells, and primary human CD4+ T lymphocytes from cord blood. (A) BJ cells were transduced with virus vectors that express either wild-type Tax or mutant Tax (Tax S258A or Tax L320G) protein. After 7 days of selection with G418 (Invitrogen, Carlsbad, CA), total RNA was extracted, and Tax expression was verified by RT-PCR. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Tax-transduced BJ cells were assayed with CM-H2DCF-DA, and the signals were visualized using fluorescence microscopy. DIC, differential interference contrast. (C) Total cell numbers in the visual field and the green fluorescent cells were counted, and the frequency of ROS/CM-H2DCF-DA-positive cells was calculated. Experiments were performed independently, three times. To obtain enough cell counts for statistical analysis, at least 500 cells in each sample were counted. The error bars represent standard deviations. Multiple data were compared by the Kruskal-Wallis H test as appropriate. When the Kruskal-Wallis test showed a significant difference, correlations between two variables were then examined by the Mann-Whitney U test with Bonferroni correction. A P value of 0.01 or less was considered statistically significant. The asterisk shows a statistically significant difference (P < 0.01). (D) Quantifications of knockdown of Tax mRNA, normalized to the level for GAPDH mRNA. Tax siRNAs were designed by using the Stealth RNA interference (RNAi) (Invitrogen) procedure. Control siRNA (irrelevant target sequence, TTT ATG TGT GCC CGT GTG GAA) and three Tax siRNAs were synthesized by Hokkaido System Science (Hokkaido, Japan). Tax-transduced cells were transfected with 1 μmol siRNA per 2 × 107 cells by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. After 48 h of incubation, the knockdown effects on Tax RNA were compared by the Mx3000P real-time PCR system (Stratagene), and we selected Tax siRNA 1 (target sequence, TCC CAG GGT TTG GAC AGA GTC TTC T) as the most effective siRNA for further experiments. The sequences for Tax siRNAs 2 and 3 are CCT TCC TCA CCA ATG TTC CCT ACA A and GCC CTA AAG ATG GCC AGC CAT CTT T, respectively. These siRNAs were not used in later experiments. (E) Tax and Tax mutant-transduced Jurkat cells were assayed with CM-H2DCF-DA and treated as indicated. The fluorescent signals were visualized. (F) Total cell numbers and green fluorescent cells were counted. The frequency of ROS/CM-H2DCF-DA-positive cells in the indicated samples was calculated, with statistical analysis performed as described for panel C.
We next investigated ROS levels using BJ, Jurkat, and primary CD4+ T cells. To measure intracellular ROS, a peroxide-sensitive reagent (CM-H2DCF-DA; Molecular Probes, Eugene, OR) was used. The cells were stained using 5 μM CM-H2DCF-DA for 10 min at 37°C and observed under a fluorescence microscope. Increased ROS was indicated by higher CM-H2DCF-DA fluorescence in the experimental cells than in the control cells. Indeed, BJ-Tax cells (Fig. 1B) showed strong fluorescent signals, with 70% of the cells staining brightly positive for ROS in a representative low-power field. In comparison, the two Tax mutants (BJ-Tax 258 and BJ-Tax 320) (Fig. 1B) elicited fewer positively stained cells while the positive-control cells (treated with hydrogen peroxide) were >80% positive for ROS (Fig. 1B). The results from three independent experiments were quantified and analyzed (Fig. 1C); these results demonstrated statistically significant differences in ROS production between wild-type Tax, Tax S258A, and Tax L320G (Fig. 1C).
To confirm further that ROS generation was due to Tax expression, we designed three small interfering RNAs (siRNAs) (siRNAs 1, 2, and 3) that target Tax (Fig. 1D). The effectiveness of the siRNAs in knocking down Tax mRNA was verified (Fig. 1D). siRNA 1 was found to be the most effective (Fig. 1D) and was employed in subsequent experiments. We repeated ROS induction using wild-type Tax in Jurkat cells (Fig. 1E) and primary CD4+ T cells (Fig. 1F). In Jurkat cells, the ROS induced by Tax was efficiently reduced by transfected siRNA that targeted Tax (Fig. 1E and F), consistent with a direct role for Tax in ROS induction.
