Abstract
The emergence of oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus highlights the need for rapid oseltamivir resistance screening. We report the development and validation of high-throughput real-time reverse transcriptase PCR assays for the detection of the H275Y substitution in the neuraminidase 1 gene that can be accomplished in 3 to 4 h.
The continuous spread of influenza A pandemic (H1N1) 2009 virus [pandemic (H1N1) 2009 virus] is compelling the use of the neuraminidase inhibitors (NAI) oseltamivir (Tamiflu) and zanamivir (Relenza) to treat infected patients in order to minimize further spread of the virus. NAI are the only available antivirals against this pandemic (H1N1) 2009 virus, because adamantanes (amantadine and rimantadine) are completely ineffective (4, 16). The extensive use of these NAI, in particular oseltamivir, is creating an unprecedented selective pressure for the emergence and spread of drug-resistant viral strains. The World Health Organization (WHO) has recommended vigilant monitoring for oseltamivir-resistant viruses, because the number of documented sporadic resistant cases is increasing, reaching nearly 100 cases worldwide by 15 December 2009 (18). Recently, the U.S. Centers for Disease Control and Prevention (CDC) reported the first human-to-human transmission of oseltamivir-resistant pandemic (H1N1) 2009 virus in two summer campers receiving oseltamivir prophylaxis (2). In addition, the WHO has announced outbreaks of oseltamivir-resistant pandemic (H1N1) 2009 virus in two immunocompromised groups, one in North Carolina and the other in Wales, United Kingdom (18). In both outbreaks, human-to-human oseltamivir-resistant virus transmission was suspected.
At least two mechanisms contribute to neuraminidase resistance in the seasonal influenza viruses H1N1 and avian influenza H5N1 (12). One mechanism involves reduction of the binding efficiency of virus hemagglutinin to its receptor. The other is associated with amino acid substitutions in and around the NA active site, of which the substitution at position 275 (histidine to tyrosine [H275Y]) is the most common (7, 11, 6). Sequence analysis of the hemagglutinin gene is not a reliable indicator of neuraminidase drug resistance phenotype, but histidine-to-tyrosine (H275Y) substitution in the active site of the NA-1 gene does indicate reduced binding affinity of the neuraminidase inhibitor oseltamivir. Recent reports characterizing the current oseltamivir-resistant pandemic (H1N1) 2009 virus confirmed the presence of the H275Y mutation (2, 3, 10).
While phenotypic analysis of oseltamivir-resistant influenza A viruses is widely accepted as the “gold standard” methodology for detecting influenza virus drug resistance, genotypic analysis has been widely utilized to detect a point mutation (cytosine to thymine) at position 823 of the NA-1 gene that results in a histidine-to-tyrosine substitution (8, 13). The genotypic assays include sequencing part of the neuraminidase gene by using the Sanger dideoxy sequencing method or by pyrosequencing. These assays are labor-intensive, with a long turnaround time ranging from 24 to 72 h, and require specialized equipment and human effort. Moreover, sequencing and pyrosequencing assays have reduced sensitivities for detecting low concentrations (<15%) of quasispecies present in a patient's sample (14). Therefore, high-throughput assays with short turnaround times are needed in order to expedite oseltamivir drug resistance detection.
