Abstract
Poxvirus-based human immunodeficiency virus (HIV) vaccine candidates are currently under evaluation in preclinical and clinical trials. Modified vaccinia virus Ankara (MVA) vectors have excellent safety and immunogenicity records, but their behavior in human cell cultures remains only partly characterized. We studied here various virological and immunological aspects of the interactions of MVA-HIV, a vaccine candidate developed by the French National Agency for AIDS Research (ANRS), with primary human cells. We report that MVA-HIV infects and drives Gag expression in primary macrophages, dendritic cells (DCs), and epithelial and muscle cells. MVA-HIV-infected DCs matured, efficiently presented Gag, Pol, and Nef antigens, and activated HIV-specific cytotoxic T lymphocytes (CTLs). As expected with this type of vector, infection was cytopathic and led to DC apoptosis. Coculture of MVA-HIV-infected epithelial cells or myotubes with DCs promoted efficient Gag antigen major histocompatibility complex class I (MHC-I) cross-presentation without inducing direct infection and death of DCs. Antigen-presenting cells (APCs) infected with MVA-HIV also activated HIV-specific CD4+ T cells. Moreover, exposure of DCs to MVA-HIV or to MVA-HIV-infected myotubes induced type I interferon (IFN) production and inhibited subsequent HIV replication and transfer to lymphocytes. Altogether, these results show that MVA-HIV promotes efficient MHC-I and MHC-II presentation of HIV antigens by APCs without facilitating HIV replication. Deciphering the immune responses to MVA in culture experiments will help in the design of innovative vaccine strategies.
Early efforts to develop an anti-human immunodeficiency virus (anti-HIV) vaccine focused on the generation of HIV-specific humoral responses. For instance, recombinant gp120 was shown to rapidly induce neutralizing antibodies, which displayed a limited capacity to control replication of primary viral isolates (38, 41) and did not confer protection from infection (15, 48). However, recent studies identified new classes of neutralizing antibodies that may form the basis for future humoral immunity-based vaccine strategies (24, 53, 59). Vaccines promoting HIV-specific cellular responses have also been studied extensively for the past 2 decades. Multiple lines of evidence suggest that T cells participate in the control of HIV-1 replication. During acute infection, expansion of HIV-specific CD8+ T cells (HS CTLs) before the appearance of neutralizing antibodies is associated with decreased viremia (60). Resistance to disease progression correlates with the detection of Gag-specific CTLs and with the presence of particular HLA alleles, such as HLA-B57 and -B27 (32, 33). Viral escape mutants are found in infected individuals (31), underscoring the selection pressure exerted by CTLs. In addition, CD8+ T-cell depletion experiments in nonhuman primates (NHP) established the importance of these cells in controlling viral replication during acute and chronic phases of infection (34).
In NHP, vaccination with attenuated simian immunodeficiency virus (SIV) mutants confers near-complete protection from homologous challenge, providing proof of concept that development of an HIV vaccine is feasible (62). However, for obvious safety reasons, attenuated HIV strains are not currently under consideration. Recombinant viral vectors encoding HIV proteins or peptide strings, based on viruses such as poxviruses, adenoviruses, vesicular stomatitis virus, and measles virus, represent promising alternatives. These viruses infect various cells and tissues. In animals and human volunteers, they elicit specific cellular and humoral responses to foreign genes inserted in the vector genome. Moreover, their natural adjuvant effect may lead to secretion of appropriate cytokines and chemokines. Each type of viral vector induces distinct qualities of T-cell and antibody responses, depending on parameters such as viral tropism, fitness, and interplay with the innate immune system (2, 18, 25, 50).
Recombinant poxviruses, such as vaccinia virus (VACV), canarypox virus, and modified vaccinia virus Ankara (MVA), are among the most-studied vectors. The recent Thai phase III clinical trial combined, in a prime-boost strategy, a canarypox vector expressing HIV Gag, Pro, and Env antigens (Ags) and a trimeric recombinant gp120 protein. Preliminary results demonstrated a partial but significant protection against HIV infection in vaccinated individuals. This raises considerable interest in characterizing other poxviruses as vaccine candidates. MVA is a nonpersisting vector with excellent safety records. It is being considered for vaccination against infectious diseases, including HIV infection, malaria, and influenza, and against cancers (for a review, see reference 19). In preclinical studies, vaccination with an MVA vector encoding HIV-1 Gag linked to a string of CTL epitopes (so called MVA-HIVA), used alone or in combination with DNA in a prime-boost strategy, induced HS T cells (21, 63). Moreover, vaccination of NHP with MVA encoding SIV Gag, Pol, Nef, and Env induced a robust polyfunctional SIV-specific T-cell response leading to reduced viral replication and prolonged survival upon simian/human immunodeficiency virus (SHIV) challenge (40). Mucosal vaccination of NHP with MVA encoding SIV Gag, Pol, and Env also led to good control of SHIV replication, with a 2-log reduction in viral loads (1). The route of MVA inoculation influences the immunogenicity of the vector and the quality of the immune response (20, 37). MVA has been studied in cell culture experiments and in animal models (5, 10, 11, 35). MVA was generated by 500 passages of the Ankara strain of vaccinia virus, leading to deletion of multiple viral genes (39). It replicates in BHK cells and chicken embryo fibroblasts but does not perform a full replication cycle in other cell lines or primary cells (5). Viral expression is limited to early gene products and to inserted foreign genes. MVA vectors generally display a broad tropism, infecting a large panel of cell lines and antigen-presenting cells (APCs), and induce rapid cell death by apoptosis. Dendritic cells (DCs) are important targets of MVA infection (7, 13, 29). Upon MVA infection, DC morphology, gene expression profiles, and maturation state are modified (23). A comparison of canarypox virus and MVA vectors revealed differences in infectivity and antigen production by DC cultures that may influence immunogenicity in vivo (64). The pathways mediating innate immune sensing were recently characterized and shown to involve Toll-like receptor 2 (TLR2) to TLR6, the cytosolic sensor MDA5, and the NALP3 inflammasome (10). Much less is known about the sensitivity of primary epithelial and muscle cells to MVA infection. These cells are likely some of the first cells, along with DCs, that may be targeted by the vector after mucosal or intramuscular vaccination. The ability of MVA-infected cells to present or cross-present antigens to human CD4+ and CD8+ virus-specific lymphocytes, as well as the susceptibility of MVA-exposed DCs to subsequent HIV infection, remain largely unknown.
In the present work, we describe and characterize an MVA-Gag-Pol-Nef (MVA-HIV) vaccine candidate developed by the French National Agency for AIDS Research (ANRS). We first studied the tropism of MVA-HIV by using a panel of human primary cells and immortalized cell lines. We report that MVA-HIV preferentially infects APCs, myotubes, and epithelial cells. MVA-HIV infection is cytopathic. Infected cells present Gag-, Pol-, and Nef-derived epitopes and directly activate HS CD8+ cells. MVA-HIV-infected APCs also stimulate HS CD4+ T-cell clones. In addition, HIV antigens expressed by MVA-HIV-infected muscle and epithelial cells are cross-presented by DCs to stimulate HS CTLs. Finally, we show that exposure of DCs to MVA induces an antiviral state that inhibits HIV replication and transfer to T cells. Our results strongly suggest that the highly immunogenic properties of MVA vector are not associated with an enhancement of HIV replication.
MATERIALS AND METHODS
MVA-HIV vaccine candidate.
MVA-HIV was developed in partnership with the ANRS. The recombinant virus (MVATG17401) was manufactured by Transgène (Strasbourg, France) according to standard procedures (39). The recombinant HIV antigens include the full-length codon-optimized sequence of gag (encoding amino acids [aa] 1 to 512) fused with fragments from pol (encoding aa 172 to 219, 325 to 383, and 461 to 519) and nef (encoding aa 66 to 147 and 182 to 206) from the Bru/Lai isolate (Los Alamos database accession number K02013). To enhance further Gag expression, poly(C), poly(G) (longer than 3 nucleotides), and poly(GC) (longer than 8 nucleotides) motifs within gag were replaced. Using PCR, the gag-pol-nef sequence was then introduced into the transfer plasmid pTG17401. The recombinant virus vaccine (MVATG17401) expressing the gag-pol-nef fusion of HIV-1 was then produced by homologous recombination between pTG17401 and the virus (MVATGN33). The gag-pol-nef sequences were inserted into the excision III site of MVA by cloning using standard procedures (39). Briefly, after transfer of the plasmid to MVATGN33, selection of MVATG17401 clones was carried out by successive subcloning. Characterization was carried out on 100 clones after 6 passages. The structure and expression profiles of the clones were determined based on PCRs and Western blotting. By use of specific primers, the absence of the wild-type (WT) virus was shown in 100% of clones. Expression of HIV recombinant protein is under the control of the early-late vaccinia virus promoter p5HR (51).
MVA infection.
Cells were infected in serum-free medium for 1 h at the indicated multiplicity of infection (MOI) (ranging from 0.1 to 10), washed three times with serum-free medium, and resuspended in the appropriate medium for further use. MVA infection was monitored using anti-Gag p24 antibodies and Western blotting, fluorescence-activated cell sorting (FACS), or confocal microscopy. When required, virus was UV inactivated for 15 min by use of a 312-nm UV bulb positioned 4 cm over the sample (Fisher Bioblock-Scientific).
Cells.
