Abstract
Tularemia is a highly contagious infectious zoonosis caused by the bacterial agent Francisella tularensis. Serology is still considered to be a cornerstone in tularemia diagnosis due to the low sensitivity of bacterial culture and the lack of standardization in PCR methodology for the direct identification of the pathogen. We developed a novel immunochromatographic test (ICT) to efficiently detect F. tularensis-specific antibodies in sera from humans and other mammalian species (nonhuman primate, pig, and rabbit). This new tool requires none or minimal laboratory equipment, and the results are obtained within 15 min. When compared to the method of microagglutination, which was shown to be more specific than the enzyme-linked immunosorbent assay, the ICT had a sensitivity of 98.3% (58 positive sera were tested) and a specificity of 96.5% (58 negative sera were tested) on human sera. On animal sera, the overall sensitivity was 100% (22 positive sera were tested) and specificity was also 100% (70 negative sera were tested). This rapid test preferentially detects IgG antibodies that may occur early in the course of human tularemia, but further evaluation with human sera is important to prove that the ICT can be a valuable field test to support a presumptive diagnosis of tularemia. The ICT can also be a useful tool to monitor successful vaccination with subunit vaccines or live vaccine strains containing lipopolysaccharide (e.g., LVS) and to detect seropositive individuals or animals in outbreak situations or in the context of epidemiologic surveillance programs in areas of endemicity as recently recommended by the World Health Organization.
Tularemia is a highly contagious infectious zoonosis caused by Francisella tularensis. This Gram-negative bacterium is widespread in North America, as well as in several parts of Europe and Asia (25). More than 200 species ranging from mice to men have been shown to develop clinical infection, but rodents and lagomorphs are more particularly susceptible and are considered to represent the main reservoir in many areas of the world (16). Transmission is often associated with handling of infected animals, but the infection can also be acquired orally, via the respiratory route, or by bites of infected vertebrates or arthropod vectors (19, 26). In addition, F. tularensis is considered a category A agent with a high potential to be misused in bio terrorism or biological warfare (9).
Regardless of the route of infection, tularemia is a serious and sometimes fatal disease in humans and several mammalian hosts. The course of infection depends on the virulence of the infectious strain, the portal of entry, the extent of systemic involvement and the immune status of the host. The occurrence of several different clinical forms of tularemia makes a clinical diagnosis very difficult (29). The predominant manifestations of human disease are the ulceroglandular, glandular, oculoglandular, pharyngeal, typhoidal, and the pneumonic form. However, overlapping of the different symptoms is observed frequently. F. tularensis is intrinsically resistant to beta-lactam antibiotics (30). Furthermore, several lines of evidence indicate that the success of antibiotic treatment depends on the timely application of effective antibacterial therapeutics. A delay of more than 14 days frequently results in treatment failure (no clinical response, recurrent or relapsing disease) in ca. 20 to 30% of all cases, but even a percentage of 65% has been described (8). For these reasons, a rapid and reliable diagnosis is needed to start adequate treatment.
Serology is a cornerstone of diagnosis in tularemia for several reasons. Bacterial culture of this fastidious organism is difficult and poses a high risk of laboratory infection. Antibodies against F. tularensis in patients appear 6 to 10 days after the onset of symptoms (17), thus at a moment when tularemia has not always been diagnosed, and in most cases IgM, IgG, and IgA antibodies arise simultaneously (10, 17, 31). Several different laboratory methods for the detection of F. tularensis-specific antibodies have been described, including indirect immunofluorescence, agglutination assays as well as enzyme-linked immunosorbent assays (ELISAs), which represent the most commonly utilized methods at present. The latter tests are recommended by the World Health Organization (WHO) and enable a confirmation of a tularemia infection within one to two working days (33), but standardized reagents are not always commercially available, and both methods require a laboratory environment, equipment, and experienced laboratory personal to be adequately performed. In addition to its application in clinical microbiology, detection of antibodies against F. tularensis is useful to confirm successful vaccination after immunization with live or subunit vaccines and can also be applied for seroepidemiologic studies in endemic regions or populations at risk (24, 27). In veterinary medicine, serology is used mainly for tularemia surveillance in rodents, hares, or surrogate animals such as boars or predators, including wolves or bears (1, 26, 34). Tularemia outbreaks in zoos or animal facilities cause additional necessities for a multispecies assay, which could be applied as a point-of-care assay (18).
In the present study, we describe the development and evaluation of a rapid test format, namely, an immunochromatographic test (ICT), which is able to detect anti-F. tularensis lipopolysaccharide (LPS) antibodies in a sensitive and specific manner in sera from human patients, vaccinees, as well as nonhuman primates (NHP; two different species), pigs, rabbits, and mice.