In the present study we validated two real-time reverse transcriptase PCR (qRT-PCR) assays by utilizing TaqMan chemistry for the detection of the point mutation (cytosine to thymine) at position 823 of the NA-1 gene of pandemic (H1N1) 2009 virus. One set of modified primers and two probes previously reported by Chutinimitkul et al. were utilized to validate both assays (5). Chutinimitkul et al. initially validated these primers and probes for the detection of oseltamivir resistance in H5N1 isolates (5). The primers utilized in the present study were modified to increase the assay's sensitivity and specificity to detect pandemic (H1N1) 2009 virus, while the two minor groove binding (MGB) probes were synthesized as described by Chutinimitkul et al. (5). The modified primers were forward, 5′-GGG GCA GTG GCT GTG TTA-3′, and reverse, 5′-AGG GCG TGG ATT GTC TCC-3′. The two MGB probes utilized were sensitive, 5′-6-carboxyfluorescein (FAM)-TCC TCA TAG TGR TAA TT-3′-nonfluorescent quencher (NFQ), and resistant, 5′-FAM-TCC TCA TAG TAR TAA TT-3′-NFQ. The point mutation in the probe was positioned 7 bases from the 3′ end to minimize cross-reactivity with similar sequences (1). Two qRT-PCR assays were designed, for H275 for the detection of cytosine (sensitive) and H275Y for the detection of thymine (resistant) at position 823. This permitted detection of mixed infections of oseltamivir-resistant and oseltamivir-sensitive viruses. Samples positive for the H275Y mutation by the TaqMan assay were confirmed by sequencing part of the neuraminidase gene by utilizing the same set of primers described above. The sequencing procedures were previously described (9).
Sensitivity of the qRT-PCR assays was optimized by evaluating different concentrations of primers (200, 300, 600, and 900 nM) and probes (100, 200, and 300 nM). The concentrations of primers used in this study that gave the best detection limits were 600 nM for the primers and 200 nM for the probes. The AgPath-ID one-step RT-PCR kit (Applied Biosystems-Ambion, CA) was used to develop both assays. Briefly, two 25-μl qRT-PCR mixtures that contained 12.5 μl of 2× RT-PCR buffer, 1 μl of 25× RT-PCR enzyme mix, a 600 nM concentration of each primer, and a 200 nM concentration of either the sensitive or the resistant probe were prepared to evaluate 5 μl of each patient sample. 5-Carboxy-X-rhodamine, succinimidyl ester (ROX) present in the 2× RT-PCR buffer was utilized as an internal reference dye. Amplification and detection were performed using an Applied Biosystems 7500 platform under the following conditions: 30 min at 48°C for reverse transcription, 10 min at 95°C to activate AmpliTaq Gold DNA polymerase, and 50 cycles of 15 s at 95°C and 1 min at 60°C.
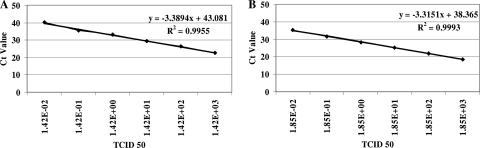
The analytical sensitivity of the H275Y qRT-PCR was determined by 10-fold serial dilution of oseltamivir-resistant pandemic (H1N1) 2009 virus RNA isolated from cultured virus of an Israeli patient [A/Israel/6290/2009(H1N1); accession number GU371269]. Briefly, viral RNA was extracted from 200 μl of A/Israel/6290/2009(H1N1) virus stock (7.8 × 105 50% tissue culture infective doses [TCID50]/ml) with a NucliSENS easyMAG extractor (bioMérieux, Boxtel, Netherlands). RNA was eluted in 55 μl of elution buffer and stored at −70°C. Analysis of the 10-fold serial dilutions of viral RNA for the H275Y mutation in triplicate by qRT-PCR revealed that the assay's analytical sensitivity was 0.014 TCID50 with 97.2% amplification efficiency (Fig. 1A). On the other hand, the analytical sensitivity of the oseltamivir-sensitive (H275) qRT-PCR assay after 10-fold serial dilutions of extracted RNA from 3.7 × 105 TCID50/ml cultured virus from an Israeli patient [A/Israel/119/2009(H1N1)] was 0.0019 TCID50 (Fig. 1B). The amplification efficiency of the H275 qRT-PCR assay was 100%. The H275 assay's analytical sensitivity was confirmed in triplicate with similar results.
FIG. 1.
Linear limits of detection of the H275Y (A) and H275 (B) qRT-PCR assays. Serial (10-fold) dilutions of influenza A pandemic (H1N1) 2009 virus resistant (A) or sensitive (B) to oseltamivir were prepared and tested in qRT-PCR assays. Ct values were obtained for each dilution and are plotted against the TCID50 value.