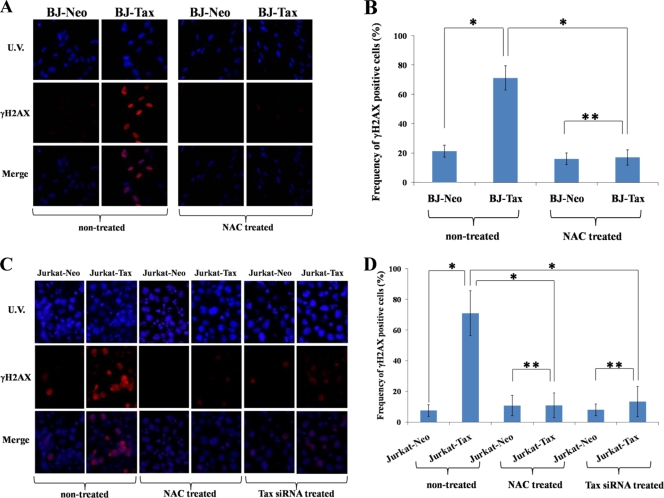
Does Tax-ROS production correlate with cellular DNA damage? To address this question, cellular damage was assayed by measuring the presence of accumulated DNA damage-associated repair protein γH2AX (4, 18). We compared Neo- and Tax-expressing BJ (Fig. 2A and B) and Jurkat (Fig. 2C and D) cells by immunostaining using mouse anti-human γH2AX antibody (GeneTex, Irvine, CA), followed by secondary incubation with anti-mouse Alexa568 (Molecular Probe, Eugene, OR). BJ-Tax and Jurkat-Tax cells had strong nuclear γH2AX staining, consistent with increased DNA damage (Fig. 2A, B, C, and D, nontreated), while BJ-Neo and Jurkat-Neo cells did not (Fig. 2A, B, C, and D, nontreated). Transfection of siRNA targeted to Tax significantly reduced the induction of γH2AX in Jurkat-Tax cells (Fig. 2C and D, Tax siRNA treated), supporting a role for Tax in γH2AX-induction. To establish that the accumulation of γH2AX in BJ-Tax and Jurkat-Tax cells was due to ROS, we treated Neo- and Tax-expressing cells with the ROS scavenger N-acetyl cysteine (NAC) and then stained for γH2AX (Fig. 2A, B, C, and D, NAC treated). NAC treatment effectively erased the γH2AX signals in both BJ-Tax and Jurkat-Tax cells, consistent with the interpretation that the DNA damage-associated γH2AX observed in these cells was a result of ROS (Fig. 2A, B, C, and D). Three independent experiments were performed, and the collective findings verified the statistically significant differences between Neo- and Tax-expressing cells and between Tax-expressing cells without and with NAC or siRNA treatments (Fig. 2B and D).
FIG. 2.
Accumulation of DNA damage responsive γH2AX protein in Tax-expressing BJ and Jurkat cells. (A) Wild-type Tax-transduced BJ cells were treated without (nontreated) or with (NAC treated) NAC treatment and were examined by immunofluorescence for γH2AX. (B) Frequency of γH2AX-positive cells in BJ-Neo and BJ-Tax cells without (nontreated) or with (NAC treated) NAC treatment. Experiments were performed three times. To obtain enough cell counts for statistical analysis, at least 500 cells in each sample were counted. The error bar represents the standard deviation. Statistical analysis was performed as described for Fig. 1C. A single asterisk shows a statistically significant difference (P < 0.01), and double asterisks show an absence of significant difference. (C) Wild-type Tax-transduced Jurkat cells were treated without (nontreated) or with (NAC treated) NAC or were transfected with Tax siRNA. The cells were examined by immunofluorescence for γH2AX. (D) Statistical analyses of the results in panel C were performed as described for Fig. 1C.
