Abstract
We report the identification and characterization of three Staphylococcus aureus isolates recovered from throat and vaginal cultures, as well as from an axillary abscess, of a 17-year-old female who died of tampon-related toxic shock syndrome. The three S. aureus isolates were unrelated as determined by pulsed-field gel electrophoresis. The vaginal isolate was mecA, Panton-Valentine leukocidin, and staphylococcal enterotoxin B and C negative, toxic shock syndrome toxin 1 positive, and staphylococcal cassette chromosome mec element (SCCmec) untypeable, which was consistent with the clinical and autopsy findings that death was due to tampon-related toxic shock syndrome.
CASE REPORT
The patient was a 17-year-old female who was found lethargic and unresponsive on the day of admission after a 4-day history of fever, vomiting, and diarrhea. She had been transferred by helicopter from an outside hospital to Vanderbilt Children's Hospital Emergency Department (ED), where she was intubated and volume resuscitated. Her temperature on admission was 37°C, pulse 137, respiration 25, and blood pressure 51/36 mm Hg. Initially, her pupils were equal in size and reactive. Auscultation of the lungs showed good breath sounds bilaterally. She had a normal CO2 on the end-tidal CO2 monitor. Her abdomen had hypoactive bowel sounds and was nondistended. She did have a tampon in place, which was removed, and a vaginal culture was obtained. Her extremities were mottled as well as cold by palpation.
While in the ED, multiple attempts were made to obtain blood pressure readings after aggressive fluid management. Despite the patient's afebrile condition, blood cultures and a throat culture were obtained in addition to the vaginal culture. The patient was quickly started on empirical antimicrobial therapy with cefepime and vancomycin due to the possibility of septic shock. She was also given 125 mg of Solu-Medrol in case there was adrenal insufficiency. The patient had venous blood gases obtained; these showed acidosis, with a pH of 6.8. She was given intravenous bicarbonate for her low pH. A chest X-ray was obtained, which showed no evidence of pulmonary infiltrate. The white cell count was 24,800/μl, and the packed cell volume (PCV) was 28%. The patient was transfused with 2 units of blood and started on dopamine, Levophed, and vasopressin drips. Her carotid pulse was the only pulse consistently palpable.
The patient underwent a head, abdominal, and pelvic computed tomography (CT), which showed evidence of extensive third spacing from resuscitation fluids. The patient was transferred to the pediatric critical care unit (PCCU), where she was noted to have unequal pupils with sluggish reactivity. No palpable peripheral or femoral pulses were obtainable; her carotid pulse was just barely palpable. Her extremities were cold and mottled with a capillary refill of 3 s. A right femoral venous central line was put in place, as well as a left tibial intraosseous line. An abscess with central purulent ulceration was noted in her left axilla; this purulent ulceration was cultured. She had been menstruating, and a tampon had been removed after her arrival in the ED. After arrival to the PCCU, a left femoral arterial art line was placed. She continued to be severely hypotensive. Chest compressions and an epinephrine infusion were started, and the patient was resuscitated with multiple doses of epinephrine, calcium, bicarbonate, and vasopressin. An additional unit of packed red blood cells (PRBC) and 1 unit of fresh frozen plasma (FFP) were rapidly transfused.
Without any improvement in her condition despite aggressive resuscitation and inotropic support, pediatric surgery was consulted regarding the possibility of extracorporeal membrane oxygenation (ECMO) cannulation. A bedside echocardiogram showed severely depressed cardiac function without a pericardial effusion. At the time of evaluation, cardiopulmonary resuscitation (CPR) had been ongoing for approximately 15 min. Reexamination of the patient demonstrated sinus bradycardia with no arterial pulsatility. In addition, the patient had fixed, dilated pupils. The patient was deemed not an appropriate candidate for ECMO due to her severe neurologic dysfunction. Family members were brought to the bedside while CPR efforts were ongoing. After >35 min of active CPR with lack of improvement or return of pulsatility in arterial line waveform, all resuscitative efforts were discontinued. The patient was pronounced dead with her family at the bedside. Consent was obtained for a full autopsy.
Bacterial culture and identification.
Bacterial cultures of the throat, vagina, and axillary abscess were set up on 5% sheep blood agar plates, and the plates were incubated overnight at 35°C with 5% CO2. Phenotypic identification and differentiation were determined by colony morphology, catalase, and Staphaurex latex agglutination as previously described (9).
Molecular detection and typing.