The specificities of the two assays were investigated by analyzing one clinical isolate of each of the common human respiratory viruses that were strongly positive by real-time PCR analysis (threshold cycle [Ct], <25). These included seasonal influenza A viruses (H1N1 and H3N2), influenza B virus, human metapneumovirus, respiratory syncytial virus (RSV) types A and B, and adenovirus type 2. Both assays were specific, since no positive signals were noted in the analysis of these viruses (data not shown).
Validation of the two assays, H275 (sensitive) and H275Y (resistant), was performed on 31 patient samples collected between 15 June 2009 and 1 December 2009 from 18 patients with a high risk of developing oseltamivir resistance. This group included hospitalized patients who were treated with at least one course of oseltamivir without improvement. Most of the patients were immunologically suppressed due to various underlying diseases. All 31 patient samples were positive for pandemic (H1N1) 2009 virus (Ct, <34) based on qRT-PCR assay as previously described by Panning et al. (15).
Of the 18 patients, 6 (33%) were positive for the H275Y mutation by qRT-PCR. Table 1 summarizes the data for all of these patients. In patient 1, oseltamivir-resistant virus was first detected in a sample obtained after 9 days of oseltamivir therapy. The first sample of patient 1 tested sensitive for oseltamivir on 30 July 2009, and mixed sensitive and resistant viruses were detected in this patient's second sample when tested on 9 August 2009. Sequence analysis of the neuraminidase genes amplified from the two samples confirmed the results of the qRT-PCR assays. Similar results were obtained for patient 4. Interestingly, only oseltamivir-resistant viruses were detected in samples from patients 2, 3, and 5. These results were also confirmed by sequence analysis. Since the initial samples collected from these three patients at the onset of clinical symptoms contained resistant viruses, it is highly unlikely that they were infected with sensitive strains. Rather, it appears that they were initially infected with resistant viruses as previously reported by the CDC and the WHO (2, 18).
TABLE 1.
Detection of oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus in Israeli patients by qRT-PCR and by sequence analysis
| Patient no. | Sample type | Date sample collected (day-mo-yr) | qRT-PCR assay (Ct)a |
N*823 sequencing resultb | |||
|---|---|---|---|---|---|---|---|
| Influenza A pandemic (H1N1) 2009 | H275 (sensitive) | H275Y (resistant) | Interpretation | ||||
| 1 | Throat/nose | 30-07-09 | 17 | 24 | Neg | WT | C |
| Throat/nose | 09-08-09 | 20 | 29 | 27 | WT and mutant | T/C | |
| 2 | Throat/nose | 14-08-2009 | 26.4 | Neg | 29.7 | Mutant | T |
| Throat/nose | 16-08-2009 | 19.4 | Neg | 25.3 | Mutant | T | |
| Throat/nose | 19-08-2009 | Neg | Neg | Neg | ND | ||
| 3 | Throat/nose | 16-08-2009 | 21.5 | Neg | 25 | Mutant | T |
| 4 | Throat/nose | 20-09-2009 | 16 | 19 | Neg | WT | C |
| Throat/nose | 03-10-2009 | 29 | 34.1 | 33.6 | WT and mutant | T/C | |
| Throat/nose | 05-10-2009 | 30 | Neg | 35.7 | Mutant | T | |
| 5 | Throat/nose | 11-10-2009 | 25.6 | Neg | 28.3 | Mutant | T |
| Throat/nose | 15-10-2009 | 25.6 | Neg | 29 | Mutant | T | |
| Throat/nose | 20-10-2009 | 26.2 | Neg | 28.5 | Mutant | T | |
| 6 | Throat/nose | 19-11-2009 | 17.5 | 24.5 | 21.8 | WT and mutant | T |
| Endotracheal | 19-11-2009 | 22.2 | 25.3 | Neg | WT | C | |
| Throat/nose | 23-11-2009 | 16.1 | 33.3 | 21 | WT and mutant | T | |
| Endotracheal | 23-11-2006 | 27.1 | 32.8 | 31 | WT and mutant | T/C | |
Neg, negative result; ND, not determined; WT, wild type.
