Abstract
The relevance of translational control in the gene expression and oncotropism of the autonomous parvoviruses was investigated with MVMp, the prototype strain of minute virus of mice (MVM), infecting normal and transformed rodent and human cells of different tissue origins. Mouse embryo fibroblasts (MEFs) and NIH 3T3 fibroblasts were resistant to MVMp infection, but 3T3 fibroblasts derived from double-stranded RNA (dsRNA)-dependent protein kinase R (PKR) knockout mice (PKRo/o) behaved in a manner that was highly permissive to productive MVMp replication. NIH 3T3 resistance correlated with significant phosphorylation of eukaryotic translation initiation factor 2 (eIF2) occurring at early time points after infection. Permissive PKRo/o cells were converted to MVMp-restrictive cells after reintroduction of the PKR gene by transfection. Conversely, regulated expression of the vaccinia virus E3 protein, a PKR inhibitor, in MEFs prevented eIF2α phosphorylation and increased MVMp protein synthesis. In vitro-synthesized genome-length R1 mRNA of MVMp was a potent activator of PKR. Virus-resistant primary MEFs and NIH 3T3 cells responded to MVMp infection with significant increases in eIF2α phosphorylation. In contrast, virus-permissive mouse (PKRo/o, BHK21, and A9) and human transformed (NB324K fibroblast, U373 glioma, and HepG2 hepatoma) cells consistently showed no significant increase in the level of eIF2α phosphorylation following MVMp infection. The synthesis of the viral NS1 protein was inversely correlated with the steady-state PKR levels. Our results show that the PKR-mediated antiviral response is an important mechanism for control of productive MVMp infection, and its impairment in human transformed cells allowed efficient MVMp gene expression. PKR translational control may therefore contribute to the oncolysis of MVMp and other autonomous parvoviruses.
The susceptibility of a given cell to a virus infection is not exclusively restricted to the presence of a virus receptor at the cell surface. Intracellular events, such as the accessibility of viral mRNA to the host translation machinery, may have great influence on the outcome of virus replication (3, 14, 33, 53). Thus, viruses have developed strategies for improving the translation of their mRNAs, either by increasing the affinity of viral mRNAs for the ribosome or by lowering the dependence of viral mRNA on initiation factors involved in the recruitment of mRNA to the ribosome (11, 66).
Translation of viral mRNAs must also overcome the antiviral barriers imposed on this process by the host cells. The double-stranded RNA (dsRNA)-activated protein kinase R (PKR) protein plays a central role in this process, as it is capable of detecting dsRNA species generated by replication of RNA viruses and by transcription of some DNA-containing viruses (3, 4, 26, 30, 35, 46). PKR can be specifically activated in vitro by dsRNA but not by RNA-DNA hybrids or DNA (7, 9, 42, 45). Active PKR phosphorylates the alpha subunit of initiation factor 2 (eIF2α), leading to inhibition of translation initiation and thus aborting virus multiplication. Phosphorylation of eIF2α at the Ser51 residue prevents eIF2 from forming the ternary complex with GTP and the initiator Met-tRNA, a tightly regulated critical step in translation initiation (21, 22, 30). The role of PKR as an antiviral sentinel became apparent when increased susceptibility to RNA virus infection was observed in cultured cells or animals devoid of this kinase (4, 70, 80).
Viruses of several families have developed different strategies for preventing or bypassing PKR activation, such as inhibiting PKR phosphorylation by direct binding (as shown by the hepatitis C virus NS5A protein) (29), binding to the RNA binding domain of PKR (as shown by adenovirus type 5 [Ad5] VA I RNA) (39), and sequestering dsRNA (as shown by the NS1 influenza virus protein) (44). Accordingly, the effect of PKR on virus replication is clearly manifested when the various gene products that inhibit its activation are genetically ablated (41, 76, 79, 82). However, little is known about the effect of the PKR/eIF2α system on infection of simple DNA viruses with limited coding capacity.
The relevance of translation control in the Parvoviridae, a family of small viruses containing a linear single-stranded DNA (ssDNA) genome of approximately 5,000 nucleotides (8, 38), has been recognized only recently. In adeno-associated virus type 5 (AAV5), a member of the Dependovirus genus that requires a helper virus to complete the infection cycle, viral replication and protein synthesis were enhanced by coexpression of an adenovirus VA I RNA (52) that binds to PKR (51), preventing its activation, which is mediated by a short RNA sequence of AAV5 (51). Whether translation control of viral mRNA and sensitivity to PKR activation are exclusive characteristics of the dependoviruses or may also apply to the other members of the Parvoviridae whose replication is not dependent on a helper virus remains unknown.
In the Parvovirus genus, the genome of the type species minute virus of mice (MVM) is organized as two temporally regulated, overlapping transcription units (15). The left-hand gene, driven by the P4 promoter, encodes the nonstructural NS1 and NS2 proteins, and the right-hand gene, driven by the P38 promoter, encodes the structural VP1 and VP2 proteins (reviewed in reference 8), which assemble into capsids in the nucleus (59). The NS polypeptides play multiple and complex roles in viral multiplication and virus-host interactions. For example, the NS1 protein performs essential activities in viral DNA replication (18), viral promoter transactivation (23, 58), and cytotoxicity (12).
A major area of research in Parvoviridae biology concerns the potential use of MVM and related autonomous parvoviruses as anticancer agents. The MVM prototype strain, MVMp, is apathogenic for newborn and adult mice (10) but infects and kills certain human tumor cell lines in vitro (49, 62). The parvoviral oncosuppressive phenomenon has been extensively evaluated with regard to infection of fibroblasts, epithelial cells (reviewed in reference 62), and neural cells (63) transformed by a wide variety of agents (16, 49) as well as in animal models of human cancer (1, 25, 77). The molecular mechanisms regulating autonomous parvovirus infection of normal and neoplastic cells operate at the recognition of cell surface receptors (55) as well as at multiple intracellular interactions which are only partially understood. Autonomous parvovirus infection may be restricted at the levels of postentry stages regulated by capsid determinants (2, 5, 56, 68), transcription activities of viral promoters (17), sensitization to cytotoxic NS proteins (12, 48, 64), and genome replication at high levels of viral gene expression (63). However, whether translational control plays a significant role in the interaction of parvoviruses with neoplastic cells has not been investigated so far.
Infection of murine fibroblasts with MVMp is reported here to activate PKR and subsequently to phosphorylate eIF2α, thus leading to a drastic inhibition of virus gene expression. Moreover, a consistent inverse correlation was established between PKR-mediated eIF2α phosphorylation and susceptibility to MVMp in different normal and tumor-derived human cell lines. These findings uncover translational control of gene expression as a major mechanism whereby autonomous parvovirus infection is regulated.