MATERIALS AND METHODS
Sera used for test evaluation.
In the present study we developed a new rapid serological test for the diagnosis of tularemia. The assay should allow the detection of F. tularensis LPS-specific antibodies in human and other mammalian species; therefore, 208 sera and 11 antibody preparations derived from humans and five different other species were used. All specimens were tested in parallel by serum agglutination, ELISA, and the new ICT (24).
Serum samples were taken from frozen aliquots stored at −40°C in the serum collection of the German reference laboratory for tularemia and included 53 sera from clinically confirmed tularemia patients (acute cases) and 53 sera from patients with suspected bacterial infection, for which tularemia was excluded by clinical and laboratory investigations. To analyze clinical sensitivity, 53 sera from 50 tularemia patients were tested. In three patients, F. tularensis subsp. holarctica had been cultured from blood or ulcer sources. In 14 patients, F. tularensis DNA had been detected by PCR (al least two different protocols were used to confirm the presence of F. tularensis subsp. holarctica). Tularemia was laboratory-confirmed by seroconversion (two persons) or a significant change in antibody titer (n = 24 samples) in 20 patients (including 2 patients with positive PCR samples and all culture-proven cases).
In nine patients from whom only one serum sample was available, there was a definite epidemiological proof (F. tularensis cultured from a frozen hare, shot and skinned from one family [two patients] or patients sharing symptoms and time of onset of disease with confirmed tularemia cases in the same household [waterborne outbreak of oropharyngeal tularemia]). In four patients, tularemia was clinically diagnosed in ulceroglandular form (n = 3) or oropharyngeal form (n = 1), and a high single titer against F. tularensis was found. According to the German legal health regulations these patients also fulfilled the case definitions for “confirmed cases.” In most cases, serum samples from tularemia patients were obtained 4 to 6 weeks after the onset of symptoms (range, 3 days to more than 4 months), although detailed information on the time between infection and sampling was scarce. Pre- and postvaccination sera from five individuals who had been vaccinated (live vaccination) with the F. tularensis subsp. holarctica LVS strain in 2004 were also tested. In addition, 44 sera obtained from two different nonhuman primate species (Macaca mulatta and M. fascicularis) recently exposed to F. tularensis subsp. holarctica (18) were analyzed. We also tested pre- and postimmunization sera (n = 8) from four pigs that were vaccinated with inactivated bacterial cells from F. tularensis subsp. holarctica LVS, F. tularensis subsp. tularensis (ATCC 6223), F. tularensis subsp. novicida (ATCC 15482), or F. philomiragia (ATCC 25018) and 40 sera from rabbits immunized with different bacterial pathogens, including F. tularensis subsp. holarctica LVS. Successful vaccination for each pathogen was tested by ELISA and immunoblot. Finally, different mouse monoclonal antibodies (MAbs) recognizing different epitopes of F. tularensis LPS were tested in all four assays (12).
Agglutination assay.
Microagglutination was performed as recently described (21) using inactivated F. tularensis subsp. holarctica LVS bacterial cells incubated overnight with serial dilution of the specimens. In this direct reaction, mainly IgM is detected, whereas IgA and IgG are only weak agglutinogens (31). All tests were performed in duplicate. Sera showing reciprocal titers ≥8 were considered to be positive.
ELISA protocol and Western blotting.
For ELISA, a commercial test kit approved for use in human diagnostics (Seramun, Dolgenbrodt, Germany), was used according to the manufacturer's instructions with slight modifications (26). For humans and NHP serum samples, a horseradish peroxidase-anti-human immunoglobulin conjugate was used. The use of specific anti-monkey conjugate (NatuTec, Frankfurt, Germany) did not improve or significantly change the results obtained with the reagent provided with the test kit, probably due to the strong cross-reactivity of human and monkey immunoglobulin. Sera were diluted 1:300 before use. For specimens obtained from pigs, rabbits, and mice, species-specific anti-immunoglobulin conjugates were used (Sigma, Munich, Germany). The optimal dilutions for serum (1:100 or 1:500) and anti-Ig conjugate (1:1,000 or 1.2,000) were determined for each species by checkerboard titration. The ELISA cutoff was calculated using results of all agglutination-negative sera (from humans and animals). The mean optical density (OD) at 405 nm for 127 sera was 0.067 with a standard deviation (SD) of 0.087. Therefore, the cutoff for the ELISA was set to 0.327 (mean OD + 3 SD). All sera showing an OD above this level were regarded as positive.
All serum samples giving positive signals in ELISA, microagglutination, or the new rapid test were further tested for the presence of anti-F. tularensis LPS-specific antibodies by Western blotting as recently described (24).
Protocol for ICT manufacturing.
