Abstract
We report a case of disseminated Scedosporium/Pseudallescheria infection due to Pseudallescheria boydii sensu stricto after lung transplantation in a patient with cystic fibrosis. Dissemination occurred under voriconazole. Despite surgery and combination therapy with voriconazole, caspofungin, and terbinafine, the patient died 8 months after transplantation. Previously reported cases are reviewed.
CASE REPORT
A 37-year-old woman suffering from cystic fibrosis (CF) was admitted to our institution in April 2008 for double-lung transplantation. Her medical history included diabetes mellitus since 2002 and more than 10 years of airway colonization with Aspergillus fumigatus and Scedosporium/Pseudallescheria. Starting in 2006, and while awaiting transplantation, she received oral voriconazole (250 mg, twice a day). Her postoperative course was relatively uncomplicated except for cytomegalovirus (CMV) infection due to a mismatch at the time of transplantation in spite of valganciclovir prophylactic treatment (900 mg/day). The immunosuppressive regimen included tacrolimus (therapeutic range, 12 to 13.5 ng/ml), mycophenolate mophetil (750 mg/day), and prednisone (37.5 mg/day). Oral voriconazole was continued as a long-term posttransplantation prophylaxis (250 mg twice a day). A fungal culture of a bronchial secretion obtained the day after transplantation was positive for a filamentous fungus routinely identified as Scedosporium apiospermum/P. boydii (isolate I), but several other respiratory specimens obtained over the following weeks were negative. The patient was discharged from the hospital on day 45 after transplantation, still taking voriconazole. The voriconazole serum levels, checked regularly (1.06, 1.96, and 1.69 mg/liter on days 26, 36, and 50, respectively) were within therapeutic limits (1 to 2.5 mg/liter) except for two occasions (0.16 and 0.35 mg/liter on days 18 and 64, respectively).
In June (day 70), she presented at our hospital with nodules on her legs that had appeared 2 weeks previously. On examination, the nodules were fibrous, dermo-hypodermic, measuring 1 to 2 cm in diameter, and slightly pigmented on the surface. A biopsy was performed. Histopathological microscopic examination (Gomori-Grocott and periodic acid-Schiff stainings) revealed an inflammatory infiltrate along with several branched and septate hyaline hyphae (fungal cultures were not performed). Continuation of voriconazole in association with a reduced dose of corticosteroid was associated with clinical improvement. However, 3 weeks later, a new biopsy was performed and septate hyphae were again seen on direct examination. A fungal culture of this biopsy specimen was positive for S. apiospermum/P. boydii (isolate II). At this time, the results for chest computed tomography (CT) were unremarkable, no sign of dissemination was noted on brain CT, and the nodules disappeared over the following weeks.
All the while, positive CMV DNAemia was still detected in spite of successive curative treatments with per os valganciclovir (1,800 mg/day from day 63 to day 80), intravenous ganciclovir (from day 80 to day 97), and finally foscarnet (from day 97 to day 115 and from day 138 to day 151). Cytomegalovirus infection was associated with fever, leucopenia, and digestive symptoms. In August (day 138), a CMV strain resistant to both ganciclovir (L595F mutation on the UL97 gene) and foscarnet (E756D mutation on the UL54 gene) was detected. Since then, CMV infection could not be controlled by antiviral therapy, leading to high viral loads, ranging from 5 log10 to 6.7 log10 copies/106 cells.
