Abstract
The incidence of rubella cases in China from 1991 to 2007 was reviewed, and the nucleotide sequences from 123 rubella viruses collected during 1999 to 2007 and 4 viral sequences previously reported from 1979 to 1984 were phylogenetically analyzed. Rubella vaccination was not included in national immunization programs in China before 2007. Changes in endemic viruses were compared with incidences of rubella epidemics. The results showed that rubella epidemics occur approximately every 6 to 8 years (1993/1994, 2001, and 2007), and a shift of disease burden to susceptible young adults was observed. The Chinese rubella virus sequences were categorized into 5 of the 13 rubella virus genotypes, 1a, 1E, 1F, 2A, and 2B; cocirculations of these different genotypes were found in China. In Anhui province, a shift in the predominant genotype from 1F and 2B to 1E coincided with the 2001 rubella epidemic. This shift may have occurred throughout China during 2001 to 2007. This study investigated the genotype distribution of rubella viruses in China over a 28-year period to establish an important genetic baseline in China during its prevaccination era.
Rubella virus infection is usually mild or asymptomatic in children and adults. The greatest public health consequence of rubella is that infections in the first trimester of pregnancy often lead to serious birth defects, including hearing impairment, cataracts, and cardiac defects, collectively known as congenital rubella syndrome (CRS) (7). The estimated annual incidence of CRS cases worldwide was 100,000 in 2003 (21).
Rubella virus is the sole member of the genus Rubivirus, in the family Togaviridae. The virus has a single-strand, positive-sense RNA genome of 9,762 nucleotides (nt) that encodes 2 nonstructural polypeptides (p150 and p90) within its 5′-terminal two-thirds and 3 structural polypeptides (C, E2, and E1) within its 3′-terminal one-third (4). The E1 glycoprotein is considered immunodominant in the humoral response induced against the structural proteins (2) and contains neutralizing and hemagglutinating determinants (4). A 739-nt region within the E1 gene (nt 8731 to 9469) is accepted as the minimum amount of sequence information required for molecular epidemiological purposes. Nine rubella virus genotypes (1B, 1C, 1D, 1E, 1F, 1G, 2A, 2B, and 2C) and 4 provisional genotypes (1a, 1 h, 1i, and 1j) based on sequence variation in the 739-nt region have been established (26, 27). The distribution of these genotypes worldwide has been reviewed elsewhere (26-28). Briefly, some of the genotypes are geographically restricted (such as genotype 1C, which is endemic only in Central and South America) and others are more broadly distributed (such as genotype 1E, which has been found in the Americas, Africa, Europe, and Asia).
Rubella is a vaccine-preventable disease, and live attenuated rubella virus vaccines have been available since the 1960s. Rubella vaccines, used singly or in combination with vaccines against mumps and measles, have proved to be highly effective in the prevention of rubella and CRS. By the end of 2006, rubella vaccine had been introduced in routine immunization programs in 123 countries, an increase from 65 countries in 1996 (19). Some countries (e.g., Cuba, the United States, Sweden, and Finland) have declared rubella elimination owing to their immunization programs (19); however, rubella is still endemic in many countries around the world, including China.
A seroprevalence survey performed in 2006 showed that about 20% of 623 women aged 20 to 39 years from Chongqing and Beijing, China, were susceptible to rubella virus infection (17), and the estimated number of CRS cases in China in 2005 was at least 20,000 (16). To control rubella epidemics and prevent CRS, routine rubella vaccination was instituted in the Chinese national immunization program in 2007. Two types of rubella vaccine are currently available in China (36). The BRDII attenuated vaccine strain of rubella virus, derived from a wild-type rubella virus isolated from a patient during an epidemic in Beijing in 1979 and attenuated through 30 low-temperature passages on a diploid cell line (23), was introduced in 1994 in some parts of China. In addition, an imported measles-mumps-rubella (MMR) vaccine containing the rubella virus vaccine strain RA27/3 has been available since 1996 in large cities (29). In a trial comparing the BRDII and RA27/3 vaccines, the seroconversion rates and mild side effects were similar (11). However, rubella surveillance was implemented only in Shandong and Heilongjiang provinces, where rubella and CRS surveillance projects were initiated and sponsored by the Ministry of Health of China and the World Health Organization (WHO) in 2009.