A loopful of each purified bacterial isolate was placed into 1 ml of distilled water and heated at 95°C for 7 min. The supernatant was used directly for PCR amplification. The mecA gene was detected by a procedure described previously (12). Real-time PCR assays with TaqMan hydrolysis probes were performed to determine the staphylococcal cassette chromosome mec element (SCCmec) types I, II, III, and IV and to detect the Panton-Valentine leukocidin (PVL) gene as described previously (9). The same TaqMan PCR format was used to detect the toxic shock syndrome toxin 1 (TSST-1) (2). The primers and fluorophore TaqMan probe were as follows: TST-522F, 5′-TCA TCA GCT AAC TCA AAT ACA TGG ATT-3′; TST-609-R, 5′-TGT GGA TCC GTC ATT CAT TGT T-3′; and TST-522-P, 5′-FAM-TCC AAT AAC CAC CCG TTT TAT CGC TTG AA-TAMRA-3′ (where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). Staphylococcal enterotoxins B (SEB) and C (SEC) genes were detected by real-time PCR assays as previously described (10). The relatedness of staphylococcal isolates was determined by a pulsed-field gel electrophoresis (PFGE) typing analysis as previously described (14).
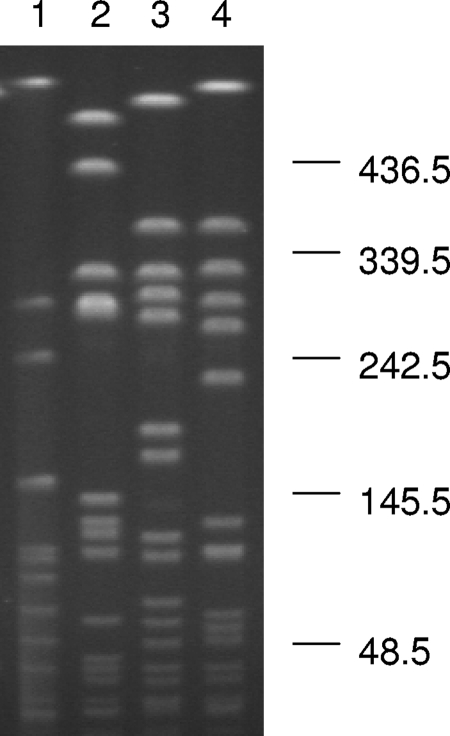
Staphylococcus aureus was isolated from throat and vaginal swab specimens as well as from the left axillary abscess; these cultures were collected at the time of admission. Both pre- and postmortem blood cultures were negative. While both throat and vaginal staphylococcal isolates were susceptible to methicillin, the axillary isolate was methicillin resistant. Further molecular analysis, including mecA, PVL, TSST-1, and SEB and SEC gene detection and SCCmec typing, was performed on the three isolates. The axilla isolate was mecA positive, PVL positive, and SCCmec type IV. Both throat and vaginal isolates were mecA negative and SCCmec untypeable. The TSST-1 gene was detected only in the vaginal isolate, and SEB and SEC genes were negative for all three isolates (Table 1). The three isolates possessed totally unrelated PFGE patterns (Fig. 1) . In comparison to the USA types available in the PulseNet (15), of the two mecA-negative and SCCmec-untypeable isolates, the vaginal one was a subtype of PFGE type USA200 (13), and the throat one was highly related to USA900. The axilla abscess isolate was highly related to USA400 (15).
TABLE 1.
Molecular characterization of the three S. aureus isolates
| Isolation site | Presence ofa: |
SCCmec type | PFGE pattern | ||||
|---|---|---|---|---|---|---|---|
| mecA | PVL gene | TSST-1 gene | SEB gene | SEC gene | |||
| Vaginal swab | − | − | + | − | − | Untypeable | Subtype of USA200 |
| Throat swab | − | − | − | − | − | Untypeable | Highly related to USA900 |
| Axilla abscess | + | + | − | − | − | Type IV | Highly related to USA400 |
+, detected; −, not detected.
FIG. 1.
Pulsed-field gel electrophoresis patterns of SmaI-digested genomic DNA of the three S. aureus isolates. Lanes 1, 2 and 3 are isolates recovered from a vaginal swab, throat swab, and axilla abscess, respectively. Lane 4 is a USA300 MRSA isolate recovered at Vanderbilt University Medical Center (9). Molecular sizes are in kilobases.