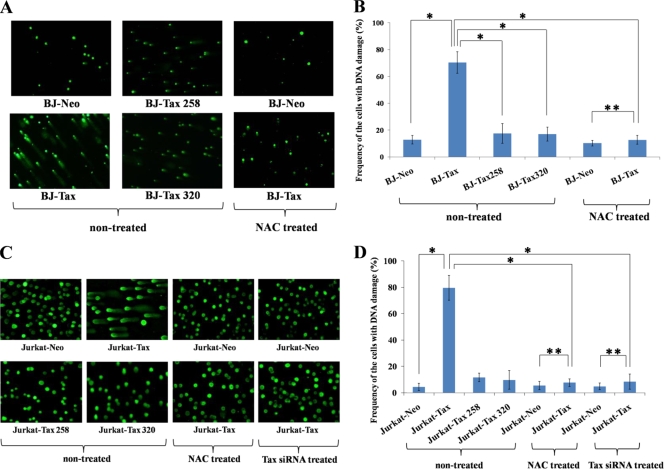
A more direct measure of DNA damage than staining for γH2AX is monitoring for single- and double-stranded DNA breaks by employing the “comet” assay (comet assay kit; Stressgene, Ann Arbor, MI). The visualization of a “comet” tail in an individual cell indicates DNA strand breakage. To perform the comet analysis, cells after 1, 3, and 5 days of Tax transduction were collected and embedded in agarose gel. They were then processed for electrophoresis after sodium hydroxide treatment. Among BJ-Tax (Fig. 3A and B) and Jurkat-Tax (Fig. 3C and D) cells, the majority showed comet tails, indicative of DNA breaks (Fig. 3A and C, nontreated). In contrast, Neo- or Tax mutant-expressing BJ and Jurkat cells displayed negligible numbers of comet cells (Fig. 3A, B, C, and D, nontreated). NAC or siRNA treatment significantly reduced the comet tailing (Fig. 3A, B, C, and D, NAC treated, and B and C, Tax siRNA treated), further corroborating a link between Tax-induced ROS and cellular DNA damage.
FIG. 3.
Tax-induced DNA damage was measured by a comet assay. (A) Wild-type or mutant Tax (Tax 258 and Tax 320)-transduced BJ cells were treated without (nontreated) or with (NAC treated) NAC, and they were examined by a comet assay after 6 days. (B) Graphic summary of the results of the comet study. The experiments were repeated three times. To obtain enough cell counts for statistical analysis, at least 500 cells in each sample were counted. Error bars show standard deviations. Statistical analysis was performed as described for Fig. 1C. A single asterisk shows a statistically significant difference (P < 0.01), and double asterisks show an absence of significant difference. (C) Wild-type or mutant Tax (Tax 258 and Tax 320)-transduced Jurkat cells were treated without (nontreated) or with (NAC treated) NAC or transfected with Tax siRNA. The cells were examined by a comet assay after 6 days. (D) Graphic summary of the results from panel C for the comet assays. The statistical analysis was performed as described for Fig. 1C.
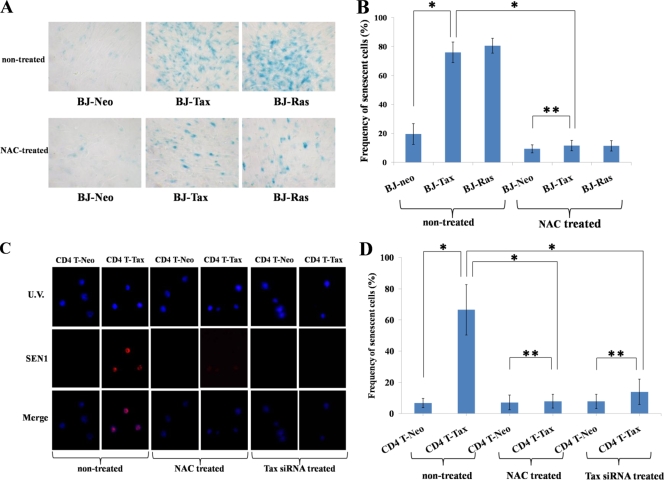
A frequent physiological reactive response of nontransformed cells to DNA damage is senescence (3, 8, 33). When wild-type Tax was expressed in BJ cells, many cells showed irregularly shaped nuclei, and the overall cell growth rate in cultured BJ-Tax cells was decreased in comparison to the level for BJ control cells (data not shown). To explore the relationship between Tax expression and cell senescence in BJ (Fig. 4A and B) and primary CD4+ T (Fig. 4C and D) cells, we stained the cells for senescence markers (staining for senescence-associated beta-galactosidase [SA β-Gal] was performed for BJ cells [senescence detection kit; Trevigen, Gaithersburg, MD], and staining for SEN1, also known as MORF4 [35], was performed for primary CD4+ T cells [Abcam, Cambridge, MA]). Elsewhere, Ras overexpression has been reported by others to induce cell senescence (8, 33). Thus, we used Ras expression as a positive senescence control. Indeed, the numbers of SA β-Gal staining cells in Tax- and Ras-expressing BJ cells were increased more than 3-fold over the number for BJ-neo control cells (Fig. 4A and B). Because primary CD4+ T cells that express Tax showed poor cytoplasmic SA β-Gal visualization due to the inherently small cytoplasmic volume of primary lymphocytes, we chose to immunostain for a nuclear senescence marker, SEN1 (also known as MORF4 [35]). Tax distinctly induced SEN1 expression in primary human CD4+ T cells, and the frequency of senescent cells was significantly increased compared to the level for control Neo-expressing CD4+ T cells (Fig. 4C and D).