In this study, we reviewed the incidence of rubella cases in China from 1991 to 2007 and analyzed the sequences of a large collection of rubella viruses collected between 1999 and 2007. Although effective rubella and CRS surveillance has not yet been established in China, rubella virological surveillance in Anhui, Henan, and Shandong provinces has been in effect since 1999, when a project was conducted (1999 to 2002) to support and evaluate the development of a case-based measles surveillance system (1). In subsequent years, rubella virological surveillance in additional provinces was integrated into the Chinese measles and rubella laboratory network (25). Viruses of different genotypes were found in the same geographical areas and time periods, and a major shift in genotype was documented.
MATERIALS AND METHODS
Specimen collection.
Throat swab specimens were collected from patients with clinically suspected rubella within the 5 days following rash onset, during outbreak investigations or in routine surveillance from 1999 to 2007 in 13 provinces (Anhui, Henan, Shandong, Shanxi, Chongqing, Hebei, Jiangxi, Sichuan, Ningxia, Hunan, Qinghai, Hainan, and Liaoning) (see the supplemental material and Table 1).
TABLE 1.
Chronological and geographical distributions of rubella viruses in China, 1979 to 2007
| Province | Genotype (no. of isolates) | Yr(s) |
|---|---|---|
| Beijing | 2A (2) | 1979, 1980 |
| Shandong | 1a (1) | 1984 |
| 1F (1) | 2000 | |
| 1E (5) | 2001, 2002, 2006 | |
| 2A (3) | 2001 | |
| Anhui | 1F (8) | 1999, 2000 |
| 2B (2) | 2000 | |
| 1E (12) | 2001, 2007 | |
| Henan | 1F (6) | 2002 |
| 1E (18) | 2001, 2006, 2007 | |
| Chongqing | 1E (11) | 2003, 2005, 2007 |
| Shanxi | 1E (2) | 2004 |
| Hebei | 1E (1) | 2005 |
| Jiangxi | 1E (5) | 2006 |
| Sichuan | 2B (2) | 2006 |
| 1E (14) | 2007 | |
| Ningxia | 1E (6) | 2006 |
| Hunan | 1E (1) | 2006 |
| Qinghai | 1E (9) | 2007 |
| Hainan | 1E (7) | 2007 |
| Liaoning | 1E (10) | 2007 |
| Hong Kong | 2B (1) | 1980 |
Rubella incidence data sources.
The numbers of rubella cases and the annual rubella incidence rates were taken directly from reports from 145 sentinel sites distributed throughout China, covering a population of 11 million (accounting for approximately 1% of the total population of China at that time) between 1991 and 2002 (5), and from the National Disease Reporting Information System (NDRIS) between 2004 and 2007. These data were used for nationwide disease surveillance and covered medical institutions and centers for disease control and prevention in the whole country.
Virus isolation and primary identification.
Specimens were inoculated onto monolayers of African green monkey kidney (Vero) cells or Vero/SLAM cells, according to standard methods (36). Cells inoculated with clinical specimens were incubated at 35°C for 7 days. The culture supernatant was harvested and used to inoculate fresh cells for up to 2 additional passages. The viral RNA was extracted from infected cells after additional passages by using the QIAamp viral RNA extraction minikit (Qiagen, Valencia, CA). The presence of viral RNA was detected via reverse transcription-PCR (RT-PCR), which amplified a 185-nt fragment of the E1 coding region as previously described (36).
RT-PCR amplification and sequence determinations.
RT-PCR was performed using the Titanium One-Step RT-PCR kit (BD Bioscience, Palo Alto, CA) to amplify a 1,107-nt (nt 8656 to 9762) product containing the 739-nt WHO-recommended sequence window (nt 8731 to 9469) as previously described (3). The PCR products were purified using a QIA gel extraction kit (Qiagen, Valencia, CA) and were sequenced bidirectionally using the dye terminator method (BigDye Terminator version 3.1 cycle sequencing kit; Applied Biosystems, Foster City, CA) and an ABI 3100 Genetic Analyzer (Applied Biosystems, Hitachi, Tokyo, Japan). Sequence data were aligned, edited, and assembled to obtain the 739-nt sequence windows, using Sequencher, version 4.0.5 (GeneCode, Ann Arbor, MI).
Phylogenetic analysis.
Sequence alignments were created with the BioEdit sequence alignment editor software 5.0.9 (Tom Hall, North Carolina State University, Raleigh, NC) (10), and a phylogenetic dendrogram was constructed using the neighbor-joining Kimura two-parameter distance method, via the MEGA 4.1 program (Sudhir Kumar, Arizona State University, Tempe, AZ) (22); the reliability of the tree was estimated with 1,000 bootstrap pseudoreplicates. According to WHO recommendations, the primary criterion for valid genotype assignments is the proper grouping of the reference virus set included in the same analysis (25). Sequence relatedness was calculated using the MEGA 4.1 program (22).