Clinical-grade DCs were prepared using a VacCell processor as described previously (43). Peripheral blood mononuclear cells (PBMCs) were cultured for 7 days in serum-free medium (Invitrogen) with 500 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Gentaur) and 50 ng/ml interleukin-13 (IL-13; Peprotech, Tebu-bio), and DCs were isolated by elutriation. Alternatively, DCs were generated using 1,000 U/ml IL-4 (R&D) and 100 ng/ml GM-CSF (Gentaur) as described previously (44). Both isolation procedures yielded immature DCs (CD1a+ MHC-I+ MHC-II+ CD64− CD83− CD80low CD86low cells). HeLa cells, 293T cells, and primary human cells (Hsk-789.Sk cells, A549 cells, BEAS-2B cells, and MRC5 cells) were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS). Hsk-789.Sk cells are primary human skin cells (ATCC 7518 [American Type Culture Collection]). MRC5 cells (ATCC CCL-171) are primary human fetal lung epithelial cells. A549 cells (ATCC CCL-185) are human malignant alveolar type II pneumocytes, and BEAS-2B cells (ATCC CRL-9609) are simian virus 40 (SV40)-transformed human airway epithelial cells. PBMCs were isolated from the blood of healthy donors by Ficoll centrifugation. CD4+ T lymphocytes were isolated by negative selection, using magnetic beads (Miltenyi Biotec), and were cryopreserved. Where stated, CD4+ T lymphocytes were activated by phytohemagglutinin (PHA) and grown with recombinant human IL-2 (rhIL-2) for 7 days. HD420 and Bre cells are Epstein-Barr virus (EBV)-immortalized B cells (44). Jurkat T cells, MT4C5 cells, CEM cells, HUT cells, PBMCs, primary lymphocytes, and monocytes were grown in RPMI medium with 10% FBS. CD14+ monocytes were isolated from PBMCs by positive selection, using magnetic beads (Miltenyi Biotec). To allow differentiation of macrophages (Mφ), monocytes were cultured for 7 days in RPMI 1640 medium with 5% human AB− serum, 5% FBS, and rhuM-CSF (12.5 ng/ml; Promokine). CHQ5 primary myoblasts, originally isolated from the quadriceps of a 5-day-old infant (9), were cultured in Ham's F10 medium with 20% heat-inactivated FBS and 50 μg/ml gentamicin. Myotubes were differentiated from myoblasts after 5-day cultures in DMEM supplemented with 50 μg/ml gentamicin, 100 μg/ml transferrin, and 10 μg/ml insulin (9). CTLs specific for Gag (recognizing the HLA-A*02-restricted SL9 epitope p17 [aa 77 to 85]) (43), Pol (recognizing the HLA-A*02-restricted IV9 epitope [aa 476 to 484]; a kind gift from Florence Buseyne, Institut Pasteur) (43), and Nef (recognizing the HLA-A*11-restricted QK10 epitope [aa 73 to 82]; a kind gift from Michèle Février, Institut Pasteur) (16) and Gag-specific CD4+ T-cell clones (44) were cultured as described previously (16, 43, 44).
ELISPOT assays. (i) Direct presentation.
Stimulator cells were exposed to MVA-HIV, UV-inactivated MVA-HIV, or cognate peptides (1 μg/ml) as a positive control, washed, and incubated for 5 h in RPMI medium containing 10% FBS to allow virus internalization. Stimulator cells were then cocultured for at least 8 h with HS T-cell clones. Gamma interferon (IFN-γ) production was measured using enzyme-linked immunospot (ELISPOT) assays as described previously (43).
(ii) Cross-presentation.
Donor cells were infected with MVA-HIV for 1 h at an MOI of 1 or incubated with 1 μg/ml of SL9 peptide, washed extensively, seeded for 5 h, and then cocultured with DCs for 1 h. Donor and stimulator cells (DCs) were then cocultured for at least 8 h with HS T-cell clones, and IFN-γ production was measured by ELISPOT assays.
Surface and intracellular staining.
Cell surface staining was performed at 4°C for 30 min, using anti-HLA-A2 (BB7.2-fluorescein isothiocyanate [BB7.2-FITC]; BD Pharmingen), anti-CD4, anti-CD8 (BD Pharmingen), anti-CD14 (BD Pharmingen), anti-CD19 (BD Pharmingen), anti-CD56 (Becton Dickinson), anti-CD86-phycoerythrin (anti-CD86-PE) (BD Pharmingen), anti-CD80-allophycocyanin (anti-CD80-APC) (BD Pharmingen), anti-CD83-FITC (BD Pharmingen), anti-major histocompatibility complex class I-FITC (anti-MHC I-FITC) (W6.32-FITC; Sigma), and anti-DC-SIGN (120507-APC/-PE) (R&D) monoclonal antibodies (MAbs). Anti-HIV Gag (KC57-FITC; Beckman-Coulter) MAb was used to detect infected cells. Briefly, cells were fixed for 10 min with phosphate-buffered saline (PBS) plus 4% paraformaldehyde, washed, and permeabilized for 15 min in PBS containing 0.1% bovine serum albumin (BSA) and 0.05% saponin prior to Ab staining. Isotype-matched MAbs were used as negative controls. Samples were analyzed by flow cytometry, using a FACSCalibur (Becton Dickinson) or FacsCanto (Becton Dickinson) flow cytometer with CellQuest or FacsDIVA software.
Confocal imaging.
Myotubes or DCs were infected with MVA-HIV (MOI of 1 or 0.1, respectively) for 6, 15, or 24 h and fixed in 4% paraformaldehyde (10 min on ice). Cells were then stained with anti-desmin rabbit polyclonal Ab (Abcam) and goat anti-rabbit-DyLight-547 (Pierce) or with rhodamine-phalloidin (4 U/ml; Molecular Probes) and anti-HIV Gag (KC57-FITC; Beckman-Coulter) Abs in PBS-5% BSA plus 0.01% saponin. Samples were analyzed by confocal microscopy on a Zeiss LSM510 instrument.
Cell viability assays. (i) 7-AAD staining.
7-Aminoactinomycin D (7-AAD)-APC (BD Pharmingen) was used according to the manufacturer's instructions. In brief, cell pellets were resuspended in 100 μl PBS (107 cells/ml), and 5 μl of 7-AAD was added to the cell suspension. Cells were incubated for 10 min on ice in the dark. As a positive control, cells were treated with actinomycin D (1 μg/ml, 12 h, 37°C) (Sigma). Samples were analyzed by flow cytometry.
(ii) Trypan blue exclusion.
Myotubes were harvested from plates by use of PBS plus 1% EDTA and were incubated with trypan blue (5 min, room temperature). Trypan blue-negative cells (viable cells) were counted in a Bürker cell-counting chamber.
Analysis of HIV replication in DCs. (i) HIV infection.
The R5-tropic virus HIVNLAD8 was produced as described previously (44).
(ii) DCs directly exposed to MVA-HIV.
DCs were exposed to MVA-HIV for 1 h, washed extensively, loaded with HIVNL-AD8 at a low viral inoculum (1 ng of p24/ml) or high viral dose (100 ng of p24/ml) with DEAE-dextran (10 μg/ml; Sigma) for 2 to 3 h at 37°C, washed, and seeded in 96-well plates. HIV replication was measured using a Gag p24 enzyme-linked immunosorbent assay (ELISA; NEN, Perkin-Elmer Life Sciences). Alternatively, DCs exposed to MVA-HIV and loaded with a low HIV inoculum (1 ng/ml) were cocultured with autologous PHA-activated CD4+ T cells at a 1/1 ratio in 96-well plates. As a control, PHA-activated T cells alone were infected with HIVNLAD8 (1 ng/ml of p24), washed, and seeded in 96-well plates with rhIL-2, and HIV replication was monitored.
DCs exposed to MVA-infected cells.
A total of 2 × 105 HeLa cells or myotubes seeded in 6-well plates were infected with MVA-HIV for 1 h, washed extensively, rested for 5 h to allow MVA-HIV internalization, washed, and cocultured with 2 × 106 DCs. After overnight incubation, DCs were harvested by gentle pipetting, infected with HIVNLAD8 (high dose of 100 ng p24/ml and low dose of 1 ng p24/ml) for 2 h, and then seeded in 96 well-plates at 106 cells/ml. HIV production in culture supernatants was measured using Gag p24 ELISA. Alternatively, DCs exposed to MVA-HIV-infected cells and loaded with a low HIV inoculum (1 ng p24/ml) were cocultured with autologous PHA-activated CD4+ T cells at a 1/1 ratio in 96-well plates. As a control, PHA-activated T cells alone were infected with HIVNLAD8 (at the indicated concentrations), washed, and seeded in 96-well plates with rhIL-2, and HIV replication was measured using Gag p24 ELISA.
IFN-α detection.
IFN-α secretion was quantified using the reporter cell line HL116 (a kind gift from Sandra Pellegrini, Institut Pasteur, France). HL116 cells carry the luciferase gene under the control of the IFN-inducible 6-16 promoter (57). HL116 cells were grown in DMEM supplemented with 10% FBS and hypoxanthine-aminopterin-thymidine (HAT) (50 mM). A total of 20,000 HL116 cells, plated in a 96-well plate 24 h prior to the assay, were incubated for 7 h with the desired culture supernatants or standards containing a titration of human IFN-α2a (Immunotools). Cells were then lysed (luciferase cell culture lysis reagent [5×]; Promega), and luciferase activity was measured using luciferase assay reagent (Promega). Samples were analyzed using a Perkin Elmer Wallac 1420 instrument. IFN levels are expressed as equivalent IFN-α2a concentrations.
Western blot analysis of MVA-HIV-infected cells.
HeLa cells were infected with MVA, MVA-HIV (MOI of 0.1 for 1 h at 37°C), or HIVNL4-3 pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) (100 ng of p24/ml for 3 h at 37°C). Sixteen or 36 h after infection with MVA, MVA-HIV, or HIVNL4-3, cells were lysed in PBS-1% Triton X-100 (Sigma) supplemented with protease inhibitors (Roche). Fifteen micrograms of protein lysate was analyzed by SDS-PAGE, using 4 to 12% NuPage gels (Invitrogen). The blots were probed with an anti-Gag p24 MAb (25A) (46). Horseradish peroxidase (HRP)-conjugated goat anti-IgG (Amersham) was used as secondary antibody. Peroxidase activity was revealed using chemiluminescence (Pierce).
