Abstract
Staphylococcus lugdunensis is a coagulase-negative staphylococcus that has several similarities to Staphylococcus aureus. S. lugdunensis is increasingly being recognized as a cause of prosthetic joint infection (PJI). The goal of the present retrospective cohort study was to determine the laboratory and clinical characteristics of S. lugdunensis PJIs seen at the Mayo Clinic in Rochester, MN, between 1 January 1998 and 31 December 2007. Kaplan-Meier survival methods and Wilcoxon sum-rank analysis were used to determine the cumulative incidence of treatment success and assess subset comparisons. There were 28 episodes of S. lugdunensis PJIs in 22 patients; half of those patients were females. Twenty-five episodes (89%) involved the prosthetic knee, while 3 (11%) involved the hip. Nine patients (32%) had an underlying urogenital abnormality. Among the 28 isolates in this study tested by agar dilution, 24 of 28 (86%) were oxacillin susceptible. Twenty of the 21 tested isolates (95%) lacked mecA, and 6 (27%) of the 22 isolates tested produced β-lactamase. The median durations of parenteral β-lactam therapy and vancomycin therapy were 38 days (range, 23 to 42 days) and 39 days (range, 12 to 60 days), respectively. The cumulative incidences of freedom from treatment failure (standard deviations) at 2 years were 92% (±7%) and 76% (±12%) for episodes treated with a parenteral β-lactam and vancomycin, respectively (P = 0.015). S. lugdunensis is increasingly being recognized as a cause of PJIs. The majority of the isolates lacked mecA. Episodes treated with a parenteral β-lactam antibiotic appear to have a more favorable outcome than those treated with parenteral vancomycin.
Staphylococcus lugdunensis, a coagulase-negative staphylococcus, was first described by Freney et al. in 1988 (6) on the basis of the analysis of 11 strains collected in Lyon, France. It is increasingly being identified as a cause of serious infections (10, 12, 13, 15-17). This organism may produce a bound coagulase via a clumping factor, a property which, if it is present, it shares with Staphylococcus aureus; but unlike S. aureus, it does not produce a free coagulase. In the laboratory, it can give a positive slide (short) coagulase test result but gives a negative tube (long) coagulase test result; hence, if appropriate tests are not performed, it is sometimes misidentified as S. aureus. Of the many coagulase-negative staphylococci that react to pyrrolidonylarylamidase (PYR), only S. lugdunensis, along with a small number of Staphylococcus epidermidis strains, is able to decarboxylate ornithine, distinguishing it from other staphylococcal species (13, 15).
Although it is classified as a coagulase-negative staphylococcus, S. lugdunensis can be virulent and can cause serious infections, including prosthetic joint infections (PJIs), endocarditis, osteomyelitis, and septicemia. This organism is considered part of the normal flora of human skin, and it has been reported to be present in the perineum and inguinal area as well (10, 16, 17, 21). In addition, this organism is capable of producing diseases similar to those caused by S. aureus. However, of more clinical significance is its propensity to be misidentified as another coagulase-negative staphylococcus, if additional laboratory testing is not performed. This may influence antimicrobial management through the misapplication of inappropriate oxacillin breakpoints. For various reasons, including small sample sizes and inadequate follow-up, data on S. lugdunensis prosthetic joint infections are scarce.
The goal of the retrospective cohort study described here was to outline the laboratory data and clinical characteristics of patients with S. lugdunensis total hip or knee prosthetic joint infections. These data will be useful as a guide to microbiologists and clinicians for the assessment and management of patients with S. lugdunensis prosthetic joint infections.
MATERIALS AND METHODS
Study population.
The records and microbiology data for all episodes with a total hip or knee arthroplasty infection due to S. lugdunensis seen at the Mayo Clinic in Rochester, MN, between 1 January 1998 and 31 December 2007 were reviewed. S. lugdunensis PJI was defined according to a strict case definition, which is outlined below. Episodes were followed from the date of PJI diagnosis until treatment failure, prosthesis removal, death, or the end of the study.
Data collection.