Nucleotide sequence accession numbers.
The nucleotide sequences of 43 viruses representative of the 123 Chinese rubella virus strains that were isolated in this study were deposited in the GenBank database, under accession numbers FJ875029 to FJ875071. An additional 4 viruses used in this study were WHO reference sequences and were previously deposited in GenBank (accession numbers AY968213, AY968218, AY968215, and AY968210). Four sequences of rubella viruses from China, which circulated during 1979 to 1984, were obtained from the GenBank database (accession numbers AY258322, AY258323, DQ255946, and AB003340).
RESULTS
Trends in rubella incidence in China.
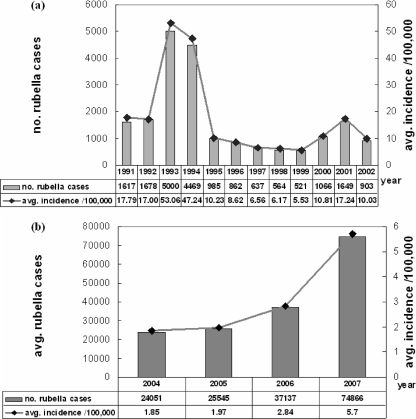
Between 1991 and 2002, the number of rubella cases was reported yearly from 145 sentinel surveillance sites throughout China (Fig. 1a) (5); between 2004 and 2007, reports were made by the NDRIS (Fig. 1b). Unfortunately, data pertaining to the number of rubella cases in 2003 were not available. Rubella epidemics occurred in 1993/1994 (reported peak annual incidence rate, 53.06/100,000) and in 2001 (reported peak annual incidence rate, 17.24/100,000). The average reported incidence rate of rubella cases increased from 1.85/100,000 in 2004 to 5.7/100,000 in 2007, indicating that another epidemic started in 2007. Surveillance inadequacies may be present in these annual incidence data. For example, the size of the population from which cases were reported by the 145 sentinel sites was not precisely known, and only approximately 1% of the total population of China between 1991 and 2002 was involved. Nevertheless, these data show that rubella epidemics occurred about every 6 to 8 years during this time period and that significant epidemics occurred in 1993/1994 and 2001, and another epidemic likely started in 2007.
FIG. 1.
Reported rubella cases in mainland China, 1991 to 2007. (a) Number of rubella cases (bars) and average rubella incidence (line with solid diamonds) between 1991 and 2002 in China, as reported by 145 sentinel surveillance sites. (b) Number of rubella cases (bars) and average rubella incidence (line with solid diamonds) between 2004 and 2007 in China, as reported by NDRIS.
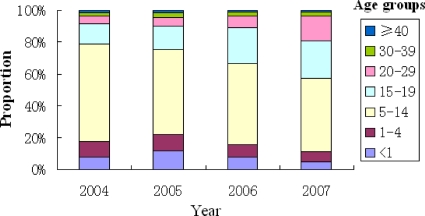
The proportion of reported rubella cases within the 15- to 29-year age group increased each year from 2004 (17.8%) to 2007 (39.0%) (Fig. 2). This is of concern because this age group contains women of childbearing age.
FIG. 2.
Rubella incidences in different age groups from 2004 to 2007, in mainland China, as reported by NDRIS.
Chronological and geographical distribution of Chinese rubella virus genotypes.
A total of 123 rubella viral isolates were obtained from throat swab specimens collected from 37 rubella outbreaks and 3 sporadic-case patients in 13 provinces in China, between 1999 and 2007. The sequences of virus isolates from each outbreak were very similar (<0.54% difference) or identical. Thus, 44 rubella viruses from outbreaks and 3 from sporadic cases were selected as representative viruses for phylogenetic analysis (see the supplemental material and Table 1). All viruses were named according to the WHO systematic nomenclature for rubella viruses (26).