MATERIALS AND METHODS
Cells.
Wild-type NIH 3T3 and 3T3 PKRo/o cells (80) were derived from the C57BL6 strain of mice. Primary mouse embryo fibroblasts (MEFs) were obtained from the 129Sv strain of mice in accordance with a previously described procedure (7). All the experiments were performed with low-passage cultures of the cells. The BHK21 and HepG2 cell lines were purchased from the ATCC. The origins of the NIH 3T3, NB324K simian virus 40 (SV40)-transformed human newborn kidney, and A9 mouse fibroblast cell lines and of the U373 glioma cells have previously been described (63, 65). Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum (FCS), except for the 3T3 PKRo/o cells, for which 10% FCS was used instead. MEF Tet-Off cells (Clontech) expressing the vaccinia virus E3 protein (vvE3) cloned into the pBI-EGFP plasmid have recently been described (24).
Virus.
Purified stocks of MVMp, the prototype strain of the parvovirus minute virus of mice (MVM) (19), were prepared from its infectious molecular clone pMM984 (31) as described previously (65). Infectious virus was quantified as numbers of PFU by a plaque assay with the NB324K cells (74). Infections were performed with growing cell monolayers at the indicated multiplicities of infection (MOI) (PFU/cell) in Ca2+-Mg2+-supplemented phosphate saline buffer (PBS) with occasional shaking, and after 1 h of viral adsorption, the inoculum was removed and replaced with fresh growth medium.
DNA transfection.
Low-confluence 3T3 PKRo/o cells growing in 24-well plates were transfected with the pcDNA3.1/Myc-His plasmid (Invitrogen) bearing pcPKR (the human PKR open reading frame [ORF]) (7) or pcβ-Gal (a fragment of β-galactosidase [β-Gal]) as a control, using the Jet-PeI reagent (PolyTransfection). Common transfection efficiency in these cells was close to 50%. After 24 h, cells were infected with MVMp and processed for immunofluorescence (IF) at 24 h postinfection (hpi) as described below.
Western blot analysis and immunofluorescence.
Cells growing in 24-well plates were quickly washed twice with cold DMEM (without serum) and lysed directly in protein sample buffer to prevent eIF2α dephosphorylation. An aliquot of these samples was applied to 10% SDS-PAGE gel and blotted to nitrocellulose membranes (Scheider-Schull) by wet transfer overnight at 4°C. Immunoblot analyses were performed as described previously (79), using the following antibodies: rabbit anti-NS1 protein polyclonal antiserum (named SP7) (27), rabbit anti-VP polyclonal antiserum recognizing MVM structural proteins (59), rabbit anti-eIF2α (Santa Cruz Biotechnology), rabbit anti-phospho eIF2α (44728G; Invitrogen) raised against the human epitope containing the S51 phosphorylation site highly conserved among eukaryotic cells, rabbit anti-PKR (Santa Cruz Biotechnology), rabbit anti-vvE3 (a gift from Bertram L. Jacobs, Arizona State University), and mouse antiactin (Sigma). For IF, a previously described protocol was followed (43), except that cells were fixed in 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.1% Triton X-100. Additional antibodies used for IF were mouse monoclonal anti-MVM capsid (59), polyclonal anti-phospho eIF2α, and mouse anti-myc antibodies (Cell Signaling).
In vitro kinase assay. The kinase assays with recombinant PKR and purified eIF2 were carried out as described previously (7). In brief, 6×His-tagged PKR was purified from HEK 293T cells transfected with the plasmid pcPKR. eIF2 was purified from rabbit reticulocyte lysates (7). R1 RNA of MVMp was synthesized in vitro with a T7 message kit (Ambion), using the complete genome of MVMp cloned in the pGEM plasmid as a template (57), under the conditions recommended by the manufacturer. The transcription reaction mixture was digested with DNase I, phenol extracted, and ethanol precipitated. R1 RNA (5 kb) was dissolved in sterile water to a concentration of 540 μg/ml (325 nM) as measured by a Nanodrop N-1000 spectrophotometer (Thermo Scientific) and its integrity analyzed with agarose gel. The kinase reaction was carried out in a 20-μl volume containing 20 mM Tris-HCl (pH 7.6), 2.5 mM MgCl2, 2.5 mM magnesium acetate [Mg(OAc)2], 0.25 mg/ml bovine serum albumin (BSA), 50 μM ATP, 0.5 μg eIF2, 3 μCi [γ-32P]ATP (3,000 Ci/mmol), and 50 ng purified PKR. Where indicated, reactions were supplemented with purified R1 (5 kb) RNA of MVMp or with synthetic dsRNA poly(I:C) (100 to 185 kDa; Sigma). Reaction mixtures were incubated at 30°C for 30 min, separated by 10% SDS-PAGE, transferred to an Immobilon-P membrane, and exposed to X-ray films.
RESULTS
Mouse 3T3 fibroblasts devoid of the PKR gene are highly permissive to MVM infection.
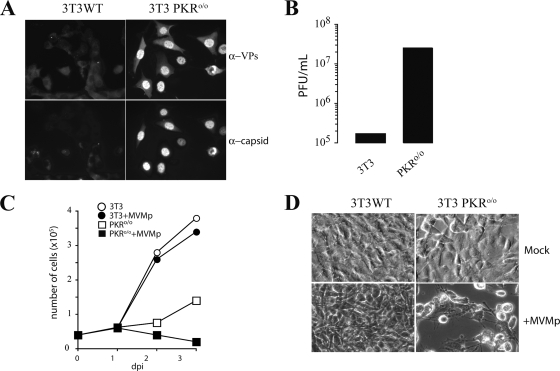
To investigate whether the PKR kinase may be important in the control of MVM infection, the replications of MVMp in normal NIH 3T3 cells and in a 3T3 cell line derived from PKR knockout mice (PKRo/o) (80) were compared. Low-passage 3T3 cells were virtually resistant to MVMp infection even when a high multiplicity of infection was used (Fig. 1A, left). No viral antigen was significantly expressed by these cells, which survived to infection and became confluent 2 days afterward, with no sign of cytotoxicity. Although MVMp replication did not progress in most 3T3 cells, a fraction of input virus was easily detected in the cytoplasm associated with endosomal vesicles (Fig. 2C), showing that the MVMp virions are internalized into the cells. In contrast, most PKRo/o cells in the cultures showed prominent VP synthesis and accumulation of viral capsids in the nucleus (Fig. 1A, right). This observation correlated with high titers of mature infectious virus released into the culture medium at late time points after infection (Fig. 1B), inhibition of cell growth (Fig. 1C), and development of a marked cytopathic effect (Fig. 1D). A nuclear phenotype of the viral capsid antigen, a high virus yield, and a marked cytopathic effect indicated high levels of permissiveness to productive MVMp infection among PKRo/o cells, these levels being similar to those observed for the most-permissive cells routinely used to grow MVMp (63).