The F. tularensis serodiagnostic test is based on the principle of immunochromatography or lateral flow assay. The test device consists of a plastic-backed nitrocellulose (NC) membrane (MTP; Whatman International, Ltd., Maidstone, United Kingdom), which is flanked at the top end by an absorbent pad (3MM; Whatman International, Ltd.) and at the bottom end by a conjugate pad (no. 7; Alchemy Laboratories, Ltd., Dundee, United Kingdom), on which gold beads (40 nm in diameter) conjugated to protein A (purchased from British Biocell International, Cardiff, United Kingdom) are adsorbed. Protein A conjugated to gold particles binds to the Fc part of antibodies of human and animal origin. Thus, antibodies from serum specimens which recognized their specific epitope on a fixed antigen are indirectly detected and visualized. Protein A preferentially binds antibodies of subclass IgG, while affinity to IgA or IgM antibodies is low. The concentration of protein A is lot specific because it depends on conjugation efficiency; therefore, the protein A concentration has to be standardized with each new lot.
A sample application pad (no. 2; Alchemy Laboratories) flanks the conjugate pad in turn. The membrane and pads are glued onto the backing of the nitrocellulose membrane resulting in a test strip 7 by 0.5 cm in size (see Fig. 1).
FIG. 1.
The new immunochromatographic test (ICT) detected F. tularensis-specific antibodies in serum from a tularemia patient and a human vaccinee, as well as the sera from four different mammalian species. Reactive sera showed a positive test line (T) within 2 to 10 min. All tests were finally read after 15 min. The application of negative serum samples resulted in a negative test line, while the positive control line (C) indicated a valid test function. In the tularemia patient and the vaccinee, seroconversion could be confirmed. CP, conjugate pad; SP, sample application pad.
The result of the test is to be read in the detection zone of the nitrocellulose membrane. This zone contains a test line and a control line, obtained by dispensing the LPS antigen from F. tularensis and a control reagent GoldLine (British Biocell International), respectively. These lines are invisible before ICT utilization. The antigen and the GoldLine were coated on the nitrocellulose membrane with an IsoFlow reagent dispenser (Imagene Technology, Hanover, NH). The concentrations, amount, and dilution buffer of LPS and conjugate applied to the test strip, as well as the dilution of the sera and the migration buffer, were optimized in a step-by-step procedure using a panel of positive and negative control sera. To obtain the optimized version of the strip, the LPS extract derived from F. tularensis holarctica (lot 60601; Micromun, Berlin, Germany) (24) was diluted at 1 mg/ml in phosphate-buffered saline (PBS [pH 7.4]) and deposited as a narrow line at a dispense rate of 1 μl/cm. The strips were then dried for 6 h at 37°C, and sealed devices may be stored at 4°C for a year at least without loss of activity. After utilization and complete drying of the strip (room temperature, 2 h), the signals read on the ICT are stable for at least another year.
Utilization of the ICT.
Assays were performed by vertically dipping the strip in a test tube or an ELISA well containing 10 μl of serum diluted with 90 μl of migration buffer (PBS [pH 7.4], 0.5% Tween 20). Driven by capillary forces, the liquid migrated along the strip into the conjugate pad, solubilizing colloidal gold beads. The protein A conjugated to beads reacted with antibodies present in the sample, while the whole complex migrated further along the nitrocellulose membrane. At the test line, antibodies specific to F. tularensis LPS, if present in the sample, bound the capture antigen, and immobilized complexes could then be visualized as a red line. In the absence of specific antibodies, no signal was visible. The GoldLine reagent is mainly composed of silver, which interacts with gold so that the control line acts as a positive control. Test results were read 15 min after initiating the migration, by visual inspection of the staining at the position of test and control lines by two independent persons who were trained for ICT interpretation and blinded regarding the origin of the serum. Tests were scored negative when no staining was observed at the test line and scored positive when the test line was visible. After utilization, all strips had a positive control line.
Statistical analysis.
The performance of the ICT was independently compared to the results of the agglutination assay and the ELISA. The sensitivity, specificity, and related confidence intervals were calculated by using the Fisher exact test. The chi-square test, as well as Cohen's kappa coefficient (2), was used to test for concordance between the ICT and each reference assay.
RESULTS
The new ICT detected specific anti-F. tularensis LPS antibodies from humans and from two nonhuman primate species (M. mulatta and M. fascicularis), as well as from pigs, rabbits, and mice in different serum dilutions within 15 min (Fig. 1). At a serum dilution of 1:10, the ICT showed an overall sensitivity of 98.8% (95% confidence interval [CI] = 93.2 to 99.9%) and an overall specificity of 98.4% (95% CI = 94.5 to 99.8%) compared to microagglutination as the “gold standard” (Table 1) . The concordance of both assays was also excellent and reached a level of 98.1% (κ = 0.98) (Table 2). Compared to the ELISA, the concordance was slightly lower (94% [κ = 0.95]) due to three discordant sera, which were later reanalyzed by Western blot and proved to be ELISA false positives, thus explaining this apparent lower concordance (data not shown).