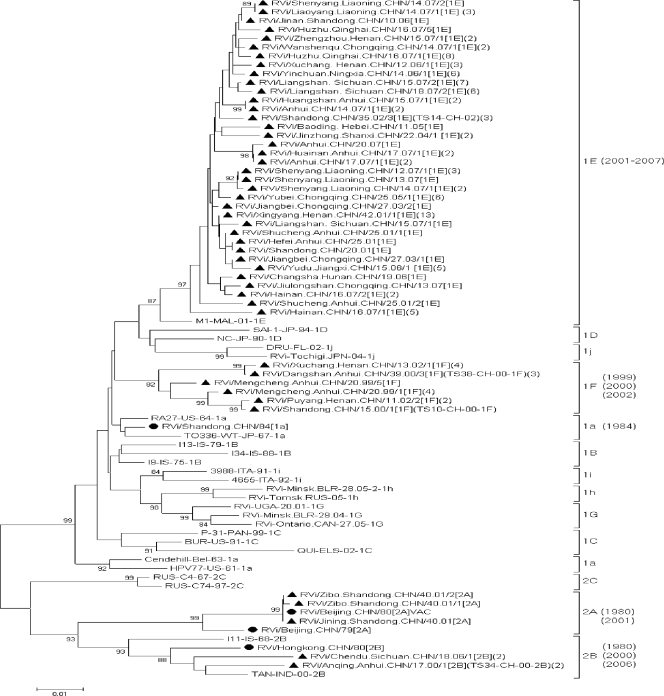
The nucleotide sequences from the 47 representative rubella viruses in this study, 4 other Chinese rubella viruses obtained between 1979 and 1984 (15, 35) (see the supplemental material and Table 1), and WHO reference viruses were analyzed (28). Note that 4 of the WHO reference viruses are also wild-type viruses from China. The 51 Chinese sequences were categorized into 5 of the 13 rubella virus genotypes, 1a, 1E, 1F, 2A, and 2B. These genotype assignments were supported by high bootstrap scores (Fig. 3).
FIG. 3.
Phylogenetic analysis of sequences of 51 representative Chinese rubella viruses from 1979 to 2007, compared to the WHO reference sequences. This tree is based on the WHO standard sequence window within the E1 gene (nt 8731 to 9469). Numbers in parentheses are the numbers of identical or similar sequences found in the same outbreak. The 47 representative rubella virus strains isolated during 1999 to 2007 are indicated by solid triangles. The 4 Chinese rubella virus sequences during 1979 to 1984, obtained from GenBank, are indicated by solid rounded diamonds. The 4 Chinese wild-type viruses, which are also WHO reference viruses, are also indicated (TS14-CH-02, TS38-CH-00-1F, TS10-CH-00-1F, and TS34-CH-00-2B).
Rubella viruses were found in China during 3 time periods (1979 to 1984, 1999 to 2000, and 2001 to 2007), with a gap where no information was available (1985 to 1998). Nucleotide sequences from 4 viruses were available between 1979 and 1984, those from 11 viruses were available between 1999 and 2000, and those from 112 viruses were available between 2001 and 2007.
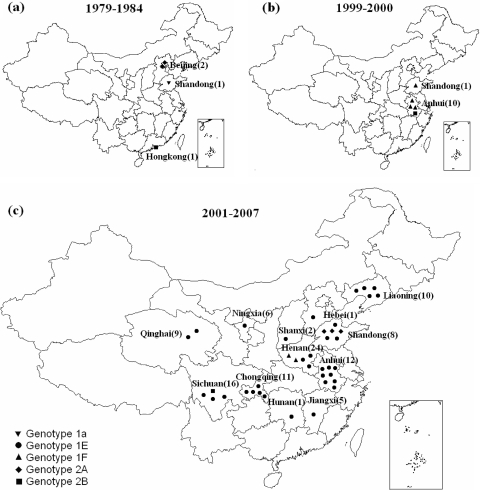
Viruses of genotypes 1a (RVi/Shandong.CHN/84[1a]), 2A (RVi/Beijing.CHN/79[2A] and RVi/Beijing.CHN/80[2A]VAC, a Chinese rubella vaccine virus), and 2B (RVi/Hongkong.CHN/80[2B]) were found during 1979 to 1984 in Beijing, Shandong, and Hong Kong (Fig. 4). Most of the rubella viruses from Anhui and Shandong provinces during 1999 to 2000 were of genotype 1F (9 virus isolates from 3 of the 4 outbreaks) or genotype 2B (2 virus isolates from the fourth outbreak). The 2 genotype 2B viruses shared 100% nucleotide similarity and shared only 96.5% similarity with the genotype 2B virus isolated in Hong Kong in 1980 (RVi/Hongkong.CHN/80[2B]).
FIG. 4.
The geographical distribution of the genotypes of rubella viruses isolated in China during 3 periods between 1979 and 2007. The provinces where the 51 representative rubella viruses of the indicated genotypes were found are shown. The location within each province is not indicated. The total number of viruses sequenced is shown for each province in each period. Genotype 2B viruses in Sichuan and Hong Kong may be classified as imports, and genotype 2A viruses in Shandong in 2001 to 2007 are likely vaccine associated (see the text).