FIG. 1.
Analysis of MVMp replication in NIH 3T3 and 3T3 PKRo/o cells. (A) IF of MVMp-infected cells probed at 24 hpi with specific antibodies for detection of total virus structural proteins (α-VPs) or the protein subunits assembled in capsids (α-capsids). (B) MVMp yield in wild-type 3T3 and 3T3 PKRo/o cells. Supernatants of infected cultures (MOI, 0.1) were sampled, and extracellular viral yield was quantified by a plaque assay. A representative result obtained at 48 hpi is shown. (C) Effect of MVMp infection (MOI, 5) on NIH 3T3 and 3T3 PKRo/o cell growth rates. The number of viable cells measured by trypan blue exclusion at the indicated days postinfection (dpi) is shown. (D) Micrographs showing the cytopathic effect provoked by MVMp on the cultures outlined in panel C at 3 dpi.
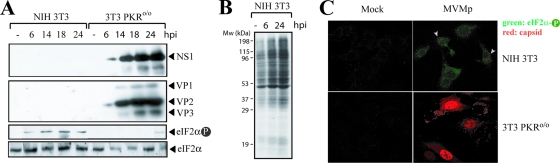
FIG. 2.
MVMp gene expression and PKR activity in 3T3 fibroblasts. (A) Time course accumulation of MVMp proteins and eIF2α phosphorylation in wild-type 3T3 and PKRo/o cells. Infected cells were lysed in sample buffer and analyzed by Western blotting at the indicated time points. Once the anti-phospho eIF2α blot was carried out, the membrane was stripped and reprobed with anti-total eIF2α. (B) Protein synthesis in MVMp-infected 3T3 cells. Cells were pulse-labeled with [35S]Met (25 μCi/ml) for 30 min at the indicated time points (hpi), and labeled proteins were resolved by 10% SDS-PAGE and autoradiography. −, mock-infected culture. (C) IF analysis of eIF2α phosphorylation in MVMp-infected 3T3 cells. At 15 hpi, cells were fixed and simultaneously probed with anticapsid (red) and anti-phospho eIF2α (green). Arrows point to infected 3T3 cells showing some MVMp input viral particles accumulated in endosomal vesicles.
MVMp protein synthesis negatively correlates with PKR activity.
To study in more detail the effects of PKR activity on MVMp replication, the accumulation levels of viral proteins and the eIF2α phosphorylation states were compared between 3T3 and PKRo/o cells. The syntheses of both nonstructural (NS1) and capsid (VP1 and VP2) proteins were nearly undetectable in 3T3 cells, whereas infected PKRo/o cells allowed syntheses of large amounts of both viral proteins (Fig. 2A). Viral protein accumulation was inversely and significantly correlated with eIF2α phosphorylation state in these cells. Indeed, a substantial phosphorylation of this factor was detected at about 6 hpi in 3T3 cells, reaching its maximal level by 16 to 18 hpi and declining at later time points (Fig. 2A, lower). Similar results were obtained when freshly prepared MEFs derived from wild-type mice of the 129Sv strain were used (see Fig. 6A). No eIF2α phosphorylation was detected in PKRo/o cells, except a faint band observed in some experiments at late time points after infection. These results agreed with previous data indicating that PKR mediates eIF2α phosphorylation in response to other viruses (79). Compared to Sindbis virus-infected cells, which showed complete eIF2α phosphorylation (79), partial eIF2α phosphorylation was observed in MVMp-infected NIH 3T3 cells (data not shown).
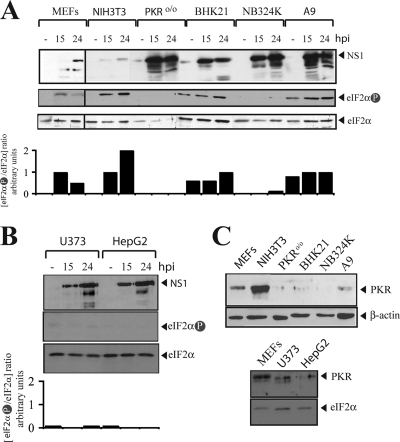
FIG. 6.
MVMp permissiveness of rodent and human transformed cells negatively correlates with PKR activity and eIF2α phosphorylation. (A, B) Rodent (MEF, NIH 3T3, PKRo/o, BHK21, and A9) and human transformed (NB324K, U373, and HepG2) cell lines, either mock (−) or MVMp infected, were analyzed for NS1 expression and eIF2α phosphorylation at the indicated time points (hpi) by Western blotting. Panels A and B show results for two representative experiments performed with parallel infections. Membranes were first probed with the anti-phospho eIF2α antibody, then stripped, and subsequently reprobed for total eIF2α accumulation. Note that substantial differences in the levels of total and phosphorylated eIF2α forms were found among the cell lines. Bands were quantified by densitometry, and the ratio between phosphorylated and total eIF2α is shown below. Since the basal level of phosphorylated eIF2α was undetectable in uninfected NIH 3T3 cells, its ratio was set to 0, and an arbitrary value of 1 was assigned to MVMp-infected 3T3 cells at 15 hpi. (C) The levels of PKR protein accumulation in the cell lines, compared to the level for the β-actin (upper) or the eIF2α (lower) housekeeping protein control, are shown. Equal amounts of total protein were probed with the indicated antisera.
Normal growth of MVMp-infected 3T3 cells suggested a possible differential effect of eIF2α phosphorylation on suppression of viral and cellular translation. To study this possibility, protein synthesis in MVMp-infected 3T3 cells was analyzed by [35S]Met pulse-labeling. As shown in Fig. 2B, the pattern of total protein synthesis in infected cells remained unaltered compared to the level for the uninfected control. To further study this phenomenon, we analyzed eIF2α phosphorylation by confocal IF. Interestingly, a faintly stippled staining of phosphorylated eIF2α was found in MVMp-infected normal 3T3 cells but not in PKRo/o cells (Fig. 2C). This result suggested a local activation of PKR in response to MVM infection.