FIG. 4.
Senescence-associated marker in Tax- and Ras-expressing human BJ fibroblasts and primary human CD4+ T lymphocytes. (A) Wild-type Tax- or Ras-expressing BJ cells were treated without (nontreated) or with (NAC-treated) NAC, and they were examined after 6 days by SA β-Gal staining. (B) The frequency of senescent β-Gal cells was tabulated as in Fig. 3B. Experiments were performed three times. To obtain enough cell counts for statistical analysis, at least 500 cells in each sample were counted. Error bars show standard deviations. Statistical analysis was performed as described for Fig. 1C. A single asterisk shows a statistically significant difference (P < 0.01), and double asterisks show an absence of significant difference. (C) Wild-type Tax- or Neo-expressing primary human CD4+ T cells were treated without (nontreated) or with (NAC-treated) NAC or with Tax siRNA, and they were examined after 6 days by immunofluorescence for SEN1. (D) The frequency of SEN1 cells was tabulated as in Fig. 3B, and the statistical analysis was as described for Fig. 1C.
We also asked if the increased expression of senescence markers could be relieved by the ROS scavenger, NAC (Fig. 4A, B, C, and D). Treatment of cells with NAC reduced SA β-Gal expression in both Tax- and Ras-transduced BJ cells (Fig. 4A), with statistically significant changes (Fig. 4B). NAC treatment of Tax-expressing primary human CD4+ T cells and transfection of siRNA targeting Tax into the corresponding cells also suppressed SEN1 in a statistically significant fashion (Fig. 4C and D).
Here, to our knowledge, we show for the first time that Tax expression in primary human cells induces ROS with accompanying DNA damage and reactive cellular senescence. Previously, Tax expression in cultured cells has been reported to result in genomic instability, cell senescence, and apoptosis (19, 24, 28, 36); however, the inciting cause(s) for these cellular changes was not characterized. Our present findings from Tax expression in normal fibroblasts, CD4+ primary T cells, and Jurkat cells suggest that some of above-mentioned changes occur via induction of ROS (Fig. 2, 3, and 4). Our results further indicate that Tax-ROS-caused DNA damage can lead to reactive cellular senescence in nontransformed human cells.
DNA damage induced by Tax and other oncoproteins has been documented previously (11, 31, 37). Earlier, it was suggested that such oncogene-induced DNA damage may have occurred indirectly through accelerated G1 cell cycle progression, which might in itself be mutagenic (11). Our current observation that Tax induces ROS is consistent with reports of ROS induction by Ras (20, 33, 38), c-Myc (37), and Epstein-Barr virus (EBV) EBNA-1 protein (13). Others have proposed that Myc and Ras may activate ROS through a mitogen-activated protein kinase (MAPK) pathway (37) and that EBNA-1 generates ROS through the NOX2 oxidase (13). We do not currently know how Tax triggers ROS, but on the basis of the results from the Tax S258A and L320G mutants, this activity likely requires the activation of both CREB and NF-κB. In addition to oxidases, cellular peroxiredoxins and thioredoxins also play roles in regulating ROS. In this regard, we note with interest that downregulation of peroxiredoxins (which increases cellular ROS) has been shown to induce NF-κB (15), which is highly activated in Tax-expressing cells (30). Further investigation is needed to understand more completely the details of how Tax influences the oxidation-reduction equilibrium in human cells, which could also involve antioxidative thioredoxin proteins (25). A more complete mechanistic insight into the redox balance in ATLL cells would be useful for potential development of anti-ROS agents that might interrupt HTLV-1 leukemogenesis.