Genotype 1E rubella viruses were found in 2001 in 2 outbreaks in Anhui and in Shandong in 2002 and later in every province where virological surveillance occurred (a total of 13 provinces). Genotype 2B viruses were also identified; 2 viruses (RVi/Chendu.Sichuan.CHN/21.06/1[2B] and RVi/Chendu.Sichuan.CHN/21.06/2[2B]) from an outbreak in Sichuan province in 2006 were considered to have been imported because they were found only in students from Vietnam (12). These 2 viruses had identical nucleotide sequences; they had only 95.7% similarity with RVi/Hongkong.CHN/80[2B] (detected in Hong Kong in 1980) and RVi/Anqing.Anhui.CHN/17.00/1[2B] (detected in Anhui province in 2000). During 2001 to 2007, genotype 1F viruses were found only in January and February of 2002 in Henan province.
Three genotype 2A viruses found in Shandong province in 2001 (RVi/Zibo.Shandong.CHN/40.01/12[2A], RVi/Jining.Shandong.CHN/40.01/13[2A], and RVi/Zibo.Shandong.CHN/40.01/14[2A]) were isolated from 3 sporadic cases. Their nucleotide sequences were very closely related to that of the BRDII vaccine virus used in China; 2 were 100% similar and the other had only a 1-nt difference from the vaccine virus. Although immunization histories were not available for the 3 cases, the sequence information indicated that these 3 isolates likely came from the rubella virus used to vaccinate these cases.
DISCUSSION
This study summarizes the rubella surveillance data and documents epidemic rubella cycles occurring approximately every 6 to 8 years (1993 to 1994, 2001, and 2007). Similar epidemic cycles have been observed in other countries, for example, in the United States before 1969 (24). Seroepidemiological surveys revealed seronegativity rates of around 20% among women of childbearing age in China (17), and this study shows that the percentage of rubella cases in individuals between 15 and 29 years of age increased between 2004 and 2007. Routine rubella vaccination was not included in the national immunization program of China until 2007; before this, vaccination was implemented on a voluntary basis in a few large cities (9). This may partly be responsible for the shift of disease burden to susceptible young adults, specifically to women of childbearing age in the prevaccination era, which could lead to an increase in the rate of CRS relative to the number of rubella cases.
This study investigated the genotype distribution of rubella viruses in China over a 28-year period to establish an important genetic baseline in China during the prevaccination era. Molecular epidemiology and phylogenetic analyses have become important tools in monitoring virus circulation and the progress of elimination efforts, and these baseline data should be useful in the near future for classifying viruses found in China as either indigenous or imported (13). For example, based on the data presented here, genotype 1G viruses, which are commonly found in Africa and Europe, would probably be considered to have been imported if found in China (3).
Among the viruses of 5 genotypes detected in China between 1979 and 2007 (1a, 1E, 1F, 2A, and 2B), those of genotypes 1a, 1F, 2A, and 2B were rarely found in recent years. Genotype 1a rubella viruses that were present during 1984 in Shandong province were not found after that year. Genotype 2A viruses, which are not closely related to the vaccine virus BRDII, have not been found since 1980. Genotype 1F viruses were first detected in 1999 in Anhui province and were likely the predominant viruses in this province during 1999 to 2000. However, viruses of this genotype have not been isolated in China or elsewhere since 2002 (in Henan). Furthermore, viruses of genotypes 2A and 1F have yet to be found outside China. Genotype 2B rubella viruses were not found frequently enough to be considered significant in indigenous transmission. Genotype 2B viruses that were not considered to be imported were found in only 1 outbreak between 1999 and 2007 (Anhui province in 2000). It would be interesting to collect samples from other Chinese provinces to determine if genotype 2B viruses are more prevalent than our data suggest.
The data presented here show that viruses of different genotypes have cocirculated in China. Genotype 1a and 2A viruses were found during 1979 to 1984, genotype 1F and 2B viruses were found during 1999 to 2000, and genotype 1F and 1E viruses were found during 2001 to 2007. Clearly, data on viruses from more locations and more years would be beneficial; the number of cocirculating viruses reported here should be considered a minimum. The more recent data presented here indicate that the number of cocirculating strains decreased after 2003. Only genotype 1E viruses were found since then (a total of 81 viruses). However, the dominance of genotype 1E viruses may not be an entirely stable situation, because recent data from 2008 indicate circulating genotypes other than 1E (data not shown).