Data were obtained from the electronic and written medical records and transferred onto a structured data sheet. These data were then analyzed by using JMP (version 7.0.1) statistical program software. Laboratory records were reviewed in conjunction with the information described above. We collected information regarding demographics, as well as the medical and surgical treatment modalities.
Isolation and identification of S. lugdunensis isolates.
Beginning in October 2001, all coagulase-negative staphylococci subjected to in vitro susceptibility testing underwent screening for the presence of ornithine decarboxylase. If the result was positive, confirmatory testing by the PYR test was performed. If the result of the PYR test was positive, final confirmation was performed by use of a tube ornithine decarboxylase test. If the results of all three tests were found to be positive, the organism was then identified as S. lugdunensis.
In vitro susceptibility testing was done by the agar dilution method. Testing for inducible β-lactamase was performed by use of a nitrocefin-based test, while PCR was used to determine whether mecA was present (13).
Statistical methods.
The number and percentage of episodes in each of the medical and surgical groups were calculated. Differences in demographic factors, clinical variables, and microbiology between the groups were compared by the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. These analyses were performed with JMP (version 7.0.1) software. The cumulative probability of treatment failure and its standard error were estimated by survival analysis (Kaplan-Meier survival method). Comparisons of the overall efficacy between the medical treatment strategies were performed by use of the Petot-Petot Wilcoxon test.
Definitions of terms used.
(i) S. lugdunensis prosthetic joint infection was said to be present if a pure culture of S. lugdunensis was isolated from at least one periprosthetic tissue or joint fluid source, along with any of the following: (a) purulence surrounding the prosthesis, which was observed at the time of periprosthetic aspiration or during surgery; (b) acute inflammation that was consistent with infection on histopathologic examination; or (c) the presence of a sinus tract, found on examination or during operative repair.
(ii) Treatment failure was defined as the occurrence of a PJI caused by S. lugdunensis or any other organism at any time after the original therapy episode.
RESULTS
Study population.
Between 1 January 1998 and 31 December 2007, we identified 28 episodes of S. lugdunensis PJIs in 22 patients who were evaluated at the Mayo Clinic in Rochester, MN. Six patients had more than one episode. The overall mean age of the cohort was 73.5 years (range, 35 to 88 years). Fourteen (50%) patients were males, and 14 (50%) patients were females. Twenty-five (89%) presented with prosthetic knee infections, while three (11%) had hip infections. Three episodes (11%) had diabetes mellitus, and five (18%) were receiving chronic steroid therapy. Nine (32%) had a urogenital abnormality, which included pelvic prolapse (one episode), urge incontinence (one episode), cystocele (one episode), rectocele (one episode), surgical involvement of the testes or prostate (two episodes), recurrent urinary tract infections (two episodes), and/or placement of a penile prosthesis (one episode). It should be noted that one of the patients had an underlying urogenital abnormality but was not treated with either intravenous (i.v.) vancomycin or a β-lactam. The median joint ages for episodes treated with parenteral β-lactam therapy and vancomycin were 961.5 days (range 145 to 2,122 days) and 1,386 days (range, 303 to 9,335) (P = 0.02), respectively.
The median number of specimens submitted per episode for the diagnosis of an S. lugdunensis PJI was four (range, one to six). The median number of positive cultures was four (range, one to six). The specimens submitted included combinations of periprosthetic tissue and synovial fluid. For 10 of the 28 episodes, the diagnosis of S. lugdunensis PJI was obtained through positive cultures of specimens from more than one source.
β-Lactam resistance and in vitro susceptibility.
All 28 isolates underwent in vitro oxacillin susceptibility testing by the agar dilution method. Among the 28 isolates tested, 24 (86%) were oxacillin susceptible. Twenty-two of the 28 isolates were tested for the presence of the β-lactamase enzyme, and 21 of the 28 isolates (75%) were tested for the mecA gene. Prior to 2001, the Mayo Clinic microbiology laboratory did not routinely identify S. lugdunensis among certain coagulase-negative staphylococci. Over the time period of the study, not all of the isolates identified were routinely tested for the presence of the mecA gene or the production of β-lactamase. Twenty of the 21 (95%) isolates tested lacked mecA, and 6 of the 22 (27%) isolates tested produced β-lactamase. Twenty seven of the 27 (100%) isolates tested were susceptible to vancomycin.