TABLE 1.
Sensitivity and specificity of the ICT compared to the microagglutination assay, utilized as a “gold standard”
| Specimen | Microagglutination as a referenced |
|
|---|---|---|
| % Sensitivity | % Specificity | |
| Human serum | 98.3 (57/58) | 96.6 (56/58) |
| NHPa serum | 100 (10/10) | 100 (34/34) |
| Pig serum | 100 (2/2) | 100 (6/6) |
| Rabbit serum | 100 (10/10) | 100 (30/30) |
| All animal serab | 100 (22/22) | 100 (70/70) |
| All human and animal serac | 98.8 (79/80) | 98.4 (126/128) |
NHP, nonhuman primate.
Pooled results from NHP, pig, and rabbit sera.
Pooled results from human, NHP, pig, and rabbit sera.
The values in parentheses indicate the number of sera giving positive results/the total number of sera tested.
TABLE 2.
Concordance and discordance between ICT and microagglutinationa
| Specimen | Result |
Concordance (%) | Result |
Discordance (%) | ||
|---|---|---|---|---|---|---|
| ICT | MA | ICT | MA | |||
| Human serum | + | + | 47.4 (55/116) | + | - | 1.8 (2/116) |
| - | - | 50.0 (58/116) | - | + | 0.9 (1/116) | |
| Total | 97.4 (113/116) | 2.7 (3/116) | ||||
| Animal serum | + | + | 23.9 (22/92) | + | - | 0.0 (0/92) |
| - | - | 76.1 (70/92) | - | + | 0.0 (0/92) | |
| Total | 100.0 (92/92) | 0.0 (0/92) | ||||
| Both human and | + | + | 37.0 (77/208) | + | - | 1.0 (2/208) |
| animal sera | - | - | 61.5 (128/208) | - | + | 0.5 (1/208) |
| Total | 98.5 (205/208) | 1.5 (3/208) | ||||
MA, microagglutination. The values in parentheses indicate the number of sera giving positive results/the total number of sera tested. The two assays showed almost perfect agreement (κ = 0.98) (2). Round-off errors account for the total of 100.1% for the combined total of the human serum values.
When pre- and postvaccination sera from five human vaccinees who had received LVS immunization were tested, the ICT detected seroconversion in all individuals (Table 3) . Seroconversion from a negative to a positive rapid test result was also confirmed in five monkeys after they were naturally exposed to F. tularensis between 2003 and 2005 (18). Similar results were obtained from three rabbits and two pigs, which had been immunized with either F. tularensis subsp. tularensis (strain ATCC 6223, one pig) or the LVS strain (one pig and three rabbits) in order to obtain hyper immune sera for diagnostic purposes.
TABLE 3.
Confirmation of seroconversion in five vaccinees who were vaccinated with attenuated LVSa
| Vaccinee | Before vaccination |
After vaccination |
||||
|---|---|---|---|---|---|---|
| ELISA (OD) | ICT | MA titer | ELISA (OD) | ICT | MA titer | |
| 1 | Neg (0.016) | - | Neg | 2.752 | +++ | 1:2,048 |
| 2 | Neg (0.008) | - | Neg | 1.862 | ++ | 1:64 |
| 3 | Neg (0.072) | - | Neg | 2.373 | +++ | 1:512 |
| 4 | Neg (0.034) | - | Neg | 1.809 | +++ | 1:256 |
| 5 | Neg (0.025) | - | Neg | 1.452 | ++ | 1:128 |
Postvaccination sera were obtained between 4 and 6 weeks after immunization. MA, microagglutination. OD, optical density; Neg, negative; +++, strongly positive; ++, moderately positive; −, negative.
The specificity of the ICT was further tested with pigs immunized with corresponding amounts of inactivated F. tularensis subsp. novicida or F. philomiragia. Sera from these animals showed a seroconversion and a high titer of specific antibodies only when tested by ELISA or immunoblot using whole bacterial antigen of F. tularensis subsp. novicida or F. philomiragia, respectively. However, these sera showed no positive results in the ICT or the other tularemia-specific tests, thus confirming the high specificity of these assays. In addition, no cross-reactivity was observed when applying rabbit sera with high titers against Yersinia pestis, Burkholderia cepacia, B. mallei, B. pseudomallei, or Pseudomonas aeruginosa.