RESULTS
The MVA-HIV vaccine candidate from the ANRS.
The MVA-HIV vaccine candidate was designed by the ANRS. It encodes an HIV polyprotein from the clade B isolate Bru, composed of the full-length Gag protein fused to three Pol and two Nef fragments (see Materials and Methods for further details). Upon MVA-HIV infection of HeLa cells, the expected 97-kDa HIV polyprotein was readily detected by Western blotting (http://www.pasteur.fr/ip/easysite/go/03b-00003g-063/virus-and-immunity/supplemental-material). Additional species with lower molecular masses, likely corresponding to processed forms of the polypeptide, were also revealed (http://www.pasteur.fr/ip/easysite/go/03b-00003g-063/virus-and-immunity/supplemental-material). Flow cytometry and confocal imaging of MVA-HIV-infected cells stained with anti-Gag MAbs confirmed the expression of HIV proteins (Fig. 1).
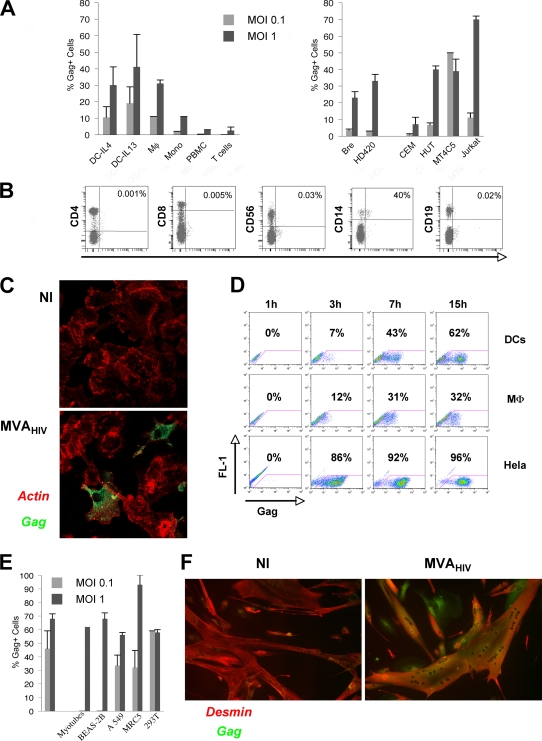
FIG. 1.
MVA-HIV preferentially infects APCs and vaccination target cells. (A) Gag expression in primary cells (left) or in cell lines (right) after MVA-HIV infection at the indicated MOI. At 15 h p.i., cells were stained for intracellular HIV Gag and analyzed by flow cytometry. DCs were differentiated in the presence of IL-4 or IL-13. The data represent the means ± standard deviations (SD) for at least three independent experiments. (B) PBMCs were infected with MVA-HIV (MOI = 1). At 15 h p.i., cells were stained for cell surface markers and intracellular HIV Gag and analyzed by flow cytometry. Uninfected PBMCs and isotype-matched Abs were used as negative controls (not shown). T cells, CD8+ and CD4+ cells; NK cells, CD56+ cells; monocytes, CD14+ cells; B cells, CD19+ cells. The percentages of Gag+ cells in the corresponding cell subsets are shown. The data are representative of three independent experiments. (C) DCs were infected with MVA-HIV (MOI = 0.1), and HIV Gag expression was further analyzed by immunofluorescence and confocal microscopy. Green, HIV Gag staining; red, actin-phalloidin-PE. NI, not infected. (D) Kinetics of HIV Gag expression in DCs, macrophages (MD-M), and HeLa cells analyzed as described for panel A. (E) HIV Gag expression in primary myotubes and epithelial cells 15 h after MVA-HIV infection (MOI = 1), assessed using flow cytometry and immunofluorescence for myotubes. The data represent the means ± SD for at least three independent experiments. (F) Myotubes were infected with MVA-HIV (MOI = 1), and HIV Gag expression was analyzed by immunofluorescence and confocal microscopy. Green, HIV Gag staining; red, anti-desmin MAb to identify myotubes. NI, not infected.
MVA-HIV infects APCs and muscle and epithelial cells.
We examined the susceptibility of various cell types to MVA-HIV infection. We first studied whether MVA-HIV infects and drives the expression of the recombinant protein in hematopoietic cells (Fig. 1). To this end, PBMCs were incubated with MVA-HIV at an MOI of 1. After 15 h, cells were stained with an anti-Gag MAb and analyzed by FACS. We observed a weak infection of whole PBMCs (3% Gag+ cells) (Fig. 1A). Double staining indicated that NK cells, CD4+ or CD8+ T lymphocytes, and B cells were marginally infected by MVA-HIV (<1% Gag+ cells), whereas CD14+ monocytes represented the majority of Gag-expressing cells (Fig. 1B). Purified CD14+ monocytes, as well as monocyte-derived Mφ and DCs, were highly susceptible to MVA infection (Fig. 1A, left panel). In DCs, confocal microscopy analysis showed a cytoplasmic and membrane-bound localization of Gag (Fig. 1C), resembling the pattern observed in HIV-infected cells. Gag expression was rapid in Mφ and DCs. It was detected as soon as 3 h postinfection (p.i.) and increased from 7 to 15 h p.i. (32 and 62% Gag+ cells for Mφ and DCs, respectively) (Fig. 1D). In contrast to primary lymphocytes, immortalized B- and T-cell lines were generally efficiently infected by MVA-HIV (Fig. 1A, right panel).
Overall, these results indicate that primary APCs are particularly sensitive to MVA infection, driving rapid expression of the vaccine antigens.
Current protocols for MVA vaccination include intramuscular, intradermal, and intramucosal applications. We thus examined the sensitivity of muscle cells and of epithelial cells to MVA-HIV infection. Primary muscle fibers (myotubes) derived from satellite muscle cells (myoblasts) were incubated with MVA-HIV. Infection was monitored with anti-Gag MAbs by use of confocal microscopy, since these cells are too large and adherent to be processed by flow cytometry. Remarkably, at 15 h p.i., up to 70% of myotubes expressed Gag (Fig. 1E and F). The epithelial cell lines 293T, HeLa, A549 (a pulmonary alveolar cell line), and BEAS-2B (a bronchial cell line), as well as primary lung fibroblasts (MRC5 cells), were efficiently infected, with 50 to 90% Gag-expressing cells (Fig. 1E). These results confirmed that MVA-HIV has a very large tropism, including relevant primary cells (myotubes and epithelial cells) that may be encountered by MVA upon injection.
MVA-HIV-infected cells present Gag, Nef, and Pol antigens.
We asked whether MVA-HIV-infected APCs present MHC-I- and MHC-II-restricted HIV-derived epitopes. To this end, DCs, Mφ, monocytes, or B cells were exposed to MVA-HIV for 1 h, washed, and seeded for 5 h to allow MVA internalization and antigen expression. Antigen presentation was revealed by overnight coculture of infected cells with HIV-specific (HS) CD8+ or CD4+ T-cell clones and was quantified by IFN-γ ELISPOT assay (Fig. 2). We first analyzed MHC-I antigen presentation by using the HS CD8 T-cell clone EM40-F21, derived from an HIV-infected patient and recognizing an immunodominant HLA-A*0201-restricted epitope from Gag p17 (SL9) (43). MVA-HIV-infected APCs induced a dose-dependent activation of EM40-F21 (Fig. 2B). At a high MOI, IFN-γ production by EM40-F21 was equivalent to that induced by the cognate peptide, added exogenously (Fig. 2B). MVA-HIV-infected DCs and Mφ efficiently activated the HS CTL clone EM40-F21, whereas whole PBMCs, primary monocytes, primary T cells, and HeLa cells were less potent (Fig. 2B). Furthermore, using Pol (aa 476 to 484)- and Nef (aa 73 to 82)-specific CTL lines restricted by HLA-A*0201 and HLA-A*11, respectively (16, 43), we demonstrated that MVA-HIV-infected B cells and DCs also present Pol and Nef epitopes (Fig. 2C and data not shown).
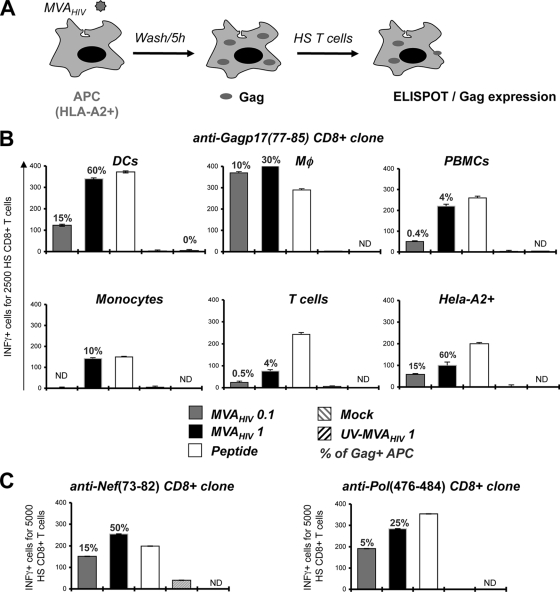
FIG. 2.
MVA-HIV-infected cells directly present antigens to HS CTLs. (A) Experimental procedure. APCs were loaded (1 h) with MVA-HIV, UV-inactivated MVA-HIV (at the indicated MOI), or peptide (1 μg/ml) or mock treated and then were washed, seeded for 5 h to allow HIV antigen expression, and cocultured for at least 8 h with HS T cells. T-cell activation was monitored by IFN-γ ELISPOT assay. (B) The CTL clone EM40-F21, specific for Gag, was used to test the capacity of diverse MVA-HIV-infected cell types to present HIV Gag-derived antigens. (C) MVA-HIV-infected APCs present Pol- and Nef-derived epitopes, leading to CTL activation. The CTL lines IV9 and P1, specific for Pol aa 476 to 484 and Nef aa 73 to 82, respectively, were cocultured with autologous B-cell lines, and T-cell activation was monitored by IFN-γ ELISPOT assay. Background IFN-γ production by target cells alone was subtracted and was at least three times lower than that with HS T cells. For each panel, data are means ± SD for triplicates and are representative of at least 2 independent experiments. Percentages of HIV Gag+ APCs, determined by flow cytometry at the end of the coculture period, are indicated.