PKR expression determines susceptibility to MVMp infection.
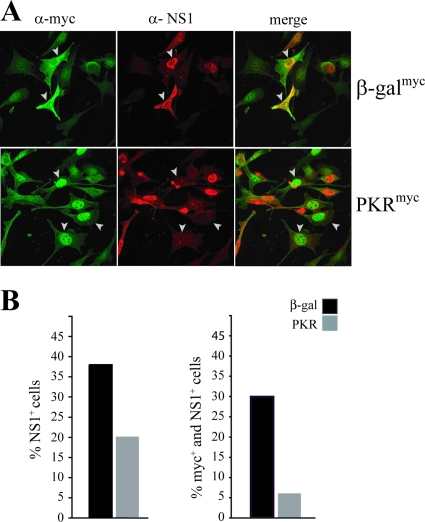
To confirm that the increased susceptibility of PKRo/o cells to MVMp infection was attributable only to PKR deficiency, the human PKR ORF was expressed in PKRo/o cells by transfection and single-cell susceptibility to MVMp was tested by IF. As shown in Fig. 3A, transfected cells expressing PKR were generally resistant to synthesis of the NS1 viral protein in comparison to control β-Gal-transfected cells inoculated in parallel. Visual estimation of the cultures showed that transfection with the PKR-expressing plasmid reduced the number of infected cells to about 50% of that observed for cells transfected with the β-Gal plasmid (Fig. 3B, left), which correlated well with the transfection efficiency generally obtained for PKRo/o cells (about 50%) (Fig. 3A). Moreover, when this analysis was focused on the population of cells actually expressing the recombinant PKR protein, only a low ratio of those cells (about 7%) was found to express MVMp antigens (Fig. 3B, right). This result showed that PKRo/o cells permissive to MVMp infection can be converted to restrictive cells upon PKR gene reintroduction, ruling out the possibility that off-target effects during the generation of the PKRo/o cell line may affect its susceptibility to MVMp.
FIG. 3.
The PKR gene blocks MVMp replication in 3T3 PKRo/o cells. (A) PKRo/o cells were transfected with the pcPKR or pcβ-Gal plasmid. Twenty-four hours afterward, cells were infected with MVMp (MOI, 5) and processed at 24 hpi for IF with the anti-myc and anti-NS1 antibodies. Representative cell fields are shown for each experiment. Note that a fraction of the PKR protein expressed from transfected plasmid is accumulated into the nucleus as described before (73). (B) Statistical analysis of the effect of PKR expression on MVMp replication. About 300 cells from 10 randomly selected fields were scored for each sample. The percentages of transfected cells expressing NS1 (left) or expressing both myc-tagged (PKR or β-Gal) proteins and the viral NS1 protein (right) are shown. The experiment was performed twice, with similar outcomes. The percentage of untransfected cells productively infected with MVMp under these conditions, tested in parallel by NS1 expression, was about 40%.
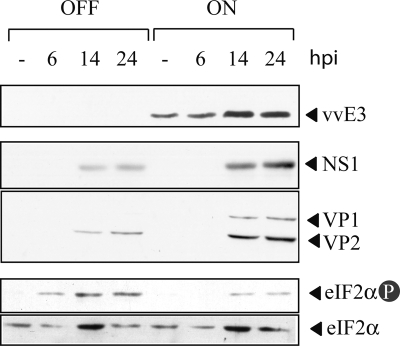
In spite of this, the artificial elimination of the PKR gene in PKRo/o cells may increase their permissiveness to MVMp infection in an artifactual manner. Therefore, the extent of MVMp permissiveness in cells whose PKR activity was inhibited but not completely eliminated was analyzed. A previously generated MEF cell line expressing the vaccinia virus E3 protein under an inducible tetracycline genetic switch was used for this purpose (24). The E3 protein is well characterized as an inhibitor of PKR activity by both allosteric binding to the kinase and competitive sequestering of dsRNA activator molecules (61, 67). As shown in Fig. 4, tetracycline withdrawal allowed E3 protein accumulation, which in turn partially prevented eIF2α phosphorylation in response to MVMp infection. Consequently, notably increased syntheses of both structural and nonstructural MVMp proteins were detected in comparison with the levels found in noninduced cells.
FIG. 4.
Inhibition of PKR activity enhances MVMp protein synthesis. Tet-Off MEFs expressing the vaccinia virus E3 protein (vvE3) were induced overnight by tetracycline withdrawal (ON) and infected with MVMp at an MOI of 10. At the indicated time points (hpi), the cells were harvested and analyzed by Western blotting with the indicated antibodies. Uninduced cells (24) were maintained with 1 μg/ml tetracycline during the experiment.
MVMp genomic RNA activates PKR in vitro.
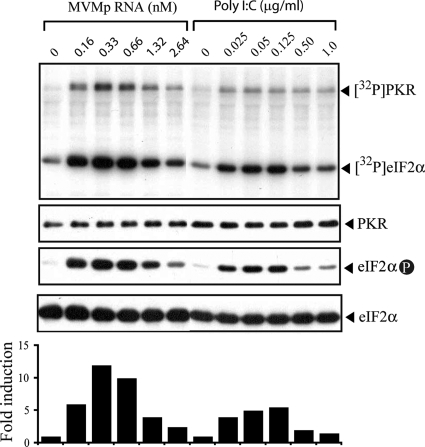
PKR activation requires dsRNA molecules with a minimal length of 30 bp (9, 42, 45). Although MVMp replicates in the nucleus and does not generate dsRNA replicative forms, which are potent PKR activators, the high degrees of secondary structure present in some viral mRNAs can also activate PKR (35). Three main viral mRNAs (R1, R2, and R3) accumulate in MVMp-infected cells as a result of the activity of two promoters (8). Among these viral mRNAs, the R1 species encompasses the full length of the MVM genome and encodes the NS1 protein. To test whether genome-length mRNA of MVMp is capable of activating PKR, an in vitro kinase assay in which the R1 messenger was incubated with purified PKR, eIF2α, and γ-[32P]ATP was carried out. The PKR basal activity in the absence of the activator was very low (Fig. 5), as described previously (45, 47). However, the addition of in vitro-synthesized R1 RNA resulted in a strong PKR activation, comparable to that obtained with a similar molar concentration of the synthetic dsRNA poly(I:C), a well-known potent PKR activator, used as a control (Fig. 5). Both the self-phosphorylated PKR and the phosphorylated eIF2α forms clearly appeared after incubation with 0.16 nM R1. Furthermore, dose-response experiments with different R1 concentrations resulted in a Gaussian bell curve of kinase activation (Fig. 5, lower), which is characteristic of PKR (45). As previously described (7), purified tRNA failed to activate PKR in this assay (data not shown). Altogether, these results indicated that the genome-length R1 RNA of MVMp is a potent activator of the PKR kinase.