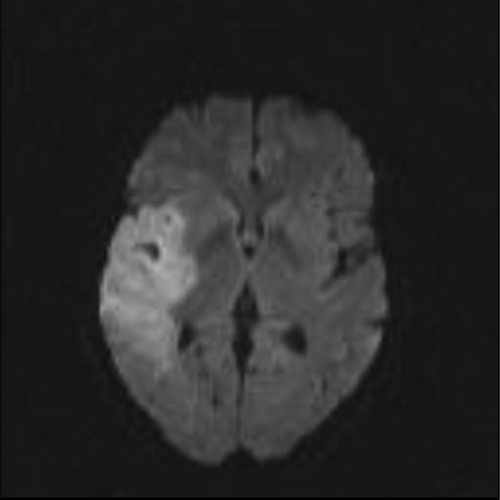
At the beginning of November (day 213), while still taking voriconazole, she was referred to our hospital for acute vestibular syndrome, dizziness, and dysarthria. On examination, the patient presented with left complete hemiplegia. Cerebral magnetic resonance imaging revealed a large ischemic region located in the right sylvian area (Fig. 1). A few days later, a blood culture (Bactec Mycosis IC/F; Becton Dickinson, Sparks, MD) performed on admission was positive on direct examination for branched and septate hyaline hyphae, with terminal conidial cells suggestive of Scedosporium/Pseudallescheria (Fig. 2A). At the same time, a large vegetation was observed on the mitral valve with transesophageal echography. No evidence of dissemination to the kidneys, liver, or spleen was detected by CT. Subcultures of the blood culture grew S. apiospermum/P. boydii (Fig. 2B) (isolate III). MICs for voriconazole, posaconazole, and caspofungin were determined with the Etest (0.002, 3, and >32 μg/ml, respectively), and the isolate was sent to the French National Reference Center for Mycoses and Antifungals (CNRMA; Institut Pasteur, Paris, France) for antifungal susceptibility testing according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standardized methodology (25). The antifungal susceptibility profile was in agreement with previous results, with a low MIC for voriconazole (0.5 μg/ml) but higher MICs for posaconazole (≥8 μg/ml) and caspofungin (2 μg/ml).
FIG. 1.
Cerebral magnetic resonance imaging scan showing a large ischemic region located in the right area.
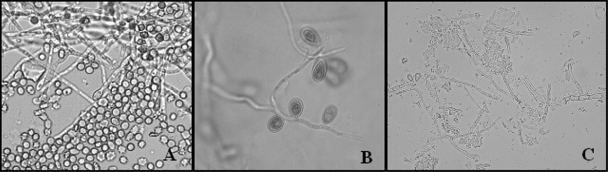
FIG. 2.
(A) Direct microscopic examination of the blood culture showing hyphae and conidia (magnification, ×500). (B) Lactophenol cotton blue stain of the fungal culture from blood showing septate hyphae and sessile conidia (magnification, ×1,000). (C) Direct examination of the cardiac vegetation showing several septate hyphae along with conidial formation (magnification, ×400).
In light of probable fungal endocarditis emerging during voriconazole therapy, caspofungin was added on day 220 (70 mg/kg of body weight/day as a loading dose, followed by 50 mg/kg/day). Three days later, the combination therapy was reinforced with terbinafine (250 mg/day) and caspofungin was increased to 150 mg/day. This combination therapy was associated with clinical improvement, allowing valve replacement and excision of the vegetation on day 228. Several septate hyphae and conidial formation typical of Scedosporium/Pseudallescheria were observed on direct examination of the vegetation (Fig. 2C). Fungal cultures yielded S. apiospermum/P. boydii, confirming the diagnosis of fungal endocarditis (isolate IV). The day after surgery, a bronchial specimen was also positive for S. apiospermum/P. boydii (isolate V). The patient's condition improved slightly in the following days, but neurological deterioration with headache was observed on day 240. Death occurred the day after, due to a massive cerebral hemorrhage.
Molecular identification of each of the five isolates was performed by amplification and sequencing of a region within the β-tubulin gene (519 bp), the calmodulin gene (633 bp), the internal transcribed spacer (575 bp), and the D1-D2 region of 28S ribosomal DNA (568 bp) and yielded 100%, 99.5%, 99.3%, and 100% homology, respectively, with the sequences (GenBank accession numbers AJ890121, AJ890207, AY213680, and AY213623, respectively) of the type strain of P. boydii sensu stricto (CBS 101.22) (14). Random amplified polymorphic DNA (RAPD) genotyping performed using the GC70, UBC-701, and UBC-703 primers as described previously revealed a unique RAPD pattern for the five P. boydii isolates (9).
Discussion.
Scedosporium/Pseudallescheria species are ubiquitous, saprophytic, filamentous fungi found widely in the environment but are also increasingly recognized as opportunistic pathogens. The clinical spectrum of these infections ranges from localized disease to disseminated infection, with a poor prognosis in solid-organ or hematopoietic stem cell transplant (HSCT) recipients and patients with haematological malignancies, especially when dissemination or fungemia occurs (7, 18, 27). Colonization of the respiratory tract by Scedosporium/Pseudallescheria is common in patients with CF, where it is the second most frequent filamentous fungus after A. fumigatus, with a prevalence ranging from 5.7 to 10% (6, 21, 31). Despite this high prevalence, disseminated Scedosporium/Pseudallescheria infections remain rare in patients with CF, even in highly immunosuppressed patients such as those undergoing lung transplantation (2, 5, 24, 26, 28). Here, we present a case involving a patient who had several years of known airway colonization with S. apiospermum/P. boydii and was admitted to our institution for double-lung transplantation. The patient subsequently developed a fatal disseminated infection due to P. boydii sensu stricto.