The nucleotide(s) found at position 823 (N*823) in the neuraminidase 1 gene.
In patient 6, qRT-PCR results indicated the presence of mixed sensitive and resistant viruses in the patient's first sample, while sequence analysis revealed only resistant virus in that sample (Table 1). qRT-PCR showed that there was a 3-Ct difference, with ∼1 log more resistant virus than sensitive virus. The lower sensitivity of the sequencing assay could in part explain why the 10% portion of sensitive virus in the pool containing mostly resistant virus was not detected. Patient 6 was indeed infected with an oseltamivir-sensitive virus, as the presence of this virus was confirmed both by the qRT-PCR assay and by sequence analysis of a positive endotracheal aspirate sample (Table 1).
The qRT-PCR assays were also used to screen 120 patient samples that tested positive for pandemic (H1N1) 2009 virus between 15 June 2009 and 1 December 2009. The purpose was to determine whether oseltamivir-resistant virus was circulating in the community and to determine if the H275Y assay cross-reacted with oseltamivir-sensitive virus in clinical samples. Patients in this study group had not been treated with oseltamivir at the time of sample collection. All samples tested positive in the H275 assay, while none tested positive using the H275Y qRT-PCR assay. Thus, the H275Y assay was specific to detecting resistant virus only, and infection with oseltamivir-resistant pandemic (H1N1) 2009 virus is apparently still a rare event at this time.
More recently, van der Vries et al. reported the development of three highly sensitive qRT-PCR assays for detection of the pandemic (H1N1) 2009 virus H1 and N1 genes and of the H275Y oseltamivir resistance mutation (17). However, oseltamivir-resistant virus was not detected in the clinical samples tested; thus, the clinical sensitivity was not evaluated.
One of the drawbacks of any qRT-PCR assay is that it is sequence specific, and any genetic shift in the primer or probe locations can alter the assay performance. The current pandemic (H1N1) 2009 virus may yet change further in the region encompassing the H275 location, other than the rare resistance mutation H275Y, and this assay will not detect such changes. This limitation can be partially overcome by sequencing or pyrosequencing, which can detect multiple nucleotide substitutions simultaneously (6). Multiplexing the two described assays would improve the flow of work and further reduce the cost.
The recent report of two oseltamivir-resistant pandemic (H1N1) 2009 virus outbreaks, in the United Kingdom and the United States, is a strong reminder that countries should follow the WHO recommendations and develop a vigilant system for detecting oseltamivir-resistant viruses (18). The qRT-PCR assay described in this report can be used to screen large numbers of clinical samples for drug resistance with a very short turnaround time (3 to 4 h) and at a very low cost. In addition, this assay detects low quantities of either sensitive or resistant viruses in a mixed infection, whereas sequencing fails to detect the minor virus type.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Bolotin, S., A. V. Robertson, A. Eshaghi, C. De Lima, E. Lombos, E. Chong-King, L. Burton, T. Mazzulli, and S. J. Drews. 2009. Development of a novel real-time reverse-transcriptase PCR method for the detection of H275Y positive influenza A H1N1 isolates. J. Virol. Methods 158:190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2009. Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis—North Carolina, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:969-972. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2009. Oseltamivir-resistant pandemic (H1N1) 2009 influenza virus, October 2009. Wkly. Epidemiol. Rec. 84:453-459. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2009. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:433-435. [PubMed] [Google Scholar]