This study provides good evidence that replacement of rubella viruses of a given genotype can occur. Rubella viruses of genotype 1F and 2B were likely replaced by viruses of genotype 1E in Anhui province. Anhui is a small, densely populated province (population, about 65,000,000; area, about 150 × 300 miles). This province has good virological surveillance, resulting in the determination of the genotypes of 10 viruses from 4 outbreaks in 1999 to 2000 (1F and 2B) and 12 viruses from 3 outbreaks in 2001 to 2007 (1E) (Table 1 and Fig. 4). Genotype 1E viruses first appeared in Anhui in 2001, an epidemic year in China. Although there are fewer viruses from Shandong, the data are consistent with a similar shift to genotype 1E in this province. Genotype 1E viruses were predominant in China during 2001 to 2007, being found in 13 provinces (101 viruses from 30 rubella outbreaks). A shift to genotype 1E similar to that observed in Anhui province may have occurred throughout China during 2001 to 2007.
Genotype 1E viruses were first identified in 1997 in the United States, Canada, the Caribbean, and Italy (20, 35). In addition to North America and Europe, 1E viruses have now been isolated in South America, Africa, and Asia (27, 36). Shifts of predominant viruses to a new genotype, such as those that occurred in Anhui and likely in all of China, have also occurred in some other countries where rubella is endemic, such as Japan, Italy, and Brazil (14, 18, 34).
Three BRDII vaccine-associated cases were found in Shandong in 2001 in this study, and isolation of the RA27/3 vaccine virus from vaccinees has been reported previously (8, 20). Vaccine virus isolations can easily occur when outbreaks and vaccination campaigns occur simultaneously; therefore, future isolations of RA27/3 and BRDII vaccine viruses are expected as vaccination increases in China.
It is also interesting that the molecular epidemiological pattern of rubella viruses in China appears to be quite different from those of the measles virus (30, 33), mumps virus (6), human enterovirus 71 (32), and coxsackievirus A16 (31). Virological surveillance for measles virus and mumps virus since 1995 shows that the genotype H1 of measles virus and genotype F of mumps virus are the only genotypes found thus far in China, and subgenotype C4 of human enterovirus 71 and genotype B1 of coxsackievirus A16 have been continuously circulating in China since they were first detected in 1998 and 1999, respectively.
Although rubella virological surveillance has been successfully implemented in China, more viruses need to be collected from other provinces to establish a complete genetic baseline for the entire country. Ongoing molecular epidemiological surveillance of circulating rubella viruses is needed, especially when routine vaccination programs are initiated. Vaccination will considerably reduce the number of susceptible individuals and, consequently, decrease or eliminate endemic viruses, allowing detection of imported viruses.
Acknowledgments
We thank all the provincial and prefectural measles and rubella laboratory staffs and the epidemiologists in mainland China for providing clinical specimens, isolates, and epidemiologic data; we thank WHO HQ and WPRO for the technical and financial support.
This work is supported by the Ministry of Health of the People's Republic of China (National Infectious Diseases Surveillance Program: 2008ZX10004-001) and WHO EPI project 2008ZX10004-008, 2009ZX10004-201, and 2009ZX10004-202.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
All authors report no conflicts of interest.
Footnotes
Published ahead of print on 29 March 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Aiqiang, X., F. Zijian, X. Wenbo, W. Lixia, G. Wanshen, X. Qing, S. Haijun, L. A. Lee, and L. Xiaofeng. 2003. Active case-based surveillance for measles in China: lessons learned from Shandong and Henan provinces. J. Infect. Dis. 187(Suppl. 1):S258-S263. [DOI] [PubMed] [Google Scholar]
- 2.Bellini, W. J., and J. P. Icenogle. 2007. Measles and rubella viruses, p. 1378-1391. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 3.Caidi, H., E. S. Abernathy, A. Benjouad, S. Smit, J. Bwogi, M. Nanyunja, R. El Aouad, and J. Icenogle. 2008. Phylogenetic analysis of rubella viruses found in Morocco, Uganda, Cote d'Ivoire and South Africa from 2001 to 2007. J. Clin. Virol. 42:86-90. [DOI] [PubMed] [Google Scholar]
- 4.Chen, M. H., and J. P. Icenogle. 2007. Molecular virology of rubella virus, p. 1-18. In J. Banatvala and C. Peckham (ed.), Rubella virus. Elsevier, Oxford, United Kingdom.