To the best of our knowledge, only 5 patients with CF who developed invasive scedosporiosis after lung transplantation have been reported in the literature since 1996 (reviewed in Table 1). Most of them had a history of airway colonization with Scedosporium/Pseudallescheria before transplantation. All but one were treated with antifungal combinations, but the mortality rate was 100%. The median time from transplantation to the onset of infection was 5 weeks (range, 2 weeks to 7.5 months). According to Husain et al., the median times to infection in solid-organ transplant recipients were 4 months in the case of S. apiospermum infection and earlier when Scedosporium prolificans was involved (18). The colonization with Scedosporium/Pseudallescheria at the time of transplantation could explain the shorter time to infection in patients with CF. Apart from the present case, dissemination to the skin was noted to occur in two other patients (patients 4 and 5). This highlights that the recovery of filamentous fungi from cutaneous lesions in patients with CF who undergo lung transplantation, even without other clinical manifestations, requires complementary investigations.
TABLE 1.
Clinical characteristics of reported cases of invasive scedosporiosis in patients with cystic fibrosis after lung transplantationa
| Patient | Age (yr) | Sex | Colonization before LTx | Antifungal prophylaxis after LTx | Time to diagnosis after LTx | Mycological organism identified | Antifungal agent(s) | Site(s)/clinical manifestation(s) | Survival time after diagnosis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | M | NA | None | 6 wkb | S. apiospermum/P. boydii | AMB | CNS | NA | 2 |
| 2 | 24 | F | No | None | 7.5 mo | S. apiospermum/P. boydii | ITC-MIC | Heart, spleen, kidneys, CNS | 4 wk | 24 |
| 3 | 30 | M | Yes | None | 2 wk | S. apiospermum/P. boydii | AMB-MIC | Neuroria, pleuritis, pericarditis | 1 wk | 5 |
| 4 | 26 | F | Yes | ITC, AMB | 3 wk | S. apiospermum/P. boydii | VRC-MICc | Skin nodules, endophtalmitis, meningitis | 6 mo | 28 |
| 5 | 19 | F | Yes | VRC | 4 wk | S. apiospermum/P. boydii | VRC-CAS-TRB followed by POS | Skin nodules, endophtalmitis, pansinusitis, chest wall cellulitis, mediastinitis, vertebral osteomyelitis, septic arthritis | 14 mo | 26 |
| 6 | 37 | F | Yes | VRC | 8 wk | P. boydii | VRC-CAS-TRB | Skin nodules, endocarditis, CNS | 6 mo | This article |
The outcome for all patients was death. M, male; F, female; NA, not available; ITC, itraconazole; MIC, miconazole; VRC, voriconazole; POS, posaconazole; AMB, amphotericin B; CAS, caspofungin; TRB, terbinafine; LTx, lung transplantation; CNS, central nervous system.
Diagnosis was made on brain biopsy.
Intraocular miconazole.
In the present case, a fatal outcome occurred despite valve replacement and salvage therapy based on a combination of voriconazole, caspofungin, and terbinafine. Regarding the pretransplantation period, it is interesting to note that Scedosporium/Pseudallescheria fungi were isolated repeatedly from respiratory tract specimens from our patient despite long-term voriconazole prophylaxis. Clearly, the fungemia and central nervous system involvement were poor prognostic factors in our patient. Indeed, fungemia has been clearly associated with a higher mortality rate in transplant recipients (18). However, the underlying, rampant, and multiresistant CMV infection in our patient must also be considered. Indeed, CMV infection has been associated with an increased risk of invasive aspergillosis in solid-organ transplant recipients, but data are still lacking for scedosporiosis (10, 19). Finally, the difficulty encountered in maintaining voriconazole plasma levels within therapeutic limits must also be considered. The pharmacokinetic variability of voriconazole levels in patients with CF, which can be responsible for the underdosage and therefore the inefficacy of antifungal therapy, has been described in a previous study showing that voriconazole levels are often undetectable in such patients (3). In our patient, cutaneous nodules appeared when the voriconazole serum level was low, despite any discontinuation of voriconazole therapy (day 64, 0.35 mg/liter). However, we are not sure that the breakthrough in our patient is the result of voriconazole underdosage, as all other dosages, performed monthly, were within the therapeutic range.