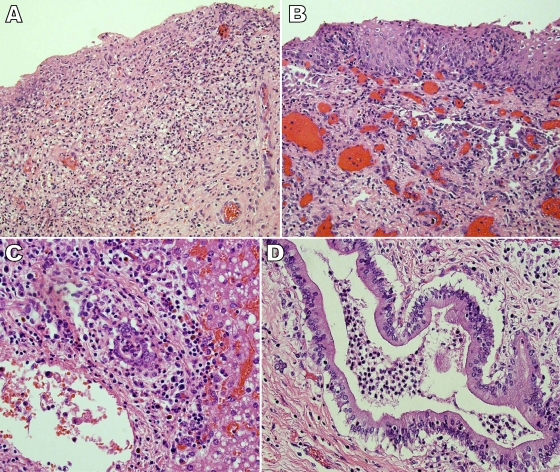
At autopsy, there was a small amount of yellow-green discharge present in the vagina. The vaginal and cervical mucosa appeared mildly hemorrhagic. Focal vaginal ulceration and necrosis as well as mixed acute and chronic vaginal and ectocervical epithelial inflammation were revealed by microscopic examination of vaginal tissues (Fig. 2A). Mixed acute and chronic ectocervical inflammation was noticed in cervix section (Fig. 2B). An ulcerated left axilla abscess was identified with no lymphadenopathy noted in the left cervical and axillary regions. Microscopic examinations of heart and lung tissues indicated no significant inflammatory reactions. Bilateral adrenal gland hemorrhage was observed microscopically, which was consistent with Waterhouse-Fredrickson syndrome. Pulmonary congestion without clear evidence of infection was observed in microscopic sections of pulmonary tissues. Liver tissue microscopy showed mixed acute and chronic periportal inflammation and acute inflammation in bile ducts, consistent with a toxic shock pattern of injury and secondary cholangitis (Fig. 2C and D) (6).
FIG. 2.
Photomicrographs of selected tissue biopsy specimens that were taken at autopsy. (A) Hematoxylin and eosin (H&E) staining (×200 magnification) of a section of the vagina illustrated focal ulceration and granulation tissue with mixed acute and chronic inflammation. There was also focal necrosis of the vaginal tissue. In addition, there were focal collections of neutrophils in blood vessels as well as vascular congestion. Finally, increased intraepithelial lymphocytes, edema, and occasional necrotic keratinocytes were observed. These findings are consistent with those from vaginal tissue taken from a fatal case of tampon-related toxic shock syndrome (1). (B) H&E staining (×200 magnification) of a section of the cervix illustrated mixed acute and chronic ectocervical inflammation. (C) H&E staining (×400 magnification) of a section of liver tissue showed mixed acute and chronic periportal inflammation and acute inflammation in bile ducts. (D) H&E staining (×400 magnification). Acute cholangitis was observed in sections of the bile ducts. These findings are consistent with the pattern of hepatic injury and secondary cholangitis associated with toxic shock syndrome (6).
Three distinct S. aureus isolates were simultaneously recovered from this patient's cultures leading to the question: which staphylococcal isolate was related to the systemic shock? Cultures of ulcerated left axilla abscess grew community-associated methicillin-resistant S. aureus (CA-MRSA), which was PVL positive but TSST-1 negative. Pathological findings associated with PVL production typically include skin furuncles, abscesses, and community-acquired necrotizing pneumonia. Sepsis has been associated with severe skin and lung infections caused by PVL-positive staphylococci (3, 9). However, in this case, the negative blood cultures and limited size of the axillary abscess did not favor this isolate and site of infection as the cause of systemic shock. On the other hand, none of the three isolates carried SEB or SEC genes, and the vaginal isolate was a subtype of PFGE type USA200 and produced TSST-1, a superantigen toxin frequently associated with toxic shock syndrome (5, 6). The USA200 strains are the predominant TSST-1-positive S. aureus strains circulating in the United States (13).
In light of the reported finding of a vaginal tampon at hospital admission as well as negative pre- and postmortem blood cultures, the pathological vaginal and cervical findings are supportive of a diagnosis of tampon-related toxic shock syndrome (1). Moreover, the patient's reported premortem prodrome of fever, nausea, vomiting, altered mental state, and erythroderma also was consistent with the diagnosis of tampon-related toxic shock syndrome, as were the liver biopsy findings at autopsy. The acute cholangitis that was identified in liver tissue sections has been associated with tampon-related toxic shock syndrome in one published report (6). The associated hepatic periportal mixed inflammation is frequently seen in shock settings and could be related to secondary ischemia. Additional findings consistent with shock included bilateral adrenal gland hemorrhage and pulmonary congestion without clear evidence of infection. The third throat isolate most likely represents S. aureus colonization.
Tampon-related toxic shock syndrome was first described in 1978 among young, healthy menstruating women (2, 6). Most reported cases have occurred in Caucasian women with a median reported age of 22 years. The most general descriptions of the syndrome include fever, rash, hypotension, and symptoms and laboratory findings involving at least three organ systems (5, 7, 11). Cultures are usually negative. The vagina during menstruation provides a good growth environment for staphylococci that can produce the TSST-1 toxic shock toxin due to temperature, pH, and glucose levels (8). Tampons are thought to further enable TSST-1 production by exposing the vagina to additional oxygen. Although superabsorbent tampons now withdrawn from the market were associated with an increase in cases of tampon-related toxic shock syndrome in the early 1980s, cases associated with tampon use continue to be reported.