Acknowledgments
We are grateful to Alicia Buckler-White and Ronald Plishka for sequence analysis. We thank Junichiro Yasunaga and Venkat Yedavalli for critical readings of the manuscript.
Work in K.-T.J.'s laboratory is supported through intramural funds from NIAID, NIH, and from the Intramural AIDS Targeted Antiviral Program (IATAP) from the office of the Director, NIH.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Afonso, P. V., A. Zamborlini, A. Saib, and R. Mahieux. 2007. Centrosome and retroviruses: the dangerous liaisons. Retrovirology 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, M. D., J. Ye, L. Xie, and P. L. Green. 2004. Transformation studies with a human T-cell leukemia virus type 1 molecular clone. J. Virol. Methods 116:195-202. [DOI] [PubMed] [Google Scholar]
- 3.Bartkova, J., N. Rezaei, M. Liontos, P. Karakaidos, D. Kletsas, N. Issaeva, L. V. Vassiliou, E. Kolettas, K. Niforou, V. C. Zoumpourlis, M. Takaoka, H. Nakagawa, F. Tort, K. Fugger, F. Johansson, M. Sehested, C. L. Andersen, L. Dyrskjot, T. Orntoft, J. Lukas, C. Kittas, T. Helleday, T. D. Halazonetis, J. Bartek, and V. G. Gorgoulis. 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444:633-637. [DOI] [PubMed] [Google Scholar]
- 4.Bonner, W. M., C. E. Redon, J. S. Dickey, A. J. Nakamura, O. A. Sedelnikova, S. Solier, and Y. Pommier. 2008. GammaH2AX and cancer. Nat. Rev. Cancer 8:957-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boxus, M., J. C. Twizere, S. Legros, J. F. Dewulf, R. Kettmann, and L. Willems. 2008. The HTLV-1 Tax interactome. Retrovirology 5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandhasin, C., R. I. Ducu, E. Berkovich, M. B. Kastan, and S. J. Marriott. 2008. Human T-cell leukemia virus type 1 Tax attenuates the ATM-mediated cellular DNA damage response. J. Virol. 82:6952-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke, M. S., M. D. Evans, M. Dizdaroglu, and J. Lunec. 2003. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 17:1195-1214. [DOI] [PubMed] [Google Scholar]
- 8.Di Micco, R., M. Fumagalli, and F. d'Adda di Fagagna. 2007. Breaking news: high-speed race ends in arrest—how oncogenes induce senescence. Trends Cell Biol. 17:529-536. [DOI] [PubMed] [Google Scholar]
- 9.Durkin, S. S., X. Guo, K. A. Fryrear, V. T. Mihaylova, S. K. Gupta, S. M. Belgnaoui, A. Haoudi, G. M. Kupfer, and O. J. Semmes. 2008. HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 283:36311-36320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, M. D., M. Dizdaroglu, and M. S. Cooke. 2004. Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. 567:1-61. [DOI] [PubMed] [Google Scholar]
- 11.Felsher, D. W., and J. M. Bishop. 1999. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 96:3940-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatza, M. L., T. Dayaram, and S. J. Marriott. 2007. Ubiquitination of HTLV-I Tax in response to DNA damage regulates nuclear complex formation and nuclear export. Retrovirology 4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruhne, B., R. Sompallae, D. Marescotti, S. A. Kamranvar, S. Gastaldello, and M. G. Masucci. 2009. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 106:2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeang, K. T., S. G. Widen, O. J. Semmes, and S. H. Wilson. 1990. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 15.Jin, D. Y., H. Z. Chae, S. G. Rhee, and K. T. Jeang. 1997. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J. Biol. Chem. 272:30952-30961. [DOI] [PubMed] [Google Scholar]
- 16.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 17.Kashanchi, F., and J. N. Brady. 2005. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene 24:5938-5951. [DOI] [PubMed] [Google Scholar]
