Abstract
In vitro antimalarial activity tests play a pivotal role in malaria drug research or for monitoring drug resistance in field isolates. We applied two isotopic tests, two enzyme-linked immunosorbent assays (ELISA) and the SYBR green I fluorescence-based assay, to test artesunate and chloroquine, the metabolic inhibitors atovaquone and pyrimethamine, our fast-acting choline analog T3/SAR97276, and doxycycline, which has a delayed death profile. Isotopic tests based on hypoxanthine and ethanolamine incorporation are the most reliable tests provided when they are applied after one full 48-h parasite cycle. The SYBR green assay, which measures the DNA content, usually requires 72 h of incubation to obtain reliable results. When delayed death is suspected, specific protocols are required with increasing incubation times up to 96 h. In contrast, both ELISA tests used (pLDH and HRP2) appear to be problematic, leading to disappointing and even erroneous results for molecules that do not share an artesunatelike profile. The reliability of these tests is linked to the mode of action of the drug, and the conditions required to get informative results are hard to predict. Our results suggest some minimal conditions to apply these tests that should give rise to a standard 50% inhibitory concentration, regardless of the mechanism of action of the compounds, and highlight that the most commonly used in vitro antimalarial activity tests do not have the same potential. Some of them might not detect the antimalarial potential of new classes of compounds with innovative modes of action, which subsequently could become promising new antimalarial drugs.
Malaria is a major global health problem, with an estimated 250 to 300 million clinical cases annually and 3.3 billion people at risk, causing nearly a million deaths, mostly among children under 5 years old in sub-Saharan Africa (18, 47). The resistance of Plasmodium falciparum, the most deadly malaria parasite to most antimalarial drugs, is a major obstacle to the eradication of this disease (46). It is also of considerable concern in the light of a recent report on decreased sensitivity to artemesinin drugs in Southeast Asia (14, 28). New chemotherapeutic approaches are thus urgently needed, based on optimization of current drugs and, more importantly, on the discovery of new antimalarial drugs. The latter implies systematic screening of drug libraries, a series of natural compounds, or a structure-based drug design targeting novel targets. In all cases, in vitro evaluation of the thousands of new molecules for their antimalarial activity is an early and necessary step. This early step aims at detecting the antimalarial potential of individual or series of compounds. It is performed in vitro against P. falciparum laboratory strains and, at a later stage, against field isolates, including multidrug-resistant strains. Assays must provide a first indication on the potency of the pharmacological activity, usually expressed as the concentration required to inhibit the parasite viability by 50% (IC50). This early step is crucial because many methods can be used to detect the effect of compounds on malaria parasites. Time-consuming diagnosis of compound effects by microscopic examination of malaria parasites have been replaced by methods using biochemical markers that can be implemented for large-scale analyses. The still largely used gold standard published in 1979 by Desjardins et al. (12) is based on the incorporation of hypoxanthine into nucleic acids. Later on, other parameters based on ethanolamine incorporation into malaria lipids were shown to be reliable substitutes (16). With both methods, experiments are carried out in multiwell plates, followed by collection of labeled macromolecules through a filtration step and counting of the incorporated radioactivity, which reflects the number of malaria parasites remaining after the drug effect, i.e., parasitemia. Nonisotopic methods were then developed, based on the detection of malaria-specific proteins/antigens or on fluorescence measurements of nucleic acid content to assess drug effects on the number of parasites.
Two enzyme-linked immunosorbent assays (ELISAs) with monoclonal antibodies, which measure the P. falciparum-specific antigen histidinerich protein 2 (HRP2) (26, 27) or lactate dehydrogenase protein (pLDH) (25) present in parasite suspensions (erythrocytes and culture medium) after incubation with the drug, are now widely used. The levels of pLDH, the last enzyme in the glycolytic pathway (23), and HRP2, a highly stable protein secreted through the membrane of infected red blood cells (RBC), are proportional to the extent of parasite growth (10, 15, 44). Parasitemia can also be quantified through the widely used SYBR green I fluorescence-based assay involving a dye whose fluorescence is enhanced upon contact with nucleic acids (20, 37). This fluorescence is restricted to Plasmodium because RBC per se do not contain nucleic acids.
In research carried out to develop new antimalarial drugs, it is important to use tests capable of estimating the antimalarial potential of the various classes of compounds, especially new drugs with novel targets and thereby novel modes of action. In 2003, Noedl et al. (30) reviewed some of these methods for in vitro assessment of malaria drug sensitivity and their implementation and feasibility limitations based on literature data. It was found that, when the above tests were applied in standard conditions, difficulties were encountered in measuring the real efficacy (IC50). These methods can even miss the antimalarial potential of compounds, notably for some anti-metabolite approaches inducing delayed death (9, 13).