Because sera from infected or immunized mice were not available, we tested the capacity of the rapid test to detect mouse antibodies by using different MAbs with known reactivity toward F. tularensis LPS. In 10 of 11 antibody preparations representing seven different antibodies derived from individual hybridoma cell lines, positive reactions were obtained with the ICT when 10 μg/ml was used. The remaining antibody showed a positive ICT at a concentration of 62.5 μg/ml.
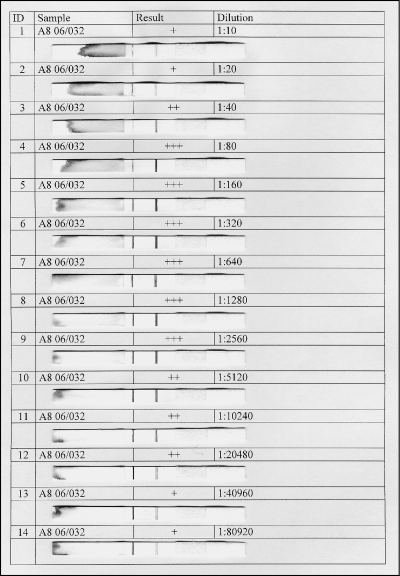
In a few serum samples (n = 4) a “hook effect” was noticed, since highly reactive sera induced only a weak signal on the test line (Fig. 2). This effect is probably due to the limiting quantity of protein A-conjugate utilized in the test, compared to the high amounts of specific immunoglobulins present in these sera. In all cases, however, positive sera could still be easily detected at a working dilution of 1:10, even though reactivity of highly positive sera was best appreciated at a serum dilutions of 1:320 or 1:640 (Fig. 2). All respective sera had reciprocal anti-F. tularensis titers of 2,048 or higher.
FIG. 2.
In 4 of 80 positive sera a “high-dose hook effect” was observed that resulted in relatively weak positive signals at the working serum dilution of 1:10. The respective sera had reciprocal anti-F. tularensis titers of 2,048 or higher (microagglutination). However, these sera could still be correctly classified as positive. At higher serum dilutions (1:1,280 to 1:20,480) the close correlation of antibody concentration and signal magnitude was apparent.
That “hook effect” set aside, we observed, with the naked eye, that the intensity of the test line was correlated with the amount of anti-F. tularensis LPS antibodies present in each positive sample. The ICT thus allows a semiquantitative analysis, with the signal evaluated by the technician as weakly positive, positive, or strongly positive. Preferably, as a more exact quantification may be required in clinical diagnostics, we also tested serial dilutions of positive samples from humans and NHP. We observed that 2-fold dilutions ranging from 1:10 to 1: 20,480 gave signals apparently correlated to the serum dilution. Using this approach, which is comparable to “endpoint titration” in ELISA, it may be possible to monitor the serum reactivity over time. Depending on the result of future trials, the newly established point-of-care test will perhaps be turned into a fully quantitative test with additional equipment.
DISCUSSION
Due to the highly infectious nature of F. tularensis, the difficulties caused by the special growth requirements, and the lack of standardized, well-evaluated PCR protocols, the clinical diagnosis of tularemia in humans is most commonly confirmed by serological proof (10, 27, 29). Definitive serological affirmation requires a 4-fold or greater rise in titers between acute and convalescent-phase sera (33). Serological tests are also needed for epidemiological studies in humans and animals and for monitoring the rise of specific antibodies after vaccination. Different investigators reported various serological methods for this purpose. For decades the whole-cell agglutination test (Widal's reaction) was the most widely used assay, but modifications such as the introduction of a microagglutination assays resulted in superior performance (6, 23). Currently, agglutination assays are still widely used and are the only commercially available and certified diagnostic test in many countries (14), even though ELISAs repeatedly proved to be more sensitive than agglutination assays (7, 28). Several different antigen preparations were used to assess the specific immune response after natural infection or vaccination, including crude bacterial sonicates (17, 28, 31), purified LPS (7, 10, 21), purified outer membrane antigens (4), ether extracts, and whole bacterial cells of the strains F. tularensis subsp. holarctica LVS and F. tularensis subsp. tularensis SCHU4 (32). These antigens were used in agglutination tests, ELISAs and for Western blotting (5, 32). Virtually all assays were able to demonstrate specific antibodies 5 to 10 days after the onset of symptoms (4, 17) or postvaccination (32). In contrast to several other infections, the role of different immunoglobulin subclasses in the diagnosis of acute tularemia and the reason for the extreme long persistence of Francisella-specific antibodies after infection, which was repeatedly demonstrated by different authors, is still not sufficiently determined (3, 11, 32). Today, a combination of a screening test (ELISA) and a confirmatory test (immunoblotting) might prove to be a feasible two-step approach for the serological diagnosis of tularemia (24). Modern techniques such as flow cytometry have the potential for high throughput and multiplexed testing, which may replace these conventional tests in the future (21). For epidemiological studies in animal populations, competitive assays or methods using protein A-peroxidase conjugates offer the advantage of being applicable for different animal species (15).