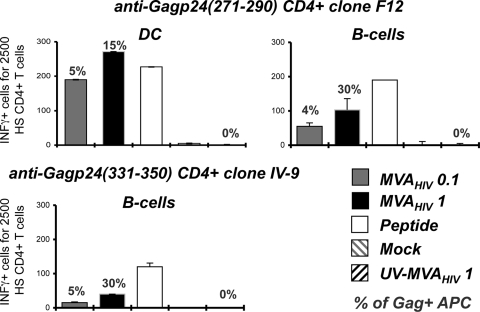
The HS CD4 T-cell clones F12 and IV-9 recognize two Gag-derived epitopes (aa 271 to 290 and aa 331 to 350, respectively) of p24, presented by HLA-DRβ*01 (44). MHC-II-restricted antigen presentation was studied using autologous primary monocyte-derived DCs or B-cell lines (HD420 and Bre). MVA-HIV-infected DCs and B cells activated F12 and IV-9 cells (Fig. 3). The HD420 B-cell line also expresses HLA-A2. We verified that HD420 activated the HS CD8 clone EM40-F21 upon MVA-HIV infection (not shown). Therefore, MVA-HIV-exposed APCs activate both HS CD4+ and CD8+ T cells.
FIG. 3.
MVA-HIV-infected cells directly present antigens to HS CD4+ T cells. As in Fig. 2, MVA-HIV-infected DCs and B cells were used to activate the HS CD4+ T-cell clone F12, which is specific for the Gag p24(271-290) amino acid sequence. MVA-HIV-infected B cells were used to stimulate the IV-9 clone, which is specific for Gag p24(331-350). T-cell activation was monitored by IFN-γ ELISPOT assay. Background IFN-γ production by target cells alone was subtracted and was at least three times lower than that with HS T cells. For each panel, data are means ± SD for triplicates and are representative of at least 2 independent experiments. Percentages of HIV Gag+ APCs, determined by flow cytometry at the end of the coculture period, are indicated.
Importantly, cells infected with wild-type MVA (not expressing any HIV antigens) did not stimulate EM40-F21 or HS CD4+ T-cell clones (not shown). UV-inactivated MVA-HIV lost viral infectivity (measured by the lack of appearance of Gag+ cells) and did not induce viral antigen presentation by DC and B cells (Fig. 2 and 3). Therefore, activation of EM40-F21 and HS CD4+ T-cell clones was not due to the presentation of incoming Gag antigens that may have been carried over with MVA-HIV virions. In agreement with these observations, the epithelial cell line HeLa-A2, which does not perform antigen cross-presentation (not shown), activated EM40-F21 cells when they were infected with MVA-HIV (Fig. 2B).
Altogether, these data demonstrate that APCs infected with MVA-HIV present HIV-derived epitopes through MHC-I and MHC-II molecules. Gag Ags are derived from neo-synthesized proteins. The capacity of MVA-HIV-infected cells to present Ag is not limited to professional APCs, thus highlighting the immunogenic potential of the vaccine candidate.
MVA-HIV stimulates DC maturation.
Upon Ag capture, immature DCs (imDCs) differentiate into a mature stage and acquire specific functions allowing Ag-specific T-cell activation. We investigated the effect of MVA-HIV on DC maturation. imDCs were exposed to MVA-HIV or treated with medium or lipopolysaccharide (LPS) as a control. DC maturation was analyzed by FACS 24 h later. As expected, LPS fully matured DCs, with an upregulation of CD83, the costimulatory molecules CD80 and CD86, and MHC-I and -II molecules (Fig. 4A). Interestingly, exposure of DCs to MVA-HIV also led to cell maturation (Fig. 4A). Only a fraction (30%) of the cells exhibited an intermediate maturation phenotype (see CD83 staining in Fig. 4A).
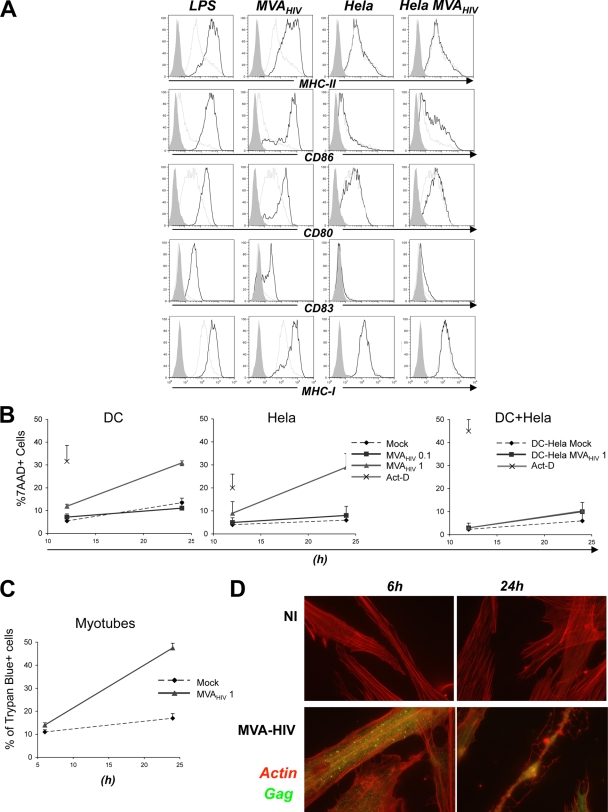
FIG. 4.
Effects of MVA-HIV on DC maturation and cell viability. (A) MVA-HIV promotes DC maturation. DCs were mock treated or infected with MVA-HIV (MOI of 1 for 1 h), washed, and incubated for 24 h in cell culture medium. As a positive control, DC maturation was induced using LPS (1 μg/ml; Sigma). For the coculture experiments, HeLa cells were infected with MVA-HIV (1 h), washed extensively, seeded for 6 h to allow MVA-HIV internalization, and cocultured with DCs for 24 h. DCs were then harvested, stained with the indicated Abs, and analyzed by FACS to monitor DC maturation. Histograms represent DC maturation within viable cells. Shaded histograms, isotype-matched Ab controls; gray lines, mock-treated imDCs; dark lines, DCs treated with the indicated stimuli. The data are representative of three independent experiments. (B) MVA-HIV induces apoptosis of infected cells. DCs and HeLa cells were infected with MVA-HIV at the indicated MOI (1 h), washed three times, and seeded in cell culture medium. Coculture experiments were performed as described for panel A. At the indicated time points, DCs exposed to MVA-HIV or MVA-HIV-infected HeLa cells were harvested and cell viability analyzed using 7-AAD and FACS. As a positive control for the induction of cell death, cells were treated with actinomycin D (Act-D; 1 μg/ml) for 12 h. Cell mortality is expressed as the percentage of 7-AAD+ cells (cells were gated to exclude cellular debris). Data are means ± SD for 3 independent experiments. (C) Primary myotubes were treated as in panel B, and cell viability was analyzed using trypan blue exclusion. Cell mortality is expressed as the percentage of trypan blue-positive cells. Data are means ± SD for duplicates and are representative of 2 independent experiments. (D) Primary myotubes used for panel C were stained intracellularly for HIV Gag and using actin-phalloidin-PE and were analyzed by confocal microscopy. Data are representative of 3 independent experiments.
DCs interact with infected cells to acquire antigens or to induce inflammation. We thus examined if MVA-infected cells induced DC activation. To this end, HeLa cells were exposed to MVA-HIV, washed thoroughly, incubated for 6 h to allow degradation of residual viral input and viral replication, and then cocultured with DCs. As expected, control, uninfected HeLa cells did not affect DCs (Fig. 4A). In contrast, MVA-HIV-infected HeLa cells induced partial DC maturation, characterized by a slight upregulation of CD83 and CD80 and a pronounced expression of CD86 (Fig. 4A). This maturation might be induced by the presence of apoptotic or dead MVA-HIV-infected HeLa cells or by the release of soluble factors by infected cells, as previously reported for canarypox virus-infected cells (27). DCs were apparently not infected after contact with MVA-HIV-infected HeLa cells, as assessed by the absence of Gag+ DCs (not shown).
Taken together, these results indicate that direct infection of DCs with MVA-HIV allowed full maturation of the APCs, whereas contact with MVA-infected cells induced an intermediate maturation phenotype.
MVA-HIV induces apoptosis of infected cells.
MVA is a cytopathic virus that induces apoptosis of infected cells (7, 23, 29, 64). We analyzed the viability of DCs, monocytes, B cells, and HeLa cells following MVA-HIV infection. Cells were infected at two MOIs, and viability was monitored by FACS, using 7-AAD (Fig. 4B and data not shown). As a positive control for induction of apoptosis, cells were treated with actinomycin D. At 24 h post-MVA-HIV infection with the high MOI, a significant proportion of the cells underwent apoptosis (30% of 7-AAD+ cells [Fig. 4B] or cells expressing active caspase-3 [not shown]). Interestingly, apoptosis was associated with direct infection of the cells, since coculture of DCs with MVA-HIV-infected HeLa cells did not induce significant DC mortality (Fig. 4B). Moreover, UV-treated MVA-HIV did not promote cell death (not shown).