Scedosporium/Pseudallescheria species are generally considered to have low in vitro susceptibilities to antifungal drugs that can also differ between species (12). According to both in vitro and in vivo studies, voriconazole could be the most effective antifungal agent, but no recommendations regarding the optimal antifungal therapy for scedosporiosis have yet emerged (12, 29). In particular, the benefit of antifungal combination therapy has not been clearly established, despite the increasing number of reports describing a favorable outcome obtained with voriconazole in combination with terbinafine and/or caspofungin (15-17, 22). The in vitro study of 35 antifungal combinations against S. apiospermum and S. prolificans describing a potent synergy between azole drugs and echinocandins will probably reopen the debate on combination therapy (8, 23). Promising results obtained with new antifungal agents such as miltefosine remain to be confirmed (20, 30). In the present case, it is interesting to note the reliability of the Etest for MIC determination for Scedosporium species, as MICs obtained by the Etest were generally correlated with those obtained by the EUCAST method, especially for voriconazole. The moderately high MIC for caspofungin found in this study has also been reported by others (8). Despite these high in vitro MICs, both caspofungin and terbinafine have been associated with a favorable outcome in clinical practice, highlighting that the results of antifungal susceptibility testing must be considered with caution since no correlation between MIC data and clinical success has been described to date for Scedosporium/Pseudallescheria (16, 17, 22).
Recent advances in molecular methods have led to a revision of Scedosporium/Pseudallescheria taxonomy, revealing that Pseudallescheria boydii is a species complex. Importantly, P. boydii, formerly considered the sexual state of Scedosporium apiospermum (i.e., teleomorph), must now be considered a distinct species, and new species have also recently been identified (11, 14). Despite being difficult and time-consuming in routine practice, identification of Scedosporium/Pseudallescheria to the species level is important because virulence and antifungal susceptibility levels can differ significantly between species (1, 12, 13). Here, molecular typing performed using DNA sequencing at four loci revealed that the five isolates belonged to P. boydii sensu stricto. Finally, RAPD genotyping revealed that the five P. boydii isolates recovered over a 7.5-month period and from different anatomic sites had the same RAPD pattern, suggesting that a single strain of P. boydii was responsible for the breakthrough infection in our patient. Unfortunately, the strains isolated before transplantation were not available for analysis.
This report demonstrates again the rare but mostly fatal risk of invasive Scedosporium/Pseudallescheria infections in patients with CF. In this setting, the utility of a screening of fungal airway colonization for detection of patients having a risk of infection needs to be discussed. According to a recent article, the choice of medium used for culture specimens could influence the rate of recovery of Scedosporium species, a better recovery rate being described with SceSel+ medium (4). Importantly, without any guidelines, the management of patients colonized with Scedosporium/Pseudallescheria remains, at present, mainly based on the individual experience of each center, and there are probably variations in practice between centers/countries. In Nantes, France, CF patients are regularly screened for fungal colonization of their respiratory tract. Those being colonized with Scedosporium/Pseudallescheria and showing a deterioration of lung function are given voriconazole, which is, in our experience, generally associated with clinical improvement. Regarding the time to infection, we suggest that both clinical and microbiological surveillance using blood cultures, as well as respiratory tract specimens, focused mainly on the first weeks after transplantation, could be beneficial for early diagnosis of these life-threatening infections. Finally, in light of this and previously reported cases, several questions must be raised. (i) Does Scedosporium/Pseudallescheria airway colonization represent a risk factor for invasive infection after transplantation in patients with CF, and should it be a contraindication to transplantation? (ii) Is antifungal prophylaxis before and after transplantation really effective in this setting, and which antifungal(s) should be administered? (iii) Regarding antifungal therapy, is voriconazole really the most effective drug, and what will be the role of new agents such as miltefosine? Larger studies are now warranted to answer to these questions and establish guidelines for the management of Scedosporium/Pseudallescheria in patients with CF.
Nucleotide sequence accession numbers.
Nucleotide sequences have been deposited in the GenBank database under accession numbers GU180097 to GU180101, GU186103 to GU186107, GU192438 to GU192442, and GU213261 to GU213265.