TSST-1 is a superantigen that demonstrates high-affinity binding for the variable beta-chain region of the T-cell receptor, allowing stimulation of much larger numbers of T cells upon exposure to the toxin and a significantly greater release of cytokines (11). Most healthy people develop humoral immunity to this superantigen during early life. However, a subset of the population that fails to develop such immunity is vulnerable to the development of toxic shock syndrome upon colonization or infection by a strain of staphylococcus that produces TSST-1. Other superantigens carried by S. aureus include staphylococcal enterotoxins in which SEB and SEC have accounted for most nonmenstrual toxic shock syndrome cases (4, 10).
This case illustrates the continued presence of fatal toxic shock syndrome. Moreover, had the characterization of these three staphylococcal strains not been done, this fatal case most likely would have been incorrectly attributed to systemic infection and/or non-tampon-related toxic shock syndrome, secondary to the MRSA axillary abscess.
Acknowledgments
We thank Haijing Li, Rosemary Verrall, Shufang Meng, Truc Minh Le, Ty Abel, Kay Washington, and Richard Goering for their excellent assistance and/or discussion.
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Blair, J. D., D. G. Livingston, and R. Vongsnichakul. 1982. Tampon-related toxic-shock syndrome. Histopathologic and clinical findings in a fatal case. Am. J. Clin. Pathol. 78:372-376. [DOI] [PubMed] [Google Scholar]
- 2.Blomster-Hautamaa, D. A., B. N. Kreiswirth, J. S. Kornblum, R. P. Novick, and P. M. Schlievert. 1986. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J. Biol. Chem. 261:15783-15786. [PubMed] [Google Scholar]
- 3.Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87:3-9. [DOI] [PubMed] [Google Scholar]
- 4.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzer, C. M. 2001. Toxic shock syndrome: broadening the differential diagnosis. J. Am. Board Fam. Pract. 14:131-136. [PubMed] [Google Scholar]
- 6.Ishak, K. G., and W. A. Rogers. 1981. Cryptogenic acute cholangitis-association with toxic shock syndrome. Am. J. Clin. Pathol. 76:619-626. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsuki, K., O. Yamasaki, S. Morizane, and T. Oono. 2006. Staphylococcal cutaneous infections: invasion, evasion and aggression. J. Dermatol. Sci. 42:203-214. [DOI] [PubMed] [Google Scholar]
- 8.Jaulhac, B., M. L. De Buyser, F. Dilasser, G. Prevost, and Y. Piedmont. 1991. Screening of staphylococci for the toxic shock syndrome toxin-1 (TSST-1) gene. Lett. Appl. Microbiol. 13:90-92. [DOI] [PubMed] [Google Scholar]
- 9.Kilic, A., H. Li, C. W. Stratton, and Y. W. Tang. 2006. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of, as well as Panton-Valentine leukocidin occurrence among, methicillin-resistant Staphylococcus aureus isolates from children and adults in middle Tennessee. J. Clin. Microbiol. 44:4436-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klotz, M., S. Opper, K. Heeg, and S. Zimmermann. 2003. Detection of Staphylococcus aureus enterotoxins A to D by real-time fluorescence PCR assay. J. Clin. Microbiol. 41:4683-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura, M. M., K. L. Rohling, M. Shashaty, H. Lu, Y. W. Tang, and K. M. Edwards. 2002. Prevalence of methicillin-resistant Staphylococcus aureus nasal carriage in the community pediatric population. Ped. Infect. Dis. J. 21:917-922. [DOI] [PubMed] [Google Scholar]
- 13.Parsonnet, J., R. V. Goering, M. A. Hansmann, M. B. Jones, K. Ohtagaki, C. C. Davis, and K. Totsuka. 2008. Prevalence of toxic shock syndrome toxin 1 (TSST-1)-producing strains of Staphylococcus aureus and antibody to TSST-1 among healthy Japanese women. J. Clin. Microbiol. 46:2731-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang, Y. W., M. G. Waddington, D. H. Smith, J. M. Manahan, P. C. Kohner, L. M. Highsmith, H. Li, F. R. Cockerill III, R. L. Thompson, S. O. Montgomery, and D. H. Persing. 2000. Comparison of protein A gene sequencing with pulsed-field gel electrophoresis and epidemiologic data for molecular typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 38:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenover, F. C., S. McAllister, G. Fosheim, L. K. McDougal, R. B. Carey, B. Limbago, D. Lonsway, J. B. Patel, M. J. Kuehnert, and R. Gorwitz. 2008. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J. Clin. Microbiol. 46:2837-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]