We also studied the fate of muscle fibers after MVA-HIV infection (Fig. 4C). At 24 h p.i., 50% of infected cells were dying or dead, as measured by trypan blue exclusion. Confocal imaging indicated that infected fiber cells expressed Gag antigens at 6 h p.i. and then displayed, at 24 h p.i., membrane blebs and fragmented nuclei, well-known markers of apoptosis (Fig. 4D).
These results confirm and extend previous works by demonstrating that DCs as well as muscle fibers undergo apoptotic death upon MVA-HIV infection.
HIV-antigens expressed by MVA-HIV-infected cells are cross-presented by DCs.
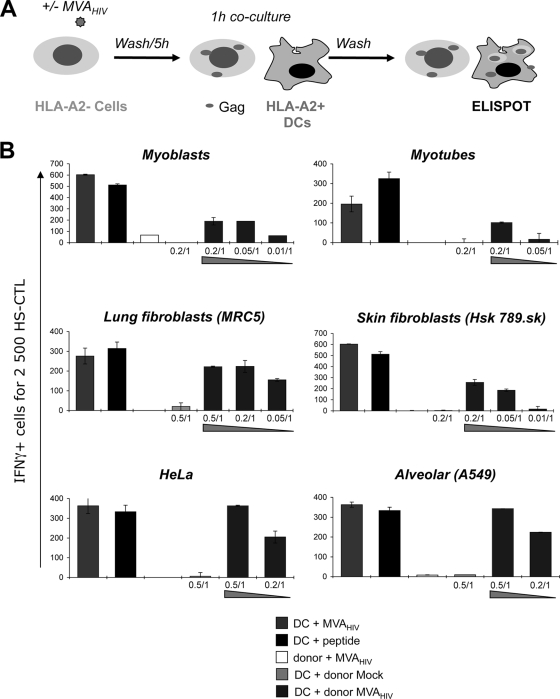
DCs display a unique capacity to take up antigens from apoptotic cells, leading to MHC-I-restricted cross-presentation. In vaccinated individuals, DCs will probably encounter MVA-infected cells. We asked whether MVA-HIV-infected cells might represent a source of antigen for cross-presentation to HS CTLs. For this purpose, HLA-A*02-negative cells (so-called donor cells) were exposed to MVA-HIV, washed extensively to remove unbound virus, and cultivated for 5 h to allow infection, potential internalization or degradation of incoming virions, and expression of HIV antigens (Fig. 5A). Infected cells were then cocultivated with HLA-A2*02+ DCs. Whatever the donor cells, we did not detect any Gag+ DCs, suggesting that no infectious MVA-HIV was transferred to DCs in the cocultures (not shown). Cross-presentation was then revealed in an IFN-γ ELISPOT assay after overnight coculture with HLA-A*02-restricted EM40-F21 CTLs. Primary skin (Hsk-789.Sk) and lung (MRC5) fibroblasts, primary myoblasts or myotubes, and a bronchiolar epithelial cell line (A549) were selected as donor cells, since they lack HLA-A*02 expression when tested by FACS (not shown). Accordingly, when infected by MVA-HIV, these HLA-A*02− donor cells did not directly activate EM40-F21 (Fig. 5). In contrast, coculture of these MVA-HIV-infected donor cells with HLA-A2+ DCs led to Gag antigen presentation and EM40-F21 stimulation. A dose-response analysis indicated that a ratio of 0.5 to 0.05 infected donor cell for 1 target DC was sufficient to activate EM40-F21 (Fig. 5B). Cross-presentation was detected with all donor cells tested (Fig. 5B). Notably, coculture of uninfected donor cells with HLA-A2+ DCs did not lead to HS CTL activation (Fig. 5B, DC + donor mock bars). HIV antigens expressed by MVA-HIV-infected HeLa cells were also cross-presented by HLA-A2+ DCs (Fig. 5B). Hence, HeLa cells are able to perform both direct (see our results with a HeLa-A2+ derivative cell line [Fig. 2]) and indirect (cross-presentation [Fig. 5]) activation of HS CTLs.
FIG. 5.
MVA-HIV-infected cells are cross-presented by DCs to HS CTLs. (A) Cross-presentation experimental procedure. HLA-A2− donor cells were mock treated or infected with MVA-HIV (MOI = 1, 1 h), washed, seeded for 5 h to allow Gag expression, and cocultured with HLA-A2+ DCs for 1 h. DCs were then harvested and incubated for at least 8 h with HS T cells. (B) HS T-cell activation monitored by IFN-γ ELISPOT assay. As a positive control, HLA-A2+ DCs were directly infected with MVA-HIV (MOI = 1) or loaded with the SL9 peptide (1 μg/ml). Importantly, in the absence of DCs, HLA-A2− donor cells infected with MVA-HIV (MOI = 1) cannot stimulate HS CTLs. Mock-treated or MVA-infected donor cell-to-DC ratios are indicated (donor cell/DC). Background IFN-γ production induced by uninfected cells and IFN-γ production by target cells alone (at least three times lower than that with HS CTLs) were subtracted. For each panel, data are means ± SD for duplicates or triplicates and are representative of 3 independent experiments.
Altogether, these results underline the immunogenic potential of MVA-HIV. Infected primary muscle fiber cells and primary fibroblasts likely represent an important source of antigens. Activation of immune cells by MVA-HIV may occur by different means. Infected cells may directly present antigens or be processed by DCs to activate CTLs.
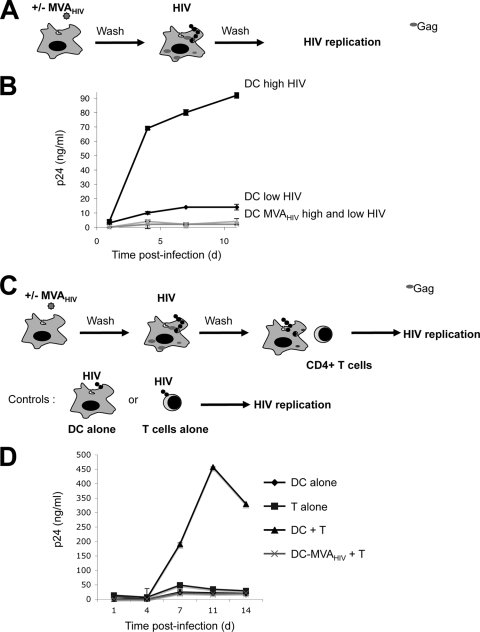
MVA-HIV-infected DCs do not efficiently spread HIV.
DCs are natural targets of HIV infection and efficiently transmit the virus to T lymphocytes. We analyzed the interactions that may exist between MVA infection of DCs and HIV replication. DCs were exposed to MVA-HIV for 1 h and then infected with HIV-1 at two MOIs. HIV replication was then monitored by measuring Gag p24 in culture supernatants by ELISA (Fig. 6A and B). In control DCs, HIV replication reached 90 and 13 ng p24/ml at day 10 p.i., at high and low MOIs, respectively (Fig. 6B). Interestingly, after DC exposure to MVA-HIV, HIV replication was barely detected (about 2 ng/ml at the high MOI) (Fig. 5B).
FIG. 6.
Exposure to MVA-HIV inhibits HIV replication in DC and DC-T-cell cocultures. (A) Impact of MVA-HIV on HIV replication in DCs. DCs were mock treated or incubated with MVA-HIV (1 h, MOI = 1), washed, and loaded with HIVNL-AD8 (2 h, high and low doses of 100 and 1 ng of HIV Gag p24/ml/106 cells, respectively), and HIV replication was monitored. (B) Kinetics of HIV replication in DC culture supernatants, assessed by HIV Gag p24 ELISA. (C) Impact of MVA-HIV on HIV transfer. DCs were treated as in panel A (HIVNL-AD8, 2 h, 1 ng of HIV Gag p24/ml/106 cells) and cocultured with activated autologous CD4+ T cells (1 DC/1 T cell). (D) Kinetics of HIV replication in DC-T-cell culture supernatants, assessed by HIV Gag p24 ELISA. As controls, DCs were cultured separately (DC alone) and CD4+ T cells were directly infected with the same HIV dose (T alone), and HIV replication was monitored. For each panel, data are means ± SD for duplicates and are representative of at least 3 independent experiments. d, day.
HIV is known to exploit the capacity of DCs to form intimate contacts with T cells to enhance its replication and to spread (44, 45, 56, 61). We thus examined the effect of MVA-HIV on HIV replication within DC-T-cell cocultures. To this end, DCs were exposed to MVA-HIV, loaded with HIV-1 (at a low MOI [1 ng p24/ml]), and then incubated with PHA-activated autologous CD4+ T cells (Fig. 6C and D). At this low MOI, HIV replicated poorly in DCs or in activated T cells cultured separately (with a peak of 25 and 48 ng of p24/ml at day 11 in DCs and T cells alone, respectively). In contrast, robust replication occurred in DC-T-cell cocultures, with a peak of 450 ng of p24/ml. Remarkably, when DCs were preexposed to MVA-HIV, there was a dramatic decrease of HIV replication in DC-T-cell cocultures (21 ng of p24/ml) (Fig. 6D).
Thus, MVA-HIV-infected DCs do not allow efficient HIV replication and transmission to T cells. This may be due in large part to the proapoptotic effects of MVA-HIV, which will reduce the number of viable DCs (Fig. 4). The fraction of dying DCs may also release apoptotic compounds that hamper HIV replication in bystander DCs, or MVA-HIV-exposed DCs may express antiviral factors (23).