Although serology is still considered to be a cornerstone in tularemia diagnosis, none of the assays described in the publications cited above addressed the need for a simple, fast, and cost-effective test, which could also be deployed in outbreak situations affecting remote areas or countries with limited resources. To overcome this problem, we developed a rapid test, which is, according to our study, well suited to detecting specific anti-F. tularensis LPS antibodies, mainly of the IgG subclass, in tularemia patients, but which might also be used in epidemiological studies and in veterinary medicine, where there is a need to confirm or exclude tularemia in livestock or pet animals.
In contrast to the microagglutination assay, a direct method to detect agglutinogens in serum samples (mainly represented by IgM antibodies [31]), our rapid test uses protein A conjugated to colloidal gold to visualize specific antibodies directed against the LPS that is fixed to the NC membrane. Due to the binding affinity of protein A, antibodies of the IgG subclass are preferentially detected. This principle might be disadvantageous in the analysis of early samples obtained from acute tularemia cases, assuming that IgM antibodies may occur earlier after infection with F. tularensis.
Although our ICT detected specific IgG antibodies in 52 of 53 serum samples from 50 tularemia cases, which would have allowed supporting the clinical diagnosis in 49 of 50 patients considered in this retrospective study, we cannot generally rule out that this assay may show negative test results during the very first days of infection.
In tularemia, IgG responses seem to occur early after infection, which was demonstrated by at least four different authors using LPS or crude bacterial sonicate as a diagnostic antigen (7, 10, 17, 31). Eliasson et al. (10) could show that there is only a very short delay of 1 to 2 days in the occurrence of IgG compared to the IgM response in 24 patients, while Koskela and Salminen (17), as well as Viljanen et al. (31), have even demonstrated that IgG tend to appear earlier than other antibodies in acute tularemia infection. ELISAs measuring IgG or IgM responses gave both positive signals well ahead of the agglutination assay (7, 10). Nevertheless, we feel that it is mandatory to further evaluate our rapid test with additional human serum samples from acute cases of tularemia.
Because this test seems to be quantitative, it might even be used to monitor serum titers over time, thus allowing tularemia diagnosis according to WHO standards. Such a use was beyond the goal of the present feasibility study but will be evaluated in the future. Our new assay is based on highly purified LPS, as utilized in recently described ELISA procedures (10, 24). The application of protein A-conjugated gold nanobeads makes it possible to detect immunoglobulins from different animal species. This approach is faster, more straightforward, and easier to apply than other “multispecies” assays such as microagglutination or competitive ELISA (4, 26).
Especially in outbreak situations with reduced laboratory capacities such as those in postwar Bosnia or Kosovo (20, 22), the availability of rapid tests would facilitate the identification of affected patients and to rapidly determine the dimension of the public health threat. The ICT is a simple, cheap, and fast tool for epidemiological studies in different host populations (sheep, hares, rabbits, beavers, mice, and boars) or in human populations. Epidemiological studies or surveillance studies in wildlife or surrogate animals such as boars (1) or predators (34) would also benefit from rapid testing “on the spot” because reliable results may guide the investigation in different directions, e.g. hotspots with high seroprevalence might be more easily identified and targeted sampling could be triggered. In this case, the serological assay should be combined with a sensitive antigen detection assay which is also available in the same test format in order to identify acute infections where antibodies had not yet been developed (13).
Due to the possible threat of bioterrorism or biological warfare posed by F. tularensis, there are several efforts to develop a safe and protective vaccine against tularemia. We could demonstrate that our rapid test is well suited to monitor the immune response after vaccination. Assuming that a completely protective vaccine had been developed, this application would be very valuable in a scenario of mass exposure, in order to rapidly identify individuals who are already protected and do not need further medical support. This situation will also appear in multinational missions involving military troops from different states with different vaccination statuses.
Further evaluation of the rapid test with human sera from tularemia patients, patients suffering from other infectious diseases, and healthy subjects is important. Prospective studies involving quantitative use of the strip on specimens from humans and additional animal species, standardized production and storage testing, and eventually the introduction of a blood separation component, allowing the direct application of whole blood, are future challenges that will be addressed.
In conclusion, a tularemia-specific rapid test based on the ICT format and highly purified LPS as the antigen showed high sensitivity and specificity when used to detect antibodies in specimens from human tularemia patients. Hence, effective treatment could be started even in regions where limited resources would otherwise prevent proper laboratory testing and identification of suspected cases. Its capacity to detect F. tularensis specific antibodies in a wide range of animal hosts turns this rapid assay also into a promising tool in the fields of veterinary medicine, epidemiology, and public health surveillance.