Acknowledgments
We acknowledge Olivier Lortholary (CNRMA, Institut Pasteur, Paris, France) for helpful discussion concerning the management of our patient, Eric Dannaoui (CNRMA, Institut Pasteur, Paris, France) for performing in vitro antifungal susceptibility testing of the isolates, and Celine Bressollette-Bodin (Laboratoire de Virologie, CHU de Nantes) for interesting comments on CMV disease. We acknowledge Adeline Maitte, Tiphaine Robert, and Bernard Besse for their technical assistance.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Alastruey-Izquierdo, A., M. Cuenca-Estrella, A. Monzon, and J. L. Rodriguez-Tudela. 2007. Prevalence and susceptibility testing of new species of Pseudallescheria and Scedosporium in a collection of clinical mold isolates. Antimicrob. Agents Chemother. 51:748-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albernaz, V., B. Huston, M. Castillo, S. Mukherji, and T. Bouldin. 1996. Pseudallescheria boydii infection of the brain: imaging with pathologic confirmation. Am. J. Neuroradiol. 17:589-592. [PMC free article] [PubMed] [Google Scholar]
- 3.Berge, M., R. Guillemain, V. Boussaud, M. H. Pham, P. Chevalier, A. Batisse, C. Amrein, E. Dannaoui, M. A. Loriot, A. Lillo-Le Louet, and E. M. Billaud. 2009. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl. Infect. Dis. 11:211-219. [DOI] [PubMed] [Google Scholar]
- 4.Blyth, C. C., A. Harun, P. G. Middleton, S. Sleiman, O. Lee, T. C. Sorrell, W. Meyer, and S. C. Chen. 11 November 2009, posting date. Detection of occult Scedosporium species in respiratory tract specimens from patients with cystic fibrosis (CF) by use of selective media. J. Clin. Microbiol. [Epub ahead of print.] doi: 10.1128/JCM.01470-09. [DOI] [PMC free article] [PubMed]
- 5.Castiglioni, B., D. A. Sutton, M. G. Rinaldi, J. Fung, and S. Kusne. 2002. Pseudallescheria boydii (anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine (Baltimore) 81:333-348. [DOI] [PubMed] [Google Scholar]
- 6.Cimon, B., J. Carrere, J. F. Vinatier, J. P. Chazalette, D. Chabasse, and J. P. Bouchara. 2000. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:53-56. [DOI] [PubMed] [Google Scholar]
- 7.Cortez, K. J., E. Roilides, F. Quiroz-Telles, J. Meletiadis, C. Antachopoulos, T. Knudsen, W. Buchanan, J. Milanovich, D. A. Sutton, A. Fothergill, M. G. Rinaldi, Y. R. Shea, T. Zaoutis, S. Kottilil, and T. J. Walsh. 2008. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 21:157-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., A. Alastruey-Izquierdo, L. Alcazar-Fuoli, L. Bernal-Martinez, A. Gomez-Lopez, M. J. Buitrago, E. Mellado, and J. L. Rodriguez-Tudela. 2008. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob. Agents Chemother. 52:1136-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defontaine, A., R. Zouhair, B. Cimon, J. Carrere, E. Bailly, F. Symoens, M. Diouri, J. N. Hallet, and J. P. Bouchara. 2002. Genotyping study of Scedosporium apiospermum isolates from patients with cystic fibrosis. J. Clin. Microbiol. 40:2108-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavalda, J., O. Len, R. San Juan, J. M. Aguado, J. Fortun, C. Lumbreras, A. Moreno, P. Munoz, M. Blanes, A. Ramos, G. Rufi, M. Gurgui, J. Torre-Cisneros, M. Montejo, M. Cuenca-Estrella, J. L. Rodriguez-Tudela, and A. Pahissa for RESITRA (Spanish Network for Research on Infection in Transplantation). 2005. Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin. Infect. Dis. 41:52-59. [DOI] [PubMed] [Google Scholar]
- 11.Gilgado, F., J. Cano, J. Gene, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilgado, F., C. Serena, J. Cano, J. Gene, and J. Guarro. 2006. Antifungal susceptibilities of the species of the Pseudallescheria boydii complex. Antimicrob. Agents Chemother. 50:4211-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilgado, F., J. Cano, J. Gene, C. Serena, and J. Guarro. 2008. Different virulence of the species of the Pseudallescheria boydii complex. Med. Mycol. 47:371-374. [DOI] [PubMed] [Google Scholar]