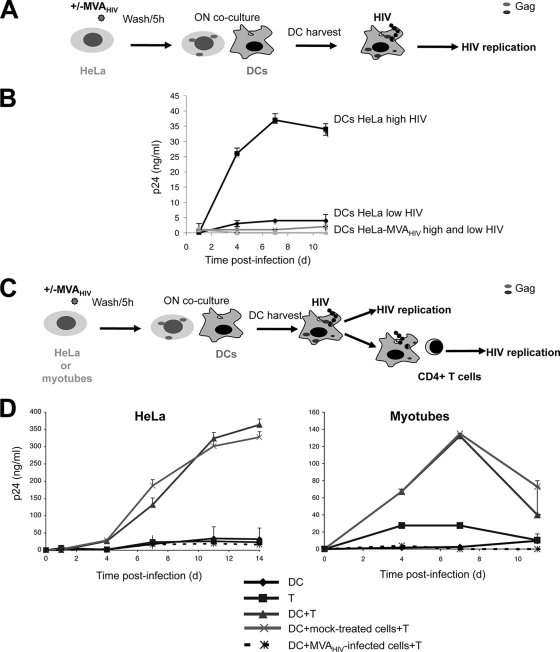
MVA-HIV-infected myotubes inhibit HIV replication in DC-T-cell cocultures.
We then examined whether MVA-HIV-infected bystander cells affect HIV replication in DCs and transfer to T cells. To this end, DCs were exposed to control or MVA-HIV-infected HeLa cells (Fig. 7A and B). Viability of DCs was not altered by contact with MVA-HIV-infected cells (Fig. 4). DCs were then exposed to two MOIs, and HIV replication was followed by measuring p24 release. MVA-HIV-infected HeLa cells inhibited HIV replication in DCs, whereas this was not the case with mock-treated HeLa cells (Fig. 7B).
FIG. 7.
Exposure to MVA-HIV-infected cells inhibits HIV replication in DCs and DC-T-cell cocultures. (A) Impact of MVA-HIV-infected cells on HIV replication in DCs. HeLa cells were infected with MVA-HIV (1 h, MOI = 1) (HeLa-MVA) or mock infected (HeLa), washed extensively, seeded (5 h) to allow MVA-HIV infection, and cocultured with DCs (1 HeLa cell for 10 DCs). After overnight (ON) coincubation, DCs were harvested by gentle pipetting and infected with HIVNL-AD8 (2 h, high and low doses of 100 and 1 ng of HIV Gag p24/ml/106 cells, respectively), and HIV replication was monitored. (B) Kinetics of HIV replication in DC culture supernatants, assessed by HIV Gag p24 ELISA. Data are means ± SD for duplicates and are representative of 2 independent experiments. (C) Impact of MVA-HIV-infected cells on HIV transfer. HeLa cells or primary myotubes were infected with MVA-HIV or mock infected and were cocultured with DCs as described for panel A. After overnight coincubation, DCs were harvested, infected with HIVNL-AD8 (2 h, 1 ng of HIV Gag p24/ml/106 cells), washed, and cocultured with activated autologous CD4+ T cells (1 DC to 1 T cell) in 96-well plates at 2 × 106 cells/ml. (D) Kinetics of HIV replication in DC-T-cell culture supernatants, assessed by HIV Gag p24 ELISA. As controls, DCs were cultured separately (DC) and CD4+ T cells were directly infected with the same HIV dose (T), and HIV replication was monitored. Data are means ± SD for duplicates and are representative of 3 independent experiments.
We then tested the effect of MVA-HIV-infected cells on HIV spread in DC-T-cell cocultures. HeLa cells or primary myotubes were infected with MVA-HIV for 1 h, washed extensively to remove unbound virus, cultivated for 5 h to allow degradation of incoming virions, and seeded with DCs. Cells were loaded with HIV for 2 h to allow viral capture by DCs and were then cocultured with activated CD4+ T cells, as outlined in Fig. 7C. A representative experiment with HeLa cells is depicted in Fig. 7D (left panel). In DC-T-cell cocultures in the absence of HeLa cells, HIV replication reached 350 ng of p24/ml, and the addition of mock-treated HeLa cells did not alter HIV replication. In sharp contrast, HIV replication was almost fully abrogated when MVA-HIV-infected HeLa cells were present. Similar results were obtained when MVA-HIV-infected myotubes were preadded to DC-T-cell cultures instead of HeLa cells (Fig. 7D, right panel). Exposure to MVA-HIV-infected cells did not induce apoptotic death of lymphocytes within DC-T-cell cocultures (not shown).
Altogether, these results demonstrate that once DCs have been in contact with MVA-HIV or MVA-HIV-infected cells, HIV does not efficiently replicate in DCs or spread to lymphocytes.
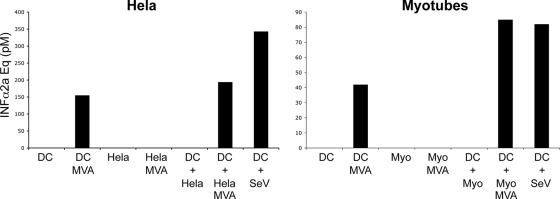
DCs exposed to MVA-HIV-infected myotubes secrete IFN-α.
Type I IFN and other IFN-inducible genes may be responsible for the anti-HIV state mediated by MVA-HIV or MVA-HIV-infected cells in DCs. MVA is indeed known to trigger IFN-α production in different cell types, such as MRC5 cells and DCs (5, 23). Interestingly, neither HeLa cells nor myotubes produced detectable levels of bioactive IFN-α when directly infected by MVA-HIV (Fig. 8). The situation was different with DCs. When these cells were infected with MVA-HIV or were cocultivated with MVA-HIV-infected HeLa cells or myotubes, they released significant levels of IFN-α, in the range of those obtained with Sendai virus (SeV), a well-characterized type I IFN inducer (Fig. 8).
FIG. 8.
DCs infected with MVA-HIV or exposed to MVA-HIV-infected cells secrete IFN-α. DCs, HeLa cells, and myotubes were directly infected with MVA-HIV (1 h, MOI = 1), washed, and cultured for 20 h, and IFN-α release in the culture supernatants was monitored. Alternatively, HeLa cells or myotubes (2 × 105 plated in 6-well plates) were infected with MVA-HIV (1 h, MOI = 1), washed extensively, seeded for 5 h to allow MVA-HIV internalization, and cocultured with DCs (2 × 106). After overnight coculture, supernatants were assessed for IFN-α. As a positive control, DCs were incubated with 4 U of hemagglutinin of SeV (55). IFN-α was quantified using a bioassay (HL116 reporter cell line) and is expressed in IFN-α2a concentration equivalents. For each sample, a serial dilution was performed and submitted to the bioassay. Data for one dilution are presented and are representative of 3 (left) and 2 (right) independent experiments.
These results show that DCs produce type I IFN when they encounter MVA-HIV-infected cells. This may restrict HIV replication and transfer in DCs.
DISCUSSION
The design of efficient candidate HIV vaccines requires a better knowledge of the mechanisms inducing appropriate immune responses. Attenuated viral vectors naturally stimulate systemic and mucosal immunity and represent attractive anti-HIV vaccine candidates. In the present work, we studied the capacity of an MVA vector coding for HIV antigens to infect primary human cells, to activate HS T-cell immunity, and to inhibit replication and transmission of HIV-1.
MVA is an attenuated strain of vaccinia virus that exhibits very limited replication in most mammalian cells (39). Traditional application routes include intramuscular and subcutaneous immunization, and alternative strategies targeting mucosal immunity are currently being developed (8, 30). We screened a panel of human primary and immortalized cells of various origins for susceptibility to MVA. We showed that muscle (primary myotubes and myoblasts) and epithelial cells are sensitive to MVA-HIV, driving the expression of the vaccine antigen. MVA infects primary lung fibroblast and alveolar and bronchial epithelial cell lines. Overall, we demonstrated that MVA infects primary cells relevant for intramuscular (myotubes), subcutaneous (epithelial cells), and intranasal (lung cells) immunization routes. We further characterized the fate of MVA-infected cells. As previously described for HeLa cells (23), we demonstrated that MVA-HIV induces apoptotic cell death, with morphological changes noticeable at 6 h postexposure, in most cell types tested. MVA is known to trigger early immigration of leukocytes at the site of infection (35). We thus tested the capacity of MVA-HIV to infect human PBMCs. Confirming previous work (52), we showed that among PBMCs, monocytes are the main targets of the vector. Moreover, monocyte-derived DCs and Mφ are particularly sensitive to MVA-HIV infection, suggesting that this may also be the case for primary blood DCs. As previously reported for murine cells (36), MVA-HIV induces phenotypic maturation of immature DCs, as well as apoptosis of a significant fraction of infected DCs. Interestingly, in contact with MVA-infected cells, DCs undergo phenotypic maturation without apoptosis. Hence, DCs attracted to the site of injection are potential targets for MVA infection and most likely undergo maturation, suggesting that MVA-HIV-infected DCs might efficiently prime CTL responses in vivo.
MVA-HIV was designed to induce HIV-specific T-cell responses. It is known that in naïve individuals, MVA primes CTL responses to the vector (vaccination to smallpox [49]) and to desired transgenes (HIV or tumor antigens [21, 54]). We assessed the impact of the vaccine candidate on T-cell activation in a cell culture system. We demonstrated that MVA-HIV-infected cells (monocytes, macrophages, and DCs) efficiently activate HIV Gag CTLs. Epitopes derived from the Nef and Pol segments of the HIV polyprotein are also presented by MVA-HIV-infected cells. Our results confirm and extend previous works showing that DCs infected with an MVA coding for a tumor antigen activate tumor-specific CTLs (12). They suggest that DCs are in part responsible for the transgene-specific responses generated in animal models and human vaccinees.