Acknowledgments
We thank Margot Ehrle and Frank Feist for their excellent technical assistance in performing this study.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Al Dahouk, S., K. Nockler, H. Tomaso, W. D. Splettstoesser, G. Jungersen, U. Riber, D. Hoffmann, H. C. Scholz, A. Hensel, and H. Neubauer. 2005. Seroprevalence of brucellosis, tularemia, and yersiniosis in wild boars (Sus scrofa) from North-Eastern Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:444-455. [DOI] [PubMed] [Google Scholar]
- 2.Altman, D. G. 1991. Practical statistics for medical research, p. 403-409. Chapman & Hall, London, United Kingdom.
- 3.Bevanger, L., J. A. Maeland, and A. I. Kvan. 1994. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin. Diagn. Lab. Immunol. 1:238-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevanger, L., J. A. Maeland, and A. I. Naess. 1989. Competitive enzyme immunoassay for antibodies to a 43,000-molecular-weight Francisella tularensis outer membrane protein for the diagnosis of tularemia. J. Clin. Microbiol. 27:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevanger, L., J. A. Maeland, and A. I. Naess. 1988. Agglutinins and antibodies to Francisella tularensis outer membrane antigens in the early diagnosis of disease during an outbreak of tularemia. J. Clin. Microbiol. 26:433-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, S. L., F. T. McKinney, G. C. Klein, and W. L. Jones. 1980. Evaluation of safranin-O-stained antigen microagglutination test for Francisella tularensis antibodies. J. Clin. Microbiol. 11:146-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, H. E., A. A. Lindberg, G. Lindberg, B. Hederstedt, K.-A. Karlsson, and B. O. Agell. 1979. Enzyme-linked immunosorbent assay for immunological diagnosis of human tularemia. J. Clin. Microbiol. 10:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celebi, G., F. Baruönü, F. Ayoğlu, F. Cinar, A. Karadenizli, M. B. Uğur, and S. Gedikoğlu. 2006. Tularemia, a reemerging disease in northwest Turkey: epidemiological investigation and evaluation of treatment responses. Jpn. J. Infect. Dis. 59:229-234. [PubMed] [Google Scholar]
- 9.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, K. Tonat, et al. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 10.Eliasson, H., P. Olcén, A. Sjöstedt, M. Jurstrand, E. Bäck, and S. Andersson. 2008. Kinetics of the immune response associated with tularemia: comparison of an enzyme-linked immunosorbent assay, a tube agglutination test, and a novel whole-blood lymphocyte stimulation test. Clin. Vaccine Immunol. 15:1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericsson, M., G. Sandstrom, A. Sjostedt, and A. Tarnvik. 1994. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J. Infect. Dis. 170:110-114. [DOI] [PubMed] [Google Scholar]
- 12.Greiser-Wilke I., C. Soiné, and V. Moennig. 1989. Monoclonal antibodies reacting specifically with Francisella sp. Zentralbl. Veterinarmed. B 36:593-600. [DOI] [PubMed] [Google Scholar]
- 13.Grunow, R., W. Splettstoesser, S. McDonald, C. Otterbein, T. O'Brien, C. Morgan, J. Aldrich, E. Hofer, E. J. Finke, and H. Meyer. 2000. Detection of Francisella tularensis in biological specimens using a capture enzyme-linked immunosorbent assay, an immunochromatographic handheld assay, and a PCR. Clin. Diagn. Lab. Immunol. 7:86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez, M. P., M. A. Bratos, J. I. Garrote, A. Duenas, A. Almaraz, R. Alamo, M. H. Rodriguez, M. J. Rodriguez Recio, M. F. Munoz, A. Orduna, and A. Rodriguez-Torres. 2003. Serologic evidence of human infection by Francisella tularensis in the population of Castilla y Leon (Spain) prior to 1997. FEMS Immunol. Med. Microbiol. 35:165-169. [DOI] [PubMed] [Google Scholar]
- 15.Guzaeva, T. V., A. M. Komarov, S. V. Yurov, S. Y. Pchelintsev, A. V. Chudinov, and S. S. Afanasiev. 1993. Protein A used in DELFIA for the determination of specific antibodies. Immunol. Lett. 35:285-289. [DOI] [PubMed] [Google Scholar]
- 16.Keim, P., A. Johansson, and D. M. Wagner. 2007. Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105:30-66. [DOI] [PubMed] [Google Scholar]