FIG. 5.
In vitro activation of PKR by the MVMp R1 genomic messenger. Purified, myc-tagged PKR was assayed for its ability to phosphorylate eIF2α in the presence of increasing concentrations of in vitro-transcribed MVMp R1 mRNA (5 kb) or of dsRNA poly(I:C) of heterogeneous molecular masses (Mw) (100 to 185 kDa). Proteins were resolved by 10% SDS-PAGE and transferred to an Immobilon-P membrane. The membrane was exposed to autoradiography (upper panel) and subsequently probed with three different antisera to detect myc-tagged PKR, phosphorylated eIF2α at serine 51 (eIF2α-P), and total eIF2α (middle panels). For the lower panel, the amount of 32P incorporated into eIF2α was quantified with a BAS-1500 phosphorimager (Fujifilm) and is presented as the fold induction value compared to the value obtained when no RNA was present in the reaction (set to 1). Results for one representative experiment out of three, producing similar results, are shown.
Degree of PKR activity correlates with permissiveness to MVMp in transformed cells.
The next experiments were aimed at evaluating the role of PKR activation in determining the susceptibility of different murine and human cell lines to MVMp infection. With this purpose, the basal levels and the activation of PKR and eIF2α phosphorylation in transformed and tumorigenic cell lines showing distinct degrees of permissiveness to MVMp replication were tested (Fig. 6A and B). MEFs and murine 3T3, PKRo/o, and A9 fibroblasts were used, the last being a common cell host for MVMp and showing a low degree of malignancy, in addition to the hamster BHK21 cell line, which is highly tumorigenic in immunodeficient mice (78). The human cell lines used were NB324K fibroblasts and U373 glioma cells, which are highly permissive to MVMp (63), in addition to HepG2 hepatoma cells. The basal levels of eIF2α phosphorylation in uninfected cells were undetectable in NB324K, PKRo/o, U373, HepG2, and NIH 3T3 cells, whereas A9 and BHK21 cells contained substantial levels of phosphorylated eIF2α. In response to MVMp infection, however, the changes in eIF2α phosphorylation were markedly different among the cell lines. In MVMp-permissive cells, eIF2α phosphorylation remained undetectable in NB324K and PKRo/o cells, while BHK21 and A9 cells showed only moderate increases of eIF2α phosphorylation at late time points after infection. However, virus-resistant MEFs and NIH 3T3 cells responded to MVMp infection with remarkably increased eIF2α phosphorylation in parallel experiments (Fig. 6A). Importantly, the amounts of NS1 viral protein accumulated in the cells were inversely correlated with the levels of induced eIF2α phosphorylation, with the NS1 levels being high in all cell lines except in virus-resistant NIH 3T3 cells and MEFs (Fig. 6A and B). Our results suggest that transformed cells show defects in PKR activation in response to MVMp infection, allowing high levels of viral gene expression.
Finally, the steady-state level of PKR kinase in these cells was quantified by Western blot analysis (Fig. 6C). Interestingly, significant differences in PKR expression were observed among the tested cell lines. NIH 3T3 cells and freshly prepared MEFs contained substantial amounts of PKR. However, very low or undetectable levels were consistently found in A9 and BHK21 cells as well as in the three transformed human cell lines. These results suggest that PKR levels may critically determine the degree of cell permissiveness to MVMp infection.
DISCUSSION
The molecular basis of the increased tropism of parvoviruses for transformed cells is complex and has only been partially understood. The pleiotropic dependence of the Parvoviridae for cellular functions may reflect the lower degree of autonomy of these viruses than of complex DNA or RNA viruses that are endowed with the capacity to counteract multiple antiviral responses of the cells. Viral entry, transcription, and genome replication can be differentially restricted at certain parvovirus-host interactions. The sensitiveness of parvovirus MVM to the antiviral effect of PKR in murine and human cells was investigated in the present work.
Mechanisms of PKR-mediated response against MVM infection.
PKR-mediated phosphorylation of eIF2α was detected 6 h after MVMp inoculation, reaching its maximal level by 15 hpi, showing that this phosphorylation is not an immediate-early event occurring after the entry of MVMp into the cells (Fig. 2A). Two findings support the notion that an accumulation of viral mRNA is required for PKR activation and eIF2α phosphorylation in 3T3 cells. First, purified genome-length MVMp RNA, corresponding to the R1 messenger, led to high-level PKR activation in vitro (Fig. 5), and second, eIF2α phosphorylation in MVMp-infected 3T3 cells correlated in time with the onset of the viral mRNA accumulation observed in the susceptible cells.
eIF2α phosphorylation in MVMp-infected 3T3 cells did not affect translation (Fig. 2B) or cell survival (Fig. 1), suggesting that only a fraction of PKR molecules were activated in response to MVMp infection. Indeed, stippled, phosphorylated eIF2α molecules in discrete cytoplasmic spots (clearly smaller than stress granules), visualized by confocal IF (Fig. 2C), suggest local PKR activation in MVMp-infected cells. Local PKR activation was proposed for other viruses to explain the differential effects of eIF2α phosphorylation on viral and host mRNA translation (30, 83). Recruitment of viral mRNA to PKR-bound ribosomes triggered local eIF2α phosphorylation, which blocked translation without affecting other distant initiation events, thus allowing the responsive cell-aborting infection without compromising its viability (Fig. 1 and 2). In addition, a moderately elevated basal level of eIF2α phosphorylation does not necessarily imply a blocked local translation, thus explaining why the higher basal levels of phosphorylated eIF2α found in some susceptible cells, such as A9 and BHK21 cells, did not prevent MVMp gene expression (Fig. 6A).
The amount of viral mRNAs synthesized in 3T3 cells was very small compared to that in PKRo/o cells inoculated at the same MOI (data not shown). This sharp transcriptional inhibition may be explained by the exacerbated effect that the initial blockade of the synthesis of the major NS1 regulatory protein had on the transactivation of MVM promoters, which is required for maximal accumulation of viral mRNAs (15, 23). A similar inhibitory effect of overall MVMp transcription was observed in permissive A9 fibroblasts constitutively expressing an antisense RNA targeted against NS1 (57). In addition, a premature translation halt due to eIF2α phosphorylation triggered rapid degradation of viral mRNA in 3T3 cells.