In vitro susceptibilities to commonly used oral antimicrobials were also determined. Ten of the 10 (100%) isolates tested for minocycline susceptibility were found to be susceptible. All five (100%) isolates tested for rifampin susceptibility were found to be rifampin susceptible. Fourteen of the 24 (58%) oxacillin-susceptible isolates tested were susceptible to penicillin, while all 24 (100%) isolates tested for susceptibility to levofloxacin were susceptible. All 27 (100%) isolates tested for susceptibility to trimethoprim-sulfamethoxazole were found to be susceptible.
Surgical intervention.
Of the total number of surgical interventions performed, 14 of the episodes (50%) underwent a two-stage revision that was associated with antibiotic therapy administered between revisions, 10 (36%) underwent irrigation and debridement followed by chronic suppression, 1 (4%) had a one-stage exchange performed with subsequent antibiotic therapy, 1 (4%) had a resection arthroplasty, and 2 (7%) underwent chronic antibiotic suppression alone without surgical intervention. Table 1 outlines the surgical modalities used by type of parenteral antibiotic therapy.
TABLE 1.
Characteristics of episodes of S. lugdunensis PJIs by type of parenteral antimicrobial therapy received
| Variable | Values for episodes treated with: |
P value | |
|---|---|---|---|
| β-Lactam (n = 14) | Vancomycin (n = 13) | ||
| Median (minimum/maximum) joint age (days) | 961.5 (145/2,122) | 1386 (303/9,335) | 0.02 |
| Median (minimum/maximum) age at time of diagnosis (yr) | 74.6 (34.9/87.7) | 74.7 (34.9/87.7) | 0.40 |
| No. of patients (% of total) with: | |||
| Sinus tract | 1 (3.7) | 3 (11.1) | 0.24 |
| Surgical therapy by two-stage exchange | 8 (29.6) | 6 (22.2) | 0.70 |
| Surgical therapy by debridement and retention of prosthesis | 5 (35.7) | 5 (35.7) | 0.70 |
| Other therapya | 1 (3.7) | 2 (7.4) | 0.70 |
| Pus | 13 (48.1) | 9 (33.33) | 0.10 |
| Total hip arthroplasty | 2 (7.4) | 1 (3.7) | 0.50 |
| Total knee arthroplasty | 12 (44.4) | 12 (44.4) | 0.50 |
| Immunocompromise | 4 (14.8) | 6 (22.2) | 0.15 |
| Chronic antimicrobial suppression | 7 (25.9) | 4 (14.8) | 0.3 |
| Genitourinary pathologyb | 4 (14.8) | 4 (14.8) | 0.9 |
| Antibiotic spacer placement | 7 (25.9) | 8 (29.6) | 0.54 |
Includes resection arthroplasty, a one-stage exchange procedure, and chronic antibiotic suppression without surgical intervention.
One patient presented with an underlying genitourinary pathology and did not receive either i.v. vancomycin or a β-lactam.
Medical therapy.
Of the two main intravenous antibiotic regimens used, parenteral β-lactam therapy with either cefazolin (11 episodes) or ceftriaxone (3 episodes) was used for a median of 38 days (range, 23 to 42 days) following surgical intervention. Parenteral vancomycin (13 episodes) was used for a median of 39 days following intervention (range 12 to 60 days). Seventeen episodes (61%) received oral antibiotic therapy following the administration of parenteral antibiotic therapy. The oral antibiotics used included β-lactam antibiotics (41%), fluoroquinolones (24%), trimethoprim-sulfamethoxazole (18%), and minocycline (18%). An antibiotic-impregnated polymethylmethacrylate spacer was used in 15 episodes (54%). The antibiotics used in these spacers contained a combination of vancomycin and gentamicin (73%) or vancomycin and tobramycin (27%).