MVA-infected cells can directly present pathogen- or tumor-derived antigens to Ag-specific T cells. Additionally, fragments of infected cells may be engulfed by DCs, leading to cross-presentation of Ags to CTLs. Here we characterized the mechanisms of MVA-HIV antigen presentation leading to CTL activation. We observed that various MVA-HIV-infected cells, including HeLa-A2+ cells (which are unable to perform cross-presentation), directly present HIV antigens to HS CTLs. Moreover, MVA induces apoptotic cell death as early as 6 h postinfection (Fig. 3) (23). In DCs, this time scale allows activation of CTL clones in culture but might not be sufficient to prime T-cell responses in vivo. Accordingly, in a mouse model, MVA-infected DCs did not prime T-cell responses, whereas injection of MHC-deficient MVA-infected cells led to Ag-specific T-cell priming (17). This study and others suggest that cross-presentation dictates the immunogenicity of MVA vaccination (22, 36). In line with these observations, we demonstrated that primary human fibroblasts, myoblasts, and myotubes infected with MVA-HIV are cross-presented by DCs to HS CTLs. Therefore, DCs recruited to the site of MVA inoculation might sample MVA-infected dying cells and then mature and migrate to lymph nodes to activate Ag-specific T cells. Priming, expansion, and maintenance of CD8+ T-cell responses require CD4+ T-cell help (4, 14). We showed that MVA-HIV-infected APCs potently activated HS CD4+ T cells.
AIDS vaccine development is facing multiple challenges. The choice of immunogens and adjuvants able to induce broad-spectrum and long-lasting memory and protection is of paramount importance. Vaccination should be safe and well tolerated and should not enhance susceptibility to infection or pathogenesis. The Merck/NIH trial using an attenuated recombinant adenovirus 5 (rAd5) expressing HIV antigens as a vector was recently stopped based on the observation that the vaccine did not provide protection (3). Even worse, initial analysis of preclinical data indicated a significant trend toward increased HIV-1 acquisition among vaccinees who had high preexisting antibody titers to Ad5. A plausible explanation is that rAd5 vectors complexed to antibodies bind to Fc receptors, induce DC maturation, and favor HIV replication and spread to T cells (42, 47). However, further analysis of the preclinical outcomes of the STEP study, presented at the AIDS Vaccine 2009 meeting, suggested that the initial weak association of Ad5 seropositivity with increased HIV acquisition was no longer valid, except perhaps among uncircumcised men (6). Whatever the outcomes, this study highlighted the importance of preexisting immunity to vaccine vectors as a potential risk associated with vaccination. Vaccine-induced enhancement of viral infection is a major problem observed not only with lentiviruses but also with flaviviruses, coronaviruses, and paramyxoviruses (26).
Preexisting immunity to MVA is rarely observed in the population because of the end of smallpox vaccine campaigns after the 1970s. This should reduce the risk of adverse effects generated by the presence of anti-MVA antibodies. On the other hand, the activation of CD4+ T cells induced by vaccination may create a milieu facilitating HIV infection. HIV is indeed known to exploit the capacity of DCs to form intimate contacts with T cells to spread to activated CD4+ T cells (44, 45, 56, 61). However, we demonstrated here that MVA-HIV-infected DCs do not allow HIV replication and transfer to lymphocytes. More importantly, exposure of DCs to MVA-HIV-infected epithelial cells and myotubes also inhibited HIV spread in DC-T-cell cultures. The underlying mechanism remains to be elucidated but probably involves type I IFN activity. We indeed showed that DCs infected with MVA or encountering MVA-infected cells produced significant levels of type I IFNs. IFN-α enhances the expression of innate cellular factors, such as APOBEC3G, Trim5α, and tetherin, that restrict different steps of the viral life cycle. Exogenously added IFN-α also inhibits HIV replication (28) and limits HIV cell-to-cell spread (58). Determining further whether IFNs directly impact HIV replication and spread will require further investigation with blocking anti-IFN or anti-IFN-receptor antibodies. A recent report demonstrated that in addition to IFNs, MVA-infected DCs secrete cytokines and chemokines, including RANTES, macrophage inflammatory protein 1α (MIP-1α), IL-6, and tumor necrosis factor (TNF) (10). It is tempting to speculate that IFNs and some of these cytokines create an antiviral state in DC-T-cell cultures, limiting further HIV infection. It will be worthwhile to determine more precisely whether MVA-HIV-infected HeLa cells or myotubes release additional cytokines or chemokines or other components, such as apoptotic debris, which may impact the sensitivity of DCs to HIV infection. This observation has important implications in vivo. Hopefully, the probability that MVA-vaccinated humans simultaneously or rapidly encounter HIV is not very high. However, these results suggest that exposure to HIV shortly after MVA-HIV vaccination might not facilitate HIV infection.
In sum, our work reveals novel aspects of the interaction between MVA and primary human cells, which is of interest for understanding poxvirus infection and for improving poxvirus-based vaccine approaches. This preclinical study also shows that the MVA-HIV vector displays important characteristics expected to induce HIV-specific cellular responses in vaccinees. It should be used in clinical trials in the near future, either alone or in a prime-boost regimen with other immunogens (such as lipopeptides or DNA).
Acknowledgments
We thank Anne de Saunière, Helen K. W. Law, and the Centre d'Immunologie Humaine for assistance and Florence Buseyne, Michèle Février, Yves Rivière, and the NIH AIDS Research and Reference Reagent Program for kind gifts of reagents. We acknowledge V. Mouly and the Human Cell Culture Platform of the Myology Institute, Paris, for providing the myoblasts.
S.B. is a fellow of the Agence Nationale de Recherche sur le SIDA (ANRS). This work was supported by grants from the ANRS HIV Vaccine Network (AHVN) and the Institut Pasteur.
The authors have no conflicting financial interests.
S.B. designed and performed experiments, A.L., M.D., F.G.-B., and P.-E.C. performed experiments and provided material, Y.L. supervised the study, and O.S. and A.M. designed experiments, supervised the study, and wrote the paper.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan, M. J. 2004. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4:595-602. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. [DOI] [PubMed] [Google Scholar]
- 6.Buchbinder, S., M. Robertson, and A. Duerr. 2009. Clinical outcomes from the STEP study, abstr. SS01-02:18. AIDS Vaccine 2009 Meet.
- 7.Chahroudi, A., D. A. Garber, P. Reeves, L. Liu, D. Kalman, and M. B. Feinberg. 2006. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J. Virol. 80:8469-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett, M., W. M. Bogers, J. L. Heeney, S. Gerber, C. Genin, A. Didierlaurent, H. Oostermeijer, R. Dubbes, G. Braskamp, S. Lerondel, C. E. Gomez, M. Esteban, R. Wagner, I. Kondova, P. Mooij, S. Balla-Jhagjhoorsingh, N. Beenhakker, G. Koopman, S. van der Burg, J. P. Kraehenbuhl, and A. Le Pape. 2008. Aerosol immunization with NYVAC and MVA vectored vaccines is safe, simple, and immunogenic. Proc. Natl. Acad. Sci. U. S. A. 105:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decary, S., V. Mouly, C. B. Hamida, A. Sautet, J. P. Barbet, and G. S. Butler-Browne. 1997. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum. Gene Ther. 8:1429-1438. [DOI] [PubMed] [Google Scholar]
- 10.Delaloye, J., T. Roger, Q. G. Steiner-Tardivel, D. Le Roy, M. Knaup Reymond, S. Akira, V. Petrilli, C. E. Gomez, B. Perdiguero, J. Tschopp, G. Pantaleo, M. Esteban, and T. Calandra. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Didierlaurent, A., J. C. Ramirez, M. Gherardi, S. C. Zimmerli, M. Graf, H. A. Orbea, G. Pantaleo, R. Wagner, M. Esteban, J. P. Kraehenbuhl, and J. C. Sirard. 2004. Attenuated poxviruses expressing a synthetic HIV protein stimulate HLA-A2-restricted cytotoxic T-cell responses. Vaccine 22:3395-3403. [DOI] [PubMed] [Google Scholar]
- 12.Drexler, I., E. Antunes, M. Schmitz, T. Wolfel, C. Huber, V. Erfle, P. Rieber, M. Theobald, and G. Sutter. 1999. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 59:4955-4963. [PubMed] [Google Scholar]
- 13.Drillien, R., D. Spehner, and D. Hanau. 2004. Modified vaccinia virus Ankara induces moderate activation of human dendritic cells. J. Gen. Virol. 85:2167-2175. [DOI] [PubMed] [Google Scholar]
- 14.Estcourt, M. J., A. J. McMichael, and T. Hanke. 2005. Altered primary CD8+ T cell response to a modified virus Ankara(MVA)-vectored vaccine in the absence of CD4+ T cell help. Eur. J. Immunol. 35:3460-3467. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, S., M. Fevrier, G. Dadaglio, H. Lecoeur, Y. Riviere, and M. L. Gougeon. 1997. Potential deleterious effect of anti-viral cytotoxic lymphocyte through the CD95 (FAS/APO-1)-mediated pathway during chronic HIV infection. Immunol. Lett. 57:53-58. [DOI] [PubMed] [Google Scholar]
- 17.Gasteiger, G., W. Kastenmuller, R. Ljapoci, G. Sutter, and I. Drexler. 2007. Cross-priming of cytotoxic T cells dictates antigen requisites for modified vaccinia virus Ankara vector vaccines. J. Virol. 81:11925-11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaucher, D., R. Therrien, N. Kettaf, B. R. Angermann, G. Boucher, A. Filali-Mouhim, J. M. Moser, R. S. Mehta, D. R. Drake III, E. Castro, R. Akondy, A. Rinfret, B. Yassine-Diab, E. A. Said, Y. Chouikh, M. J. Cameron, R. Clum, D. Kelvin, R. Somogyi, L. D. Greller, R. S. Balderas, P. Wilkinson, G. Pantaleo, J. Tartaglia, E. K. Haddad, and R. P. Sekaly. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 205:3119-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gherardi, M. M., and M. Esteban. 2005. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 86:2925-2936. [DOI] [PubMed] [Google Scholar]