- 5.Chinese Center for Disease Control and Prevention. 2003. An analysis of 35 notifiable communicable diseases of national DSPs in December 2002. Dis. Surveill. 18:3. [Google Scholar]
- 6.Cui, A., R. Myers, W. Xu, and L. Jin. 2009. Analysis of the genetic variability of the mumps SH gene in viruses circulating in the UK between 1996 and 2005. Infect. Genet. Evol. 9:71-80. [DOI] [PubMed] [Google Scholar]
- 7.Frey, T. K. 1994. Molecular biology of rubella virus. Adv. Virus. Res. 44:69-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey, T. K., E. S. Abernathy, T. J. Bosma, W. G. Starkey, K. M. Corbett, J. M. Best, S. Katow, and S. C. Weaver. 1998. Molecular analysis of rubella virus epidemiology across three continents, North America, Europe, and Asia, 1961-1997. J. Infect. Dis. 178:642-650. [DOI] [PubMed] [Google Scholar]
- 9.Gao, L., and H. Hethcote. 2006. Simulations of rubella vaccination strategies in China. Math Biosci. 202:371-385. [DOI] [PubMed] [Google Scholar]
- 10.Hall, T. A. 1999. A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 41:95-98. [Google Scholar]
- 11.Han, Y. R., K. Zhao, Y. X. Gong, S. L. Hao, S. Z. Wang, and C. T. Wang. 1985. Rubella vaccine in the People's Republic of China. Rev. Infect. Dis. 7(Suppl. 1):S79. [PubMed] [Google Scholar]
- 12.He, J. L., Z. Zhu, L. Sun, W. B. Tong, and W. B. Xu. 2007. Genotypic analysis of wild-type rubella viruses first isolated in Sichuan province. Mod. Prev. Med. 34:1751-1752. [Google Scholar]
- 13.Icenogle, J. P., T. K. Frey, E. Abernathy, S. E. Reef, D. Schnurr, and J. A. Stewart. 2006. Genetic analysis of rubella viruses found in the United States between 1966 and 2004: evidence that indigenous rubella viruses have been eliminated. Clin. Infect. Dis. 43(Suppl. 3):S133-S140. [DOI] [PubMed] [Google Scholar]
- 14.Katow, S. 2004. Surveillance of congenital rubella syndrome in Japan, 1978-2002: effect of revision of the immunization law. Vaccine 22:4084-4091. [DOI] [PubMed] [Google Scholar]
- 15.Katow, S., H. Minahara, M. Fukushima, and Y. Yamaguchi. 1997. Molecular epidemiology of rubella by nucleotide sequences of the rubella virus E1 gene in three East Asian countries. J. Infect. Dis. 176:602-616. [DOI] [PubMed] [Google Scholar]
- 16.Li, H., J. Y. Hu, L. N. Tao, and J. G. Zhang. 2005. Epidemiology characterization and preventive strategies of CRS. Shanghai J. Prev. Med. 17:72-74. [Google Scholar]
- 17.Long, Q. J., N. Y. Mao, C. S. Li, S. T. Xu, L. Li, X. H. Jiang, and W. B. Xu. 2007. A survey on rubella antibody level of women at childbearing age in Beijing and Chongqing. Chin. J. Vaccine Immun. 12:144-149. [Google Scholar]
- 18.Pan American Health Organization. 2009. Rubella watch. Pan American Health Organization, Washington, DC. http://www.paho.org/English/AD/FCH/IM/NL_RubellaWatch2008_extra_e.pdf.