Our results suggest that, unlike some RNA and larger DNA viruses, MVMp may not harbor a molecular mechanism for directly preventing or overcoming robust PKR activations such as those found in untransformed, infected mouse fibroblasts. The replication of the nonautonomous parvovirus AAV5 was recently shown to depend on the anti-PKR activity of adenovirus VAI RNA (51, 52), which binds PKR in an inhibitory fashion. This finding offers a molecular explanation for the inability of AAV to carry out an autonomous replication cycle and highlights the role played by PKR in restricting AAV5 replication. Interestingly, in a related approach, we found that the expression of the vaccinia virus E3 protein in MEFs partially prevented eIF2α phosphorylation and increased the synthesis of MVMp proteins (Fig. 4), suggesting that the MVMp infection cycle may also be enhanced in nature in coinfection with larger viruses carrying mechanisms interfering with PKR.
PKR activation requires binding to a dsRNA molecule of minimal length, with no sequence requirement (45). A few natural mRNA molecules were proven to be good PKR activators, and in some complex DNA viruses (such as herpesvirus and poxvirus), aberrant transcription from the 3′ ends of viral genes generated dsRNA species which activate PKR (35, 41). The genome-length R1 messenger of MVMp was proven to be a potent PKR activator (Fig. 5), and it is conceivable that such a large RNA molecule may fold in cells, thus giving rise to stretches of dsRNA long enough to activate PKR. Interestingly, a short region of the leader sequence of the AAV5 capsid gene RNA, comprised between nt 2131 and 2312 of the viral genome, was proven sufficient to activate PKR in cells (51). However, this region shares no significant homology with the entire MVMp genome (data not shown). Therefore, the RNA region(s) of the MVMp genome which can activate PKR remains an open issue deserving of further research.
Translational control in MVMp oncolysis.
Tumor cells commonly offer an ideal environment for virus replication, due at least in part to the reduction or complete elimination of the antiviral defense mechanisms associated with the transformation process (54). According to this, most tumor cell lines were refractory to the antiviral and antiproliferative effects of interferon (IFN) (71). This work shows that efficient replication of the autonomous parvovirus MVMp in three transformed cell lines proceeded with no eIF2α phosphorylation increase (Fig. 6). These transformed cells were therefore unable to respond to MVMp infection, allowing efficient translation of viral mRNA and virus gene expression. The loss of PKR responsiveness allowed efficient replication of some naturally oncotropic viruses, such as vesicular stomatitis virus (VSV) and reovirus (3, 72), as well as of genetically engineered oncolytic adenovirus and herpesvirus (13, 28), which are currently under research as biological weapons for combating human cancer. Likewise, the oncolytic capacity of some parvoviruses may be largely related to the failure of PKR activation and efficient eIF2α phosphorylation in transformed cells.
The mechanisms regulating PKR activation in transformed cells may differ. Whereas a loss of the PKR gene was reported to occur in some leukemia-derived cell lines (6), other transformed cell lines, such as HeLa, showed elevated PKR levels, despite the high proliferation capability of these cell lines (82). Moreover, transformation of NIH 3T3 with some oncogenes, such as H-Ras, hampered PKR activation without affecting its expression (50), and remarkably, H-Ras expression sensitized rat and human fibroblasts to MVMp cytotoxic products (49, 64). Further, important associations of PKR activity with parvovirus oncolysis may be traced at the level of the tumor suppressor genes. PKR has recently been described to play an important role in the tumor suppressor function of the p53 gene (81), a gene commonly altered in human cancers. The p53 protein, in its functionally active state, contributed to the resistance of cytopathic effects provoked by the parvovirus H1 protein (75). Inhibition of tumor suppressor proteins by expression of the polyomavirus large T antigen may also account for the sensitization of SV40-transformed 3T3 fibroblasts to MVM (49).
In addition to its role in translational control as a major cellular pathogen recognition receptor (PRR) (37), PKR acts as a signal transducer, activating NF-κB and interferon regulatory factors (IRFs), among other transcription factors (34, 40), which ultimately induce IFN-β expression (20, 34, 36). Secreted IFN-β binds to the IFN-α/β receptor, which, signaling through the JAK/STAT pathway, mediates the transcriptional regulation of more than 100 IFN-stimulated genes, including the PKR gene (69). It is therefore expected that the level of PKR activity generally correlates with IFN-β production in response to viral infection, a phenomenon that may control MVM tissue tropism. While this article was under review, Grekova et al. validated this assumption by describing that MEFs, but not A9 cells, are able to induce the expression of and to secrete type I IFNs in response to MVMp infection (32), paralleling our observations on the distinct PKR steady-state and activation levels found in these two mouse fibroblast cell lines (Fig. 6). Interestingly, MVMp infection was partly inhibited in A9 cells upon stimulation of PKR expression by exogenous IFN-β (32), a result that was hypothesized to possibly reflect an MVMp-triggered evasion mechanism in the innate antiviral response. Our studies of eIF2α phosphorylation performed with several mouse and human cell lines indicate, however, that if MVMp encodes an inhibitory mechanism for counteracting PKR activation, this mechanism is functional only for low PKR levels, such as those found in transformed cells, not for physiological or IFN-β-induced high PKR levels.
In summary, PKR inactivation, together with some previously reported molecular mechanisms, such as P4 promoter activity (17), NS1 cytotoxicity (12, 48, 64), and capsid protein phosphorylation by the Raf-1 kinase (60), may synergistically contribute to enhancement of parvovirus multiplication in transformed cells, accounting for the associated oncosuppressive phenomenon (reviewed in references 38 and 62). Genetically simple parvoviruses seem able to exploit the diversity of physiological processes perturbed during cell transformation. This unique biological feature may be applied in virotherapies against precise cancer types.
Acknowledgments
We are indebted to N. Salomé and J. Rommelaere (DKFZ, Heidelberg, Germany) for kindly providing the SP7 polyclonal antibody. Thanks to B. R. Williams (Monash University, Australia) for the PKRo/o cells and to B. L. Jacobs (Arizona State University) for the rabbit anti-vvE3 antibody.
This work was supported by the following grants from the Plan Nacional I+D of the Spanish Ministerio de Ciencia e Innovación (MICINN): SAF 2006-09810 to I.V., BFU 2006-12822 and BFU 2009-09469 to J.J.B., and SAF2008-03238 and S-SAL/0185/2006 from the VIRHOST consortium, funded by Comunidad Autónoma de Madrid, to J.M.A. The institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa (CSIC-UAM) is also acknowledged. I.V. and J.J.B. were recipients of a contract from the Ramón y Cajal Programme (MICINN).