Outcome and survival analysis.
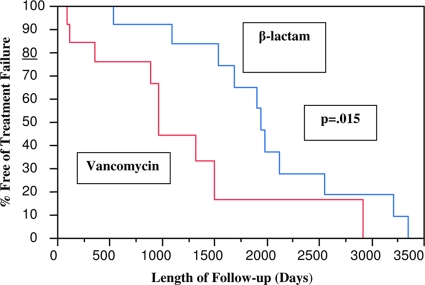
The overall 2-year cumulative incidence (standard deviation) of freedom from treatment failure was 85% (±7%). Six (21%) episodes had a relapse of S. lugdunensis PJIs; three (50%) of these episodes were treated with i.v. vancomycin, two (33%) were given i.v. ceftriaxone, and one (17%) was given cefazolin (Ancef). Two episodes were lost to follow-up. The cumulative incidences of freedom from treatment failure at 2 years among episodes treated with two-stage exchange and debridement/component retention were 86% (±9%) and 79% (±13%), respectively (P = 0.8). The cumulative incidences of freedom from treatment failure at 2 years among episodes treated with parenteral vancomycin and a β-lactam were 76% (±12%) and 92% (±7.4), respectively (P = 0.015; Fig. 1). The differences in outcomes between episodes treated with a β-lactam and those treated with vancomycin were more pronounced in recurrent episodes than in initial episodes of S. lugdunensis PJIs. Table 1 compares selected characteristics by receipt of parenteral β-lactam versus vancomycin therapy.
FIG. 1.
Kaplan-Meier time-dependent analysis of S. lugdunensis PJIs by type of medical therapy. Administration of parenteral β-lactam therapy was associated with a longer infection-free period.
DISCUSSION
To our knowledge, this is the largest published evaluation of a cohort of patients with S. lugdunensis prosthetic joint infections. It is now established that S. lugdunensis has considerable potential as an aggressive pathogen (4-8, 11-17). In addition, it behaves quite similarly to S. aureus; furthermore, misidentification as another coagulase-negative Staphylococcus species may lead to suboptimal medical management. Like S. aureus, S. lugdunensis may be positive for clumping factor, and in that case, it will show a positive reaction by latex agglutination tests. The PYR reaction and ornithine decarboxylation tests are positive for S. lugdunensis. Among PYR-positive coagulase-negative staphylococci such as S. haemolyticus, S. schleiferi, S. xylosus, S. simulans, S. intermedius, S. caprae, and S. lugdunensis, S. lugdunensis is also ornithine decarboxylase positive.
S. lugdunensis appears to have a predilection for individuals with an underlying urogenital pathology. In this study, 32% of the episodes had a urogenital abnormality. This finding is reinforced by the findings previously presented in case reports (8, 10, 16, 17, 21). It is important to point out that our study cannot definitively associate these urogenital abnormalities with patients who have S. lugdunensis PJIs, given the design of a single-cohort study. Future studies, such as case-control studies, would likely determine if, in fact, an actual association exists between S. lugdunensis PJIs and the presence of urogenital abnormalities. S. lugdunensis has been reported to colonize the perineum. Therefore, one can hypothesize that this organism either spreads into the urogenital system from the perineum and subsequently into prosthetic joints perioperatively or spreads via the hematogenous route into prosthetic joint sites via the translocation of this organism through incisions made during surgery.
On the basis of our findings, we believe that S. lugdunensis PJIs behave more like S. aureus PJIs rather than other coagulase-negative staphylococcal PJIs, in that these infections tend to be more aggressive and invasive. S. aureus and S. lugdunensis share similar clinical characteristics, and infections with these organisms are treated in similar manners. We believe that, given the nature of S. lugdunensis PJIs, all prosthetic joint fluid and tissue samples that contain a staphylococcal organism be further evaluated in order to determine if S. lugdunensis is present. However, we believe that it is more important to make the distinction between coagulase-negative staphylococci and S. lugdunensis, given the differences in the in vitro susceptibility breakpoints, clinical characteristics, and therapeutic management. An oxacillin MIC of 1 μg/ml for coagulase-negative staphylococci would be interpreted as resistant, according to current Clinical and Laboratory Standards Institute guidelines (9, 14, 18, 23); however, for S. lugdunensis, this value would be interpreted as susceptible.