- 14.Gilgado, F., J. Cano, J. Gene, D. A. Sutton, and J. Guarro. 2008. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J. Clin. Microbiol. 46:766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenouillet, F., F. Botterel, J. Crouzet, F. Larosa, Y. Hicheri, J. M. Forel, P. Helias, S. Ranque, and L. Delhaes. 2008. Scedosporium prolificans: an emerging pathogen in France? Med. Mycol. 47:343-350. [DOI] [PubMed] [Google Scholar]
- 16.Guignard, S., D. Hubert, B. Dupont, P. Anract, D. Alioua, H. Guerini, A. Paugam, and M. Dougados. 2008. Multifocal Scedosporium apiospermum spondylitis in a cystic fibrosis patient. J. Cyst. Fibros. 7:89-91. [DOI] [PubMed] [Google Scholar]
- 17.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-1113. [DOI] [PubMed] [Google Scholar]
- 18.Husain, S., P. Munoz, G. Forrest, B. D. Alexander, J. Somani, K. Brennan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 19.Husni, R. N., S. M. Gordon, D. L. Longworth, A. Arroliga, P. C. Stillwell, R. K. Avery, J. R. Maurer, A. Mehta, and T. Kirby. 1998. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin. Infect. Dis. 26:753-755. [DOI] [PubMed] [Google Scholar]
- 20.Kesson, A. M., M. C. Bellemore, T. J. O'Mara, D. H. Ellis, and T. C. Sorrell. 2009. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexadecylphospocholine (miltefosine), in combination with terbinafine and voriconazole: a case report. Clin. Infect. Dis. 48:1257-1261. [DOI] [PubMed] [Google Scholar]
- 21.L'Hirondel, J., A. Espern, V. David, D. Horeau-Langlard, M. Besse, and F. Morio. 2008. Prévalence de la colonisation fongique au cours de la mucoviscidose: étude rétrospective sur 30 mois au CHU de Nantes, abstr. 522/80A, p. 235. Abstr. 28th Reun. Interdiscip. Chimiother. Anti Infect., Paris, France.
- 22.Li, J. Y., T. Y. Yong, D. I. Grove, and P. T. Coates. 2008. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient Transpl. Infect. Dis. 10:63-65. [DOI] [PubMed] [Google Scholar]
- 23.Montoya, J. G., and F. Rosso. 2006. Is combination therapy indicated for invasive fungal infections? Yes and no. Curr. Opin. Infect. Dis. 19:357-359. [DOI] [PubMed] [Google Scholar]
- 24.Morin, O., A. Haloun, M. Treilhaud, C. Sagan, and P. Boiron. 1999. Systemic mycosis due Pseudallescheria boydii in a double transplant patient with a cystic fibrosis. Study of isolated strains with RAPD (random amplification of polymorphic DNA). J. Med. Mycol. 9:119-123. [Google Scholar]
- 25.Rodriquez-Tudela, J. L., M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. W. Denning, J. P. Donnelly, W. Fegeler, C. Lass-Flörl, C. Moore, M. Richardson, P. Gaustad, A. Schmalreck, A. Velegraki, and P. Verweij for the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982-984. [DOI] [PubMed] [Google Scholar]
- 26.Sahi, H., R. K. Avery, O. A. Minai, G. Hall, A. C. Mehta, P. Raina, and M. Budev. 2007. Scedosporium apiospermum (Pseudallescheria boydii) infection in lung transplant recipients. J. Heart Lung Transplant. 26:350-356. [DOI] [PubMed] [Google Scholar]
- 27.Solé, A., and M. Salavert. 2008. Fungal infections after lung transplantation Transplant. Rev. 22:89-104. [DOI] [PubMed] [Google Scholar]
- 28.Symoens, F., C. Knoop, M. Schrooyen, O. Denis, M. Estenne, N. Nolard, and F. Jacobs. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J. Heart Lung Transplant. 25:603-607. [DOI] [PubMed] [Google Scholar]
- 29.Troke, P., K. Aguirrebengoa, C. Arteaga, D. Ellis, C. H. Heath, I. Lutsar, M. Rovira, Q. Nguyen, M. Slavin, and S. C. Chen. 2008. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 52:1743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widmer, F., L. C. Wright, D. Obando, R. Handke, R. Ganendren, D. H. Ellis, and T. C. Sorrell. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson, E. C., D. Speers, I. H. Arthur, G. Harnett, G. Ryan, and T. J. Inglis. 2001. Molecular epidemiology of Scedosporium apiospermum infection determined by PCR amplification of ribosomal intergenic spacer sequences in patients with chronic lung disease. J. Clin. Microbiol. 39:47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]