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Angelova, A. L., M. Aprahamian, G. Balboni, H. J. Delecluse, R. Feederle, I. Kiprianova, S. P. Grekova, A. S. Galabov, M. Witzens-Harig, A. D. Ho, J. Rommelaere, and Z. Raykov. 2009. Oncolytic rat parvovirus H-1PV, a candidate for the treatment of human lymphoma: In vitro and in vivo studies. Mol. Ther. 17:1164-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonietti, J. P., R. Sahli, P. Beard, and B. Hirt. 1988. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J. Virol. 62:552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 5.Ball-Goodrich, L. J., and P. Tattersall. 1992. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J. Virol. 66:3415-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beretta, L., M. Gabbay, R. Berger, S. M. Hanash, and N. Sonenberg. 1996. Expression of the protein kinase PKR in modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene 12:1593-1596. [PubMed] [Google Scholar]
- 7.Berlanga, J. J., I. Ventoso, H. P. Harding, J. Deng, D. Ron, N. Sonenberg, L. Carrasco, and C. de Haro. 2006. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J. 25:1730-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berns, N., and C. R. Parrish. 2007. Parvoviridae, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 9.Bevilacqua, P. C., and T. R. Cech. 1996. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry 35:9983-9994. [DOI] [PubMed] [Google Scholar]
- 10.Brownstein, D. G., A. L. Smith, R. O. Jacoby, E. A. Johnson, G. Hansen, and P. Tattersall. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Invest. 65:357-364. [PubMed] [Google Scholar]
- 11.Bushell, M., and P. Sarnow. 2002. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 158:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caillet-Fauquet, P., M. Perros, A. Brandenburger, P. Spegelaere, and J. Rommelaere. 1990. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 9:2989-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascallo, M., G. Capella, A. Mazo, and R. Alemany. 2003. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 63:5544-5550. [PubMed] [Google Scholar]
- 14.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 15.Clemens, K. E., and D. J. Pintel. 1988. The two transcription units of the autonomous parvovirus minute virus of mice are transcribed in a temporal order. J. Virol. 62:1448-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, J. J., P. Becquart, N. Duponchel, N. Salome, B. L. Avalosse, M. Namba, and J. Rommelaere. 1988. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J. Virol. 62:1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis, J. J., Y. Q. Chen, N. Spruyt, N. Duponchel, S. F. Cotmore, P. Tattersall, and J. Rommelaere. 1990. Susceptibility of human cells to killing by the parvoviruses H-1 and minute virus of mice correlates with viral transcription. J. Virol. 64:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotmore, S. F., and P. Tattersall. 1995. DNA replication in the autonomous parvovirus. Semin. Virol. 6:271-281. [Google Scholar]
- 19.Crawford, L. V. 1966. A minute virus of mice. Virology 29:605-612. [DOI] [PubMed] [Google Scholar]
- 20.Der, S. D., and A. S. Lau. 1995. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc. Natl. Acad. Sci. U. S. A. 92:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 22.Dey, M., C. Cao, A. C. Dar, T. Tamura, K. Ozato, F. Sicheri, and T. E. Dever. 2005. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell 122:901-913. [DOI] [PubMed] [Google Scholar]
- 23.Doerig, C., B. Hirt, P. Beard, and J. P. Antonietti. 1988. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J. Gen. Virol. 69(10):2563-2573. [DOI] [PubMed] [Google Scholar]
- 24.Domingo-Gil, E., E. Perez-Jimenez, I. Ventoso, J. L. Najera, and M. Esteban. 2008. Expression of the E3L gene of vaccinia virus in transgenic mice decreases host resistance to vaccinia virus and Leishmania major infections. J. Virol. 82:254-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupressoir, T., J. M. Vanacker, J. J. Cornelis, N. Duponchel, and J. Rommelaere. 1989. Inhibition by parvovirus H-1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res. 49:3203-3208. [PubMed] [Google Scholar]
- 26.Elde, N. C., S. J. Child, A. P. Geballe, and H. S. Malik. 2009. Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature 457:485-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faisst, S., S. R. Faisst, T. Dupressoir, S. Plaza, A. Pujol, J. C. Jauniaux, S. L. Rhode, and J. Rommelaere. 1995. Isolation of a fully infectious variant of parvovirus H-1 supplanting the standard strain in human cells. J. Virol. 69:4538-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farassati, F., A. D. Yang, and P. W. Lee. 2001. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 3:745-750. [DOI] [PubMed] [Google Scholar]
- 29.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 70:1032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardiner, E. M., and P. Tattersall. 1988. Mapping of the fibrotropic and lymphotropic host range determinants of the parvovirus minute virus of mice. J. Virol. 62:2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grekova, S., R. Zawatzky, R. Horlein, C. Cziepluch, M. Mincberg, C. Davis, J. Rommelaere, and L. Daeffler. 2010. Activation of an antiviral response in normal but not transformed mouse cells: a new determinant of minute virus of mice oncotropism. J. Virol. 84:516-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gromeier, M., L. Alexander, and E. Wimmer. 1996. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. U. S. A. 93:2370-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 35.Jacquemont, B., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J. Virol. 15:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston, J. B., S. H. Nazarian, R. Natale, and G. McFadden. 2005. Myxoma virus infection of primary human fibroblasts varies with cellular age and is regulated by host interferon responses. Virology 332:235-248. [DOI] [PubMed] [Google Scholar]
- 37.Kawai, T., and S. Akira. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr, J. R., S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish. 2006. Parvoviruses. Hodder Arnold, London, United Kingdom.