Several studies have compared the efficacies of β-lactam antibiotics to those of glycopeptides, such as vancomycin, in patients with serious oxacillin-susceptible Staphylococcus aureus infections. Those studies have shown that β-lactam therapy is superior to vancomycin therapy for a number of reasons. First, β-lactams have a more rapid bactericidal effect than glycopeptides. Second, vancomycin has poorer penetration into bone than β-lactam antibiotics (1, 3, 23). Given this, we favor the use of β-lactams over vancomycin for the treatment of S. lugdunensis PJIs, whenever possible. This is further supported by the fact that the widespread use of vancomycin can be associated with adverse events more serious than those associated with the use of β-lactams, such as hypotension and red-man syndrome, in addition to its effects on the skin and gastrointestinal flora (1, 3, 23). On the basis of these data, PJIs caused by oxacillin-susceptible S. aureus strains tend to be managed with β-lactam therapy, and on the basis of our findings, PJIs caused by S. lugdunensis should be managed similarly.
We have analyzed a number of potential confounders that might have affected the outcomes of the PJIs. None except for joint age were found to be significant. Although a statistically significant difference in joint age was noted between episodes treated with a parenteral β-lactam versus vancomycin, the median joint age was more than 2 years in both treatment groups. Prior studies did not show a significant impact of joint age on the outcomes of PJIs that far out (19). Therefore, we believe that the outcome of episodes with PJIs was not confounded by this finding.
Of the S. lugdunensis isolates evaluated in this study, 86% were susceptible to oxacillin, and of those tested, 73% were susceptible to penicillin. β-Lactamase production has been reported in 24 to 29% of American isolates but less frequently in isolates tested from Europe (8). In the present study cohort, a β-lactam and vancomycin were the two parenteral antibiotics used for the treatment of S. lugdunensis PJIs. Therapy with parenteral β-lactam antibiotics was associated with a lower rate of treatment failure than therapy with vancomycin. The assessment of other confounders was unrevealing. Vancomycin therapy was used in a number of episodes with mecA-negative isolates, possibly due to differences in Clinical and Laboratory Standards Institute guidelines for the interpretation of oxacillin MICs over time.
Since the mecA gene product confers β-lactam antibiotic resistance, the lack of this gene in the majority of the isolates tested would suggest that therapy with a β-lactam antibiotic, such as cephalosporins and antistaphylococcal penicillins, is acceptable when the isolate is oxacillin susceptible. Our data also show that S. lugdunensis is more common in prosthetic knee infections than in prosthetic hip infections. This may suggest that this organism has some predilection for prosthetic knees; however, this cannot be ascertained, given the lack of a control group. This observation would warrant confirmation in future studies.
In conclusion, S. lugdunensis PJIs have distinctive microbiologic and clinical characteristics (15). The majority of the S. lugdunensis isolates tested were susceptible to β-lactams. Episodes treated with a β-lactam appeared to have a more favorable outcome than episodes treated with vancomycin. A high proportion of underlying urogenital abnormalities and total knee arthroplasty infections were observed in this cohort. Future cohort studies are warranted to confirm these findings.
Footnotes
Published ahead of print on 24 February 2010.