- 17.Koskela, P., and A. Salminen. 1985. Humoral immunity against Francisella tularensis after natural infection. J. Clin. Microbiol. 22:973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mätz-Rensing, K., A. Floto, A. Schrod, T. Becker, E. J. Finke, E. Seibold, W. D. Splettstoesser, and F. J. Kaup. 2007. Epizootic of tularemia in an outdoor housed group of cynomolgus monkeys (Macaca fascicularis). Vet. Pathol. 44:327-334. [DOI] [PubMed] [Google Scholar]
- 19.Petersen, J. M., P. S. Mead, and M. E. Schriefer. 2009. Francisella tularensis: an arthropod-borne pathogen. Vet. Res. 40:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen, J. M., and M. E. Schriefer. 2005. Tularemia: emergence/re-emergence. Vet. Res. 36:455-467. [DOI] [PubMed] [Google Scholar]
- 21.Porsch-Ozcürümez, M., N. Kischel, H. Priebe, W. Splettstoesser, E. J. Finke, and R. Grunow. 2004. Comparison of enzyme-linked immunosorbent assay, Western blotting, microagglutination, indirect immunofluorescence assay, and flow cytometry for serological diagnosis of tularemia. Clin. Diagn. Lab. Immunol. 11:1008-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reintjes, R., I. Dedushaj, A. Gjini, T. R. Jorgensen, B. Cotter, A. Lieftucht, F. D'Ancona, D. T. Dennis, M. A. Kosoy, G. Mulliqi-Osmani, R. Grunow, A. Kalaveshi, L. Gashi, and I. Humolli. 2002. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg. Infect. Dis. 8:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato, T., H. Fujita, Y. Ohara, and M. Homma. 1990. Microagglutination test for early and specific serodiagnosis of tularemia. J. Clin. Microbiol. 28:2372-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt, P., W. Splettstoesser, M. Porsch-Oz̈cürümez, E. J. Finke, and R. Grunow. 2005. A novel screening ELISA and a confirmatory Western blot useful for diagnosis and epidemiological studies of tularaemia. Epidemiol. Infect. 133:57-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjöstedt, A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1-29. [DOI] [PubMed] [Google Scholar]
- 26.Splettstoesser, W. D., K. Mätz-Rensing, E. Seibold, H. Tomaso, S. Al Dahouk, R. Grunow, S. Essbauer, A. Buckendahl, E. J. Finke, and H. Neubauer. 2007. Re-emergence of Francisella tularensis in Germany: fatal tularaemia in a colony of semi-free-living marmosets (Callithrix jacchus). Epidemiol. Infect. 135:1256-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splettstoesser, W. D., H. Tomaso, S. Al Dahouk, H. Neubauer, and P. Schuff-Werner. 2005. Diagnostic procedures in tularaemia with special focus on molecular and immunological techniques. J. Vet. Med. B Infect. Dis. Vet. Public Health 52:249-261. [DOI] [PubMed] [Google Scholar]
- 28.Syrjala, H., P. Koskela, T. Ripatti, A. Salminen, and E. Herva. 1986. Agglutination and ELISA methods in the diagnosis of tularemia in different clinical forms and severities of the disease. J. Infect. Dis. 153:142-145. [DOI] [PubMed] [Google Scholar]
- 29.Tärnvik, A. M., and M. C. Chu. 2007. New approaches to diagnosis and therapy of tularemia. Ann. N. Y. Acad. Sci. 1105:378-404. [DOI] [PubMed] [Google Scholar]
- 30.Urich, S. K., and J. M. Petersen. 2008. In vitro susceptibility of isolates of Francisella tularensis types A and B from North America. Antimicrob. Agents Chemother. 52:2276-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viljanen, M. K., T. Nurmi, and A. Salminen. 1983. Enzyme-linked immunosorbent assay (ELISA) with bacterial sonicate antigen for IgM, IgA, and IgG antibodies to Francisella tularensis: comparison with bacterial agglutination test and ELISA with lipopolysaccharide antigen. J. Infect. Dis. 148:715-720. [DOI] [PubMed] [Google Scholar]
- 32.Waag, D. M., K. T. McKee, G. Sandstrom, L. L. Pratt, C. R. Bolt, M. J. England, G. O. Nelson, and J. C. Williams. 1995. Cell-mediated and humoral immune responses after vaccination of human volunteers with the live vaccine strain of Francisella tularensis. Clin. Diagn. Lab. Immunol. 2:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization, Epidemic and Pandemic Alert and Response. 2007. WHO guidelines on tularaemia. World Health Organization, Geneva, Switzerland.
- 34.Zarnke, R. L., J. M. Ver Hoef, and R. A. DeLong. 2004. Serologic survey for selected disease agents in wolves (Canis lupus) from Alaska and the Yukon Territory, 1984-2000. J. Wildl. Dis. 40:632-638. [DOI] [PubMed] [Google Scholar]