- 39.Kitajewski, J., R. J. Schneider, B. Safer, S. M. Munemitsu, C. E. Samuel, B. Thimmappaya, and T. Shenk. 1986. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell 45:195-200. [DOI] [PubMed] [Google Scholar]
- 40.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133-141. [DOI] [PubMed] [Google Scholar]
- 42.Lebleu, B., G. C. Sen, S. Shaila, B. Cabrer, and P. Lengyel. 1976. Interferon, double-stranded RNA, and protein phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 73:3107-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lombardo, E., J. C. Ramirez, M. Agbandje-McKenna, and J. M. Almendral. 2000. A beta-stranded motif drives capsid protein oligomers of the parvovirus minute virus of mice into the nucleus for viral assembly. J. Virol. 74:3804-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 45.Manche, L., S. R. Green, C. Schmedt, and M. B. Mathews. 1992. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol. 12:5238-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maran, A., and M. B. Mathews. 1988. Characterization of the double-stranded RNA implicated in the inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA. Virology 164:106-113. [DOI] [PubMed] [Google Scholar]
- 47.Meurs, E., K. Chong, J. Galabru, N. S. Thomas, I. M. Kerr, B. R. Williams, and A. G. Hovanessian. 1990. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62:379-390. [DOI] [PubMed] [Google Scholar]
- 48.Mousset, S., Y. Ouadrhiri, P. Caillet-Fauquet, and J. Rommelaere. 1994. The cytotoxicity of the autonomous parvovirus minute virus of mice nonstructural proteins in FR3T3 rat cells depends on oncogene expression. J. Virol. 68:6446-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mousset, S., and J. Rommelaere. 1982. Minute virus of mice inhibits cell transformation by simian virus 40. Nature 300:537-539. [DOI] [PubMed] [Google Scholar]
- 50.Mundschau, L. J., and D. V. Faller. 1992. Oncogenic ras induces an inhibitor of double-stranded RNA-dependent eukaryotic initiation factor 2 alpha-kinase activation. J. Biol. Chem. 267:23092-23098. [PubMed] [Google Scholar]
- 51.Nayak, R., and D. J. Pintel. 2007. Adeno-associated viruses can induce phosphorylation of eIF2alpha via PKR activation, which can be overcome by helper adenovirus type 5 virus-associated RNA. J. Virol. 81:11908-11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nayak, R., and D. J. Pintel. 2007. Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production. J. Virol. 81:2205-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niepmann, M. 2009. Activation of hepatitis C virus translation by a liver-specific microRNA. Cell Cycle 8:1473-1477. [DOI] [PubMed] [Google Scholar]
- 54.Parato, K. A., D. Senger, P. A. Forsyth, and J. C. Bell. 2005. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer 5:965-976. [DOI] [PubMed] [Google Scholar]
- 55.Parker, J. S., W. J. Murphy, D. Wang, S. J. O'Brien, and C. R. Parrish. 2001. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 75:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Previsani, N., S. Fontana, B. Hirt, and P. Beard. 1997. Growth of the parvovirus minute virus of mice MVMp3 in EL4 lymphocytes is restricted after cell entry and before viral DNA amplification: cell-specific differences in virus uncoating in vitro. J. Virol. 71:7769-7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez, J. C., J. F. Santaren, and J. M. Almendral. 1995. Transcriptional inhibition of the parvovirus minute virus of mice by constitutive expression of an antisense RNA targeted against the NS-1 transactivator protein. Virology 206:57-68. [DOI] [PubMed] [Google Scholar]
- 58.Rhode, S. L., III, and S. M. Richard. 1987. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J. Virol. 61:2807-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riolobos, L., J. Reguera, M. G. Mateu, and J. M. Almendral. 2006. Nuclear transport of trimeric assembly intermediates exerts a morphogenetic control on the icosahedral parvovirus capsid. J. Mol. Biol. 357:1026-1038. [DOI] [PubMed] [Google Scholar]
- 60.Riolobos, L., N. Valle, E. Hernando, B. Maroto, M. Kann, and J. M. Almendral. 2010. Viral oncolysis that targets Raf-1 signaling control of nuclear transport. J. Virol 84:2090-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rommelaere, J., and P. Tattersall. 1990. Oncosuppression by parvovirus, p. 41-57. In P. Tijssen (ed.), Handbook of parvoviruses, vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 63.Rubio, M. P., S. Guerra, and J. M. Almendral. 2001. Genome replication and postencapsidation functions mapping to the nonstructural gene restrict the host range of a murine parvovirus in human cells. J. Virol. 75:11573-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salome, N., B. van Hille, N. Duponchel, G. Meneguzzi, F. Cuzin, J. Rommelaere, and J. J. Cornelis. 1990. Sensitization of transformed rat cells to parvovirus MVMp is restricted to specific oncogenes. Oncogene 5:123-130. [PubMed] [Google Scholar]
- 65.Santarén, J. F., J. C. Ramírez, and J. M. Almendral. 1993. Protein species of the parvovirus minute virus of mice strain MVMp: involvement of phosphorylated VP-2 subtypes in viral morphogenesis. J. Virol. 67:5126-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider, R. J., and I. Mohr. 2003. Translation initiation and viral tricks. Trends Biochem. Sci. 28:130-136. [DOI] [PubMed] [Google Scholar]
- 67.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250:302-315. [DOI] [PubMed] [Google Scholar]
- 68.Spalholz, B. A., and P. Tattersall. 1983. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J. Virol. 46:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 70.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 72.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takizawa, T., C. Tatematsu, M. Watanabe, M. Yoshida, and K. Nakajima. 2000. Three leucine-rich sequences and the N-terminal region of double-stranded RNA-activated protein kinase (PKR) are responsible for its cytoplasmic localization. J. Biochem. 128:471-476. [DOI] [PubMed] [Google Scholar]
- 74.Tattersall, P., and J. Bratton. 1983. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J. Virol. 46:944-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Telerman, A., M. Tuynder, T. Dupressoir, B. Robaye, F. Sigaux, E. Shaulian, M. Oren, J. Rommelaere, and R. Amson. 1993. A model for tumor suppression using H-1 parvovirus. Proc. Natl. Acad. Sci. U. S. A. 90:8702-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thimmappaya, B., C. Weinberger, R. J. Schneider, and T. Shenk. 1982. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell 31:543-551. [DOI] [PubMed] [Google Scholar]
- 77.Toolan, H. W., and N. Ledinko. 1968. Inhibition by H-1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology 35:475-478. [DOI] [PubMed] [Google Scholar]
- 78.Tseng, J. C., B. Levin, A. Hurtado, H. Yee, I. Perez de Castro, M. Jimenez, P. Shamamian, R. Jin, R. P. Novick, A. Pellicer, and D. Meruelo. 2004. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 22:70-77. [DOI] [PubMed] [Google Scholar]
- 79.Ventoso, I., M. A. Sanz, S. Molina, J. J. Berlanga, L. Carrasco, and M. Esteban. 2006. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 20:87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoon, C. H., E. S. Lee, D. S. Lim, and Y. S. Bae. 2009. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc. Natl. Acad. Sci. U. S. A. 106:7852-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, P., B. L. Jacobs, and C. E. Samuel. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82:840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu, S., P. R. Romano, and R. C. Wek. 1997. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 272:14434-14441. [DOI] [PubMed] [Google Scholar]