REFERENCES
- 1.Bolon, M. K., M. Morlote, S. G. Weber, B. Koplan, Y. Carmeli, and S. B. Wright. 2004. Glycopeptides are no more effective than β-lactam agents for prevention of surgical site infection after cardiac surgery: a meta-analysis. Clin. Infect. Dis. 38:1357-1363. [DOI] [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Finkelstein, R., G. Rabino, T. Mashiah, Y. Bar-El, Z. Adler, V. Kertzman, O. Cohen, and S. Milo. 2002. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J. Thorac. Cardiovasc. Surg. 123:326-332. [DOI] [PubMed] [Google Scholar]
- 4.Fleurette, J., M. Bes, Y. Brun, J. Freney, M. Coulet, M. E. Reverdy, and J. Etienne 1989. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacterial characteristics and susceptibility to antimicrobial agents. Res. Microbiol. 140:107-118. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Freney, J., Y. Brun, M. Bes, H. Meugnier, F. Grimont, P. Grimont, C. Nervi, and J. Fleurette. 1988. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Bacteriol. 38:168-172. [Google Scholar]
- 7.Herchline, T. E., J. Barnishan, L. W. Ayers, and R. J. Fass. 1990. Penicillinase production and in vitro susceptibilities of Staphylococcus lugdunensis. Antimicrob. Agents Chemother. 34:2434-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herchiline, T. E., and L. W. Ayers. 1991. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation and infection. J. Clin. Microbiol. 29:419-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain, Z., L. Stoakes, V. Massey, D. Diagre, V. Fitzgerald, S. El Sayed, and R. Lannigan. 2000. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J. Clin. Microbiol. 38:752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling, M. L., and M. Yeo. 2000. Staphylococcus lugdunensis: report of first case of skin and soft tissue infection in Singapore. Singapore Med. J. 41:177-178. [PubMed] [Google Scholar]
- 11.Monsen, T., H. Abd, K. Leonardsson, H. Edebro, and J. Wistrom. 2002. Prediction of mecA-positive coagulase-negative staphylococci: assessment of different phenotypic methods, breakpoints, culture media and culture conditions. J. Antimicrob. Chemother. 49:197-200. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch, D. R., R. J. Everts, S. T. Chambers, and I. A. Cowan. 1996. Vertebral osteomyelitis due to Staphylococcus lugdunensis. J. Clin. Microbiol. 34:993-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel, R., K. E. Piper, M. S. Rouse, J. R. Uhl, F. R. Cockerill III, and J. M. Steckelberg. 2000. Frequency of isolation of Staphylococcus lugdunensis among staphylococcal isolates causing endocarditis: a 20-year experience. J. Clin. Microbiol. 38:4262-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perazzi, B., M. R. Fermepin, A. Malimovka, S. D. Garcia, M. Orgambide, C. A. Vay, R. de Torres, and A. M. R. Famiglietti. 2006. Accuracy of cefoxitin disk testing for characterization of oxacillin resistance mediated by penicillin-binding protein 2a in coagulase-negative staphylococci. J. Clin. Microbiol. 44:3634-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampathkumar, P., D. R. Osmon, and F. R. Cockerill III. 2000. Prosthetic joint infection due to Staphylococcus lugdunensis. Mayo Clinic Proc. 75:511-512. [DOI] [PubMed] [Google Scholar]
- 16.Seenivasan, M. H., and V. L Yu. 2003. Staphylococcus lugdunensis endocarditis: the hidden peril of coagulase-negative staphylococcus in blood cultures. Eur. J. Clin. Microbiol. Infect. Dis. 22:489-491. [DOI] [PubMed] [Google Scholar]
- 17.Shuttleworth, R., and W. D. Colby. 1992. Staphylococcus lugdunensis endocarditis. J. Clin. Microbiol. 30:1948-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan, T. Y., S. Y. Ng, and J. He. 2008. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J. Clin. Microbiol. 46:2393-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukayama, D. T., V. M. Goldberg, and R. Kyle. 2003. Diagnosis and management of infection after total knee arthroplasty. J. Bone Joint Surg. Am. 85:S75-S80. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Van der Mee-Marquet, N., A. Achard, L. Mereghetti, A. Danton, M. Minier, and R. Quentin. 2003. Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J. Clin. Microbiol. 41:1404-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Wood, C. A., and R. M. Wisniewski. 1994. Beta-lactams versus glycopeptides in treatment of subcutaneous abscesses infected with Staphylococcus aureus. Antimicrob. Agents Chemother. 38:1023-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]