Our laboratory is developing a new class of antimalarial drugs targeting membrane biogenesis, which occurs throughout the P. falciparum cycle. The compounds mimic the choline structure and prevent biosynthesis of the parasite phosphatidylcholine, which accounts for 40% of its lipid molecule (40-42). The compounds inhibit P. falciparum asexual blood stages at single-digit nanomolar concentrations and cure P. vinckei malaria infection in mice at doses under 1 mg/kg (1-3, 45). The potency and specificity of these antiphospholipid effectors are likely associated with their unique property of accumulating in a nonreversible way inside intraerythrocytic parasites (43). The last generation of compounds with thiazolium cationic heads led to T3/SAR97276, a clinical candidate developed by Sanofi-Aventis that was successfully evaluated for its toxicity and pharmacokinetic parameters in phase I clinical trials. Phase 2 clinical trials to treat severe malaria by the parenteral route are under way.
The cytotoxic affect of T3/SAR97276 is outstandingly rapid, i.e., killing P. falciparum asexual blood stages after exposure of <2 h with an IC50 of 3 nM (43). However, during drug testing against field isolates of P. falciparum and P. vivax, the lack of response of a currently used diagnostic test, leading to an unreliable IC50 of this class of compounds (F. Nosten, unpublished data), intrigued us. This prompted us to compare the different drug susceptibility tests.
Bis-thiazolium compounds exert their rapid cytotoxic (i.e., nonreversible) effect after 1 to 2 h of contact with the drug (43). This is due to the rapid irreversible accumulation of the compounds within intracellular parasites at all stages (43, 45). However, although the parasite is condemned to death, its morphology remains unaffected for many hours until its collapse at the schizont stage (24). This mechanism likely induces erroneous results, according to the in vitro antimalarial activity test, and may be a problem for clinicians who do not detect rapid clearance of the parasites after treatment. To avoid problems linked with delayed parasite collapse, we routinely use a modified Desjardins test which measures [3H]hypoxanthine incorporation into nucleic acids after a full infected-erythrocyte incubation cycle (3). With the aim of evaluating choline analogs under the most suitable conditions, we determined which of these tests were actually effective for determining the antimalarial potential of novel drug series, including antiphospholipid effectors. For the present study, we used T3/SAR97276, our fast-acting cytotoxic choline analog compound, with artesunate, whose onset of action is also very rapid. Finally, four classes of current antimalarials were tested in order to determine the possible relation between the mechanism of action and the in vitro test reliability. We suggest some minimal conditions on how to apply these tests, which should lead to a standard IC50, regardless of the mechanism of action of the compounds.
MATERIALS AND METHODS
Biological materials.
The P. falciparum 3D7 strain (MRA-102 from MR4) was cultured in human RBC (EFS, Montpellier, France) at 5% hematocrit in complete medium composed of RPMI 1640 medium supplemented with 25 mM HEPES buffer (pH 7.4) and 10% AB+ human serum (39). Petri dishes were incubated at 37°C under a gaseous mixture of 5% CO2, 5% O2, and 90% N2. Parasites were synchronized by using 5% sorbitol (22).
Incubation of P. falciparum blood stage with drugs.
P. falciparum-infected erythrocytes synchronized at the ring stage were incubated in 96-well microtiter plates in 200 μl of complete medium at 1.5% hematocrit, with an initial parasitemia of 0.1 to 0.5%. Incubations at 37°C were done in gas incubation chambers under 5% O2, 5% CO2, and 90%N2. Drugs were dissolved in HEPES-buffered RPMI 1640 (T3/SAR97276, CQ) or dimethyl sulfoxide (DMSO; artesunate, atovaquone, pyrimethamine, and doxycycline) and then further diluted in complete medium. The final DMSO concentration never exceeded 0.25%. In each plate, infected erythrocytes cultured in the absence of drug served as positive controls for parasite growth, whereas uninfected erythrocytes served as negative controls (background).
In some experiments, drugs were removed from the culture medium at 48 h and replaced by complete medium without drug. Cells were washed three times, thus reducing the drug concentration by >64-fold. For incubations of at least 72 h, 50% of the medium of each well was replaced from 48 h and every 24 h by fresh medium containing or not the appropriate concentration of drug.
After incubation with or without the drugs, as indicated, the different tests were applied to determine the drug effect. Radioactive precursors were added at 48, 72, or 96 h to measure nucleic acids or phospholipid biosynthetic metabolic activity, and the reactions were pursued as indicated for 18 h or 24 h before freezing at −80°C. For nonradioactive tests, plates were frozen at the indicated times and stored at −80°C until assay to determine the parasite growth patterns.
In vitro drug sensitivity using radioactive precursors (isotopic tests).
These assays were based on a modified Desjardins test (3, 12). Metabolic activities of the parasites were assessed by measuring