- 5.Chutinimitkul, S., K. Suwannakarn, T. Chieochansin, Q. Mai le, S. Damrongwatanapokin, A. Chaisingh, A. Amonsin, O. Landt, T. Songserm, A. Theamboonlers, and Y. Poovorawan. 2007. H5N1 oseltamivir-resistance detection by real-time PCR using two high sensitivity labeled TaqMan probes. J. Virol. Methods 139:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deyde, V. M., T. G. Sheu, A. A. Trujillo, M. Okomo-Adhiambo, R. Garten, A. I. Klimov, and L. V. Gubareva. 22 December 2009, posting date. Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by using pyrosequencing. Antimicrob. Agents Chemother. 54:1102-1110. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubareva, L. V. 2004. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 103:199-203. [DOI] [PubMed] [Google Scholar]
- 8.Gubareva, L. V., R. G. Webster, and F. G. Hayden. 2002. Detection of influenza virus resistance to neuraminidase inhibitors by an enzyme inhibition assay. Antiviral Res. 53:47-61. [DOI] [PubMed] [Google Scholar]
- 9.Hindiyeh, M. Y., Y. Aboudy, M. Wohoush, L. M. Shulman, D. Ram, T. Levin, T. Frank, F. Riccardo, M. Khalili, E. S. Sawalha, M. Obeidi, G. Sabatinelli, Z. Grossman, and E. Mendelson. 2009. Characterization of large mumps outbreak among vaccinated Palestinian refugees. J. Clin. Microbiol. 47:560-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurt, A. C., J. K. Holien, M. W. Parker, and I. G. Barr. 2009. Oseltamivir resistance and the H274Y neuraminidase mutation in seasonal, pandemic and highly pathogenic influenza viruses. Drugs 69:2523-2531. [DOI] [PubMed] [Google Scholar]
- 11.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437:1108. [DOI] [PubMed] [Google Scholar]
- 12.McKimm-Breschkin, J. L. 2000. Resistance of influenza viruses to neuraminidase inhibitors: a review. Antiviral Res. 47:1-17. [DOI] [PubMed] [Google Scholar]
- 13.Meijer, A., A. Lackenby, A. Hay, and M. Zambon. 2007. Influenza antiviral susceptibility monitoring activities in relation to national antiviral stockpiles in Europe during the winter 2006/2007 season. Euro Surveill. 12:E3-E4. [PubMed] [Google Scholar]
- 14.Palmer, S., V. Boltz, F. Maldarelli, M. Kearney, E. K. Halvas, D. Rock, J. Falloon, R. T. Davey, Jr., R. L. Dewar, J. A. Metcalf, J. W. Mellors, and J. M. Coffin. 2006. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. AIDS 20:701-710. [DOI] [PubMed] [Google Scholar]
- 15.Panning, M., M. Eickmann, O. Landt, M. Monazahian, S. Olschlager, S. Baumgarte, U. Reischl, J. J. Wenzel, H. H. Niller, S. Gunther, B. Hollmann, D. Huzly, J. F. Drexler, A. Helmer, S. Becker, B. Matz, A. Eis-Hubinger, and C. Drosten. 2009. Detection of influenza A(H1N1)v virus by real-time RT-PCR. Euro Surveill. 14:3. [PubMed] [Google Scholar]
- 16.Rungrotmongkol, T., P. Intharathep, M. Malaisree, N. Nunthaboot, N. Kaiyawet, P. Sompornpisut, S. Payungporn, Y. Poovorawan, and S. Hannongbua. 2009. Susceptibility of antiviral drugs against 2009 influenza A (H1N1) virus. Biochem. Biophys. Res. Commun. 385:390-394. [DOI] [PubMed] [Google Scholar]
- 17.van der Vries, E., M. Jonges, S. Herfst, J. Maaskant, A. Van der Linden, J. Guldemeester, G. I. Aron, T. M. Bestebroer, M. Koopmans, A. Meijer, R. A. Fouchier, A. D. Osterhaus, C. A. Boucher, and M. Schutten. 24 October 2009, posting date. Evaluation of a rapid molecular algorithm for detection of pandemic influenza A (H1N1) 2009 virus and screening for a key oseltamivir resistance (H275Y) substitution in neuraminidase. J. Clin. Virol. 47:34-37. [Epub ahead of print.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. 2009. Oseltamivir resistance in immunocompromised hospital patients. http://www.who.int/csr/disease/swineflu/notes/briefing_20091202/en/index.html.