- 20.Gherardi, M. M., E. Perez-Jimenez, J. L. Najera, and M. Esteban. 2004. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J. Immunol. 172:6209-6220. [DOI] [PubMed] [Google Scholar]
- 21.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, I. Cebere, A. Mahmoud, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, J. De Bont, C. Verlinde, D. Vooijs, C. Schmidt, M. Boaz, J. Gilmour, P. Fast, L. Dorrell, T. Hanke, and A. J. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greiner, S., J. Y. Humrich, P. Thuman, B. Sauter, G. Schuler, and L. Jenne. 2006. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clin. Exp. Immunol. 146:344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra, S., J. L. Najera, J. M. Gonzalez, L. A. Lopez-Fernandez, N. Climent, J. M. Gatell, T. Gallart, and M. Esteban. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J. Virol. 81:8707-8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigwood, N. L., and V. M. Hirsch. 2009. Blocking and tackling HIV. Nat. Med. 15:841-842. [DOI] [PubMed] [Google Scholar]
- 25.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037-1045. [DOI] [PubMed] [Google Scholar]
- 26.Huisman, W., B. E. Martina, G. F. Rimmelzwaan, R. A. Gruters, and A. D. Osterhaus. 2009. Vaccine-induced enhancement of viral infections. Vaccine 27:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignatius, R., M. Marovich, E. Mehlhop, L. Villamide, K. Mahnke, W. I. Cox, F. Isdell, S. S. Frankel, J. R. Mascola, R. M. Steinman, and M. Pope. 2000. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J. Virol. 74:11329-11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpov, A. V. 2001. Endogenous and exogenous interferons in HIV-infection. Eur. J. Med. Res. 6:507-524. [PubMed] [Google Scholar]
- 29.Kastenmuller, W., I. Drexler, H. Ludwig, V. Erfle, C. Peschel, H. Bernhard, and G. Sutter. 2006. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 350:276-288. [DOI] [PubMed] [Google Scholar]
- 30.Kastenmuller, W., G. Gasteiger, L. Stross, D. H. Busch, and I. Drexler. 2009. Cutting edge: mucosal application of a lyophilized viral vector vaccine confers systemic and protective immunity toward intracellular pathogens. J. Immunol. 182:2573-2577. [DOI] [PubMed] [Google Scholar]
- 31.Kawashima, Y., K. Pfafferott, J. Frater, P. Matthews, R. Payne, M. Addo, H. Gatanaga, M. Fujiwara, A. Hachiya, H. Koizumi, N. Kuse, S. Oka, A. Duda, A. Prendergast, H. Crawford, A. Leslie, Z. Brumme, C. Brumme, T. Allen, C. Brander, R. Kaslow, J. Tang, E. Hunter, S. Allen, J. Mulenga, S. Branch, T. Roach, M. John, S. Mallal, A. Ogwu, R. Shapiro, J. G. Prado, S. Fidler, J. Weber, O. G. Pybus, P. Klenerman, T. Ndung'u, R. Phillips, D. Heckerman, P. R. Harrigan, B. D. Walker, M. Takiguchi, and P. Goulder. 2009. Adaptation of HIV-1 to human leukocyte antigen class I. Nature 458:641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769-775. [DOI] [PubMed] [Google Scholar]
- 33.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 34.Koff, W. C., P. R. Johnson, D. I. Watkins, D. R. Burton, J. D. Lifson, K. J. Hasenkrug, A. B. McDermott, A. Schultz, T. J. Zamb, R. Boyle, and R. C. Desrosiers. 2006. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 7:19-23. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann, M. H., W. Kastenmuller, J. D. Kandemir, F. Brandt, Y. Suezer, and G. Sutter. 2009. Modified vaccinia virus Ankara triggers chemotaxis of monocytes and early respiratory immigration of leukocytes by induction of CCL2 expression. J. Virol. 83:2540-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, L., R. Chavan, and M. B. Feinberg. 2008. Dendritic cells are preferentially targeted among hematolymphocytes by modified vaccinia virus Ankara and play a key role in the induction of virus-specific T cell responses in vivo. BMC Immunol. 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahe, B., A. Vogt, C. Liard, D. Duffy, V. Abadie, O. Bonduelle, A. Boissonnas, W. Sterry, B. Verrier, U. Blume-Peytavi, and B. Combadiere. 2009. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J. Invest. Dermatol. 129:1156-1164. [DOI] [PubMed] [Google Scholar]
- 38.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, and D. S. Burke. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 40.Mooij, P., S. S. Balla-Jhagjhoorsingh, G. Koopman, N. Beenhakker, P. van Haaften, I. Baak, I. G. Nieuwenhuis, I. Kondova, R. Wagner, H. Wolf, C. E. Gomez, J. L. Najera, V. Jimenez, M. Esteban, and J. L. Heeney. 2008. Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates. J. Virol. 82:2975-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas III, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, J. P., P. J. Klasse, M. J. Dolan, and S. K. Ahuja. 2008. AIDS/HIV. A STEP into darkness or light? Science 320:753-755. [DOI] [PubMed] [Google Scholar]
- 43.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 44.Moris, A., A. Pajot, F. Blanchet, F. Guivel-Benhassine, M. Salcedo, and O. Schwartz. 2006. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108:1643-1651. [DOI] [PubMed] [Google Scholar]
- 45.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nobile, C., D. Rudnicka, M. Hasan, N. Aulner, F. Porrot, C. Machu, O. Renaud, M. C. Prevost, C. Hivroz, O. Schwartz, and N. Sol-Foulon. 2010. HIV-1 Nef inhibits ruffles, induces filopodia and modulates migration of infected lymphocytes. J. Virol. 84:2282-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perreau, M., G. Pantaleo, and E. J. Kremer. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 205:2717-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 49.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Querec, T. D., R. S. Akondy, E. K. Lee, W. Cao, H. I. Nakaya, D. Teuwen, A. Pirani, K. Gernert, J. Deng, B. Marzolf, K. Kennedy, H. Wu, S. Bennouna, H. Oluoch, J. Miller, R. Z. Vencio, M. Mulligan, A. Aderem, R. Ahmed, and B. Pulendran. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosel, J. L., P. L. Earl, J. P. Weir, and B. Moss. 1986. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J. Virol. 60:436-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Puig, J. M., L. Sanchez, G. Roy, and R. Blasco. 2004. Susceptibility of different leukocyte cell types to vaccinia virus infection. Virol. J. 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheid, J. F., H. Mouquet, N. Feldhahn, M. S. Seaman, K. Velinzon, J. Pietzsch, R. G. Ott, R. M. Anthony, H. Zebroski, A. Hurley, A. Phogat, B. Chakrabarti, Y. Li, M. Connors, F. Pereyra, B. D. Walker, H. Wardemann, D. Ho, R. T. Wyatt, J. R. Mascola, J. V. Ravetch, and M. C. Nussenzweig. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636-640. [DOI] [PubMed] [Google Scholar]
- 54.Smith, C. L., P. R. Dunbar, F. Mirza, M. J. Palmowski, D. Shepherd, S. C. Gilbert, P. Coulie, J. Schneider, E. Hoffman, R. Hawkins, A. L. Harris, and V. Cerundolo. 2005. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int. J. Cancer 113:259-266. [DOI] [PubMed] [Google Scholar]
- 55.Strahle, L., D. Garcin, and D. Kolakofsky. 2006. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology 351:101-111. [DOI] [PubMed] [Google Scholar]
- 56.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 57.Uze, G., S. Di Marco, E. Mouchel-Vielh, D. Monneron, M. T. Bandu, M. A. Horisberger, A. Dorques, G. Lutfalla, and K. E. Mogensen. 1994. Domains of interaction between alpha interferon and its receptor components. J. Mol. Biol. 243:245-257. [DOI] [PubMed] [Google Scholar]
- 58.Vendrame, D., M. Sourisseau, V. Perrin, O. Schwartz, and F. Mammano. 2009. Partial inhibition of HIV replication by type-I interferons: impact of cell-to-cell viral transfer. J. Virol. 83:10527-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, L. M., S. K. Phogat, P. Y. Chan-Hui, D. Wagner, P. Phung, J. L. Goss, T. Wrin, M. D. Simek, S. Fling, J. L. Mitcham, J. K. Lehrman, F. H. Priddy, O. A. Olsen, S. M. Frey, P. W. Hammond, G. Miiro, J. Serwanga, A. Pozniak, D. McPhee, O. Manigart, L. Mwananyanda, E. Karita, A. Inwoley, W. Jaoko, J. Dehovitz, L. G. Bekker, P. Pitisuttithum, R. Paris, S. Allen, S. Kaminsky, T. Zamb, M. Moyle, W. C. Koff, P. Poignard, and D. R. Burton. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Y. E., B. Li, J. M. Carlson, H. Streeck, A. D. Gladden, R. Goodman, A. Schneidewind, K. A. Power, I. Toth, N. Frahm, G. Alter, C. Brander, M. Carrington, B. D. Walker, M. Altfeld, D. Heckerman, and T. M. Allen. 2009. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J. Virol. 83:1845-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, L., and V. N. KewalRamani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, H., T. Dong, E. Turnbull, S. Ranasinghe, B. Ondondo, N. Goonetilleke, N. Winstone, K. di Gleria, P. Bowness, C. Conlon, P. Borrow, T. Hanke, A. McMichael, and L. Dorrell. 2007. Broad TCR usage in functional HIV-1-specific CD8+ T cell expansions driven by vaccination during highly active antiretroviral therapy. J. Immunol. 179:597-606. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, X., F. Cassis-Ghavami, M. Eller, J. Currier, B. M. Slike, X. Chen, J. Tartaglia, M. Marovich, and P. Spearman. 2007. Direct comparison of antigen production and induction of apoptosis by canarypox virus- and modified vaccinia virus Ankara-human immunodeficiency virus vaccine vectors. J. Virol. 81:7022-7033. [DOI] [PMC free article] [PubMed] [Google Scholar]