- 19.Reef, S. E., S. B. Redd, E. Abernathy, L. Zimmerman, and J. P. Icenogle. 2006. The epidemiological profile of rubella and congenital rubella syndrome in the United States, 1998-2004: the evidence for absence of endemic transmission. Clin. Infect. Dis. 43(Suppl. 3):S126-S132. [DOI] [PubMed] [Google Scholar]
- 20.Reef, S. E., T. K. Frey, K. Theall, E. Abernathy, C. L. Burnett, J. Icenogle, M. M. McCauley, and M. Wharton. 2002. The changing epidemiology of rubella in the 1990s: on the verge of elimination and new challenges for control and prevention. JAMA 287:464-472. [DOI] [PubMed] [Google Scholar]
- 21.Robertson, S. E., D. A. Featherstone, M. Gacic-Dobo, and B. S. Hersh. 2003. Rubella and congenital rubella syndrome: global update. Rev. Panam. Salud Publica 14:306-315. [DOI] [PubMed] [Google Scholar]
- 22.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 23.Wang, S. S., Y. R. Han, W. N. Su, J. Chen, and K. Zhao. 1984. Studies on the reactogenicity and immunogenicity of the BRD-2 and RA27/3 live attenuated rubella vaccines. Vaccine 2:277-280. [DOI] [PubMed] [Google Scholar]
- 24.Witte, J. J., A. W. Karchmer, G. Case, K. L. Herrmann, E. Abrutyn, I. Kassanoff, and J. S. Neill. 1969. Epidemiology of rubella. Am. J. Dis. Child. 118:107-111. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2005. Global measles and rubella laboratory network—update. Wkly. Epidemiol. Rec. 80:384-388. [PubMed] [Google Scholar]
- 26.World Health Organization. 2006. Global distribution of measles and rubella genotypes—update. Wkly. Epidemiol. Rec. 81:474-479. [PubMed] [Google Scholar]
- 27.World Health Organization. 2005. Standardization of the nomenclature for genetic characteristics of wild-type rubella viruses. Wkly. Epidemiol. Rec. 80:126-132. [PubMed] [Google Scholar]
- 28.World Health Organization. 2007. Update of standard nomenclature for wild-type rubella viruses, 2007. Wkly. Epidemiol. Rec. 82:216-222. [PubMed] [Google Scholar]
- 29.Xu, A., L. Song, C. Wang, A. Wang, Q. Xu, Z. Xiao, S. Wang, M. Li, S. Hao, and Z. Li. 2000. An observation of the immuno-persistence after inoculating with the domestic BRD II strain rubella vaccine among infants and young children. Zhonghua Liu Xing Bing Xue Za Zhi 21:117-120. (In Chinese.) [PubMed] [Google Scholar]
- 30.Zhang, Y., Y. Ji, X. Jiang, S. Xu, Z. Zhu, L. Zheng, J. He, H. Ling, Y. Wang, Y. Liu, W. Du, X. Yang, N. Mao, and W. Xu. 2008. Genetic characterization of measles viruses in China, 2004. Virol. J. 5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, Y., D. Wang, D. Yan, S. Zhu, J. Liu, H. Wang, S. Zhao, D. Yu, L. Nan, J. An, L. Chen, H. An, A. Xu, and W. Xu. 2010. Molecular evidence of persistent epidemic and evolution of subgenotype B1 coxsackievirus A16-associated hand, foot, and mouth disease in China. J. Clin. Microbiol. 48:619-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, Y., X. J. Tan, H. Y. Wang, D. M. Yan, S. L. Zhu, D. Y. Wang, F. Ji, X. J. Wang, Y. J. Gao, L. Chen, H. Q. An, D. X. Li, S. W. Wang, A. Q. Xu, Z. J. Wang, and W. B. Xu. 2009. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 44:262-267. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., Z. Zhu, P. A. Rota, X. Jiang, J. Hu, J. Wang, W. Tang, Z. Zhang, C. Li, C. Wang, T. Wang, L. Zheng, H. Tian, H. Ling, C. Zhao, Y. Ma, C. Lin, J. He, J. Tian, P. Li, R. Guan, W. He, J. Zhou, G. Liu, H. Zhang, X. Yan, X. Yang, J. Zhang, Y. Lu, S. Zhou, Z. Ba, W. Liu, Y. Liang, Y. Li, Y. Ji, D. Featherstone, W. J. Bellini, S. Xu, G. Liang, and W. Xu. 2007. Molecular epidemiology of measles viruses in China, 1995-2003. Virol. J. 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng, D. P., H. Zhu, M. G. Revello, G. Gerna, and T. K. Frey. 2003. Phylogenetic analysis of rubella virus isolated during a period of epidemic transmission in Italy, 1991-1997. J. Infect. Dis. 187:1587-1597. [DOI] [PubMed] [Google Scholar]
- 35.Zheng, D. P., T. K. Frey, J. Icenogle, S. Katow, E. S. Abernathy, K. J. Song, W. B. Xu, V. Yarulin, R. G. Desjatskova, Y. Aboudy, G. Enders, and M. Croxson. 2003. Global distribution of rubella virus genotypes. Emerg. Infect. Dis. 9:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, Z., W. Xu, E. S. Abernathy, M. H. Chen, Q. Zheng, T. Wang, Z. Zhang, C. Li, C. Wang, W. He, S. Zhou, and J. Icenogle. 2007. Comparison of four methods using throat swabs to confirm rubella virus infection. J. Clin. Microbiol. 45:2847-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]