- 18.Kinner, A., W. Wu, C. Staudt, and G. Iliakis. 2008. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 36:5678-5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo, Y. L., and C. Z. Giam. 2006. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 25:1741-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, A. C., B. E. Fenster, H. Ito, K. Takeda, N. S. Bae, T. Hirai, Z. X. Yu, V. J. Ferrans, B. H. Howard, and T. Finkel. 1999. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274:7936-7940. [DOI] [PubMed] [Google Scholar]
- 21.Majone, F., and K. T. Jeang. 2000. Clastogenic effect of the human T-cell leukemia virus type I Tax oncoprotein correlates with unstabilized DNA breaks. J. Biol. Chem. 275:32906-32910. [DOI] [PubMed] [Google Scholar]
- 22.Majone, F., R. Luisetto, D. Zamboni, Y. Iwanaga, and K. T. Jeang. 2005. Ku protein as a potential human T-cell leukemia virus type 1 (HTLV-1) Tax target in clastogenic chromosomal instability of mammalian cells. Retrovirology 2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majone, F., O. J. Semmes, and K. T. Jeang. 1993. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology 193:456-459. [DOI] [PubMed] [Google Scholar]
- 24.Marriott, S. J., and O. J. Semmes. 2005. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 24:5986-5995. [DOI] [PubMed] [Google Scholar]
- 25.Masutani, H., K. Hirota, T. Sasada, Y. Ueda-Taniguchi, Y. Taniguchi, H. Sono, and J. Yodoi. 1996. Transactivation of an inducible anti-oxidative stress protein, human thioredoxin by HTLV-I Tax. Immunol. Lett. 54:67-71. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270-280. [DOI] [PubMed] [Google Scholar]
- 27.Maynard, S., S. H. Schurman, C. Harboe, N. C. de Souza-Pinto, and V. A. Bohr. 2009. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 30:2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merling, R., C. Chen, S. Hong, L. Zhang, M. Liu, Y. L. Kuo, and C. Z. Giam. 2007. HTLV-1 Tax mutants that do not induce G1 arrest are disabled in activating the anaphase promoting complex. Retrovirology 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peloponese, J. M., Jr., K. Haller, A. Miyazato, and K. T. Jeang. 2005. Abnormal centrosome amplification in cells through the targeting of Ran-binding protein-1 by the human T cell leukemia virus type-1 Tax oncoprotein. Proc. Natl. Acad. Sci. U. S. A. 102:18974-18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peloponese, J. M., M. L. Yeung, and K. T. Jeang. 2006. Modulation of nuclear factor-kappaB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol. Res. 34:1-12. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan, E., M. Ward, X. Guo, S. S. Durkin, A. Sawyer, M. Vilela, C. Osgood, A. Pothen, and O. J. Semmes. 2008. Physical and in silico approaches identify DNA-PK in a Tax DNA-damage response interactome. Retrovirology 5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semmes, O. J., and K. T. Jeang. 1992. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J. Virol. 66:7183-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 34.Takatsuki, K. 2005. Discovery of adult T-cell leukemia. Retrovirology 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tominaga, K., E. Tominaga, M. J. Ausserlechner, and O. M. Pereira-Smith. 2010. The cell senescence inducing gene product MORF4 is regulated by degradation via the ubiquitin/proteasome pathway. Exp. Cell Res. 316:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripp, A., P. Banerjee, M. Sieburg, V. Planelles, F. Li, and G. Feuer. 2005. Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression. J. Virol. 79:14069-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vafa, O., M. Wade, S. Kern, M. Beeche, T. K. Pandita, G. M. Hampton, and G. M. Wahl. 2002. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell 9:1031-1044. [DOI] [PubMed] [Google Scholar]
- 38.Wu, C., I. Miloslavskaya, S. Demontis, R. Maestro, and K. Galaktionov. 2004. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432:640-645. [DOI] [PubMed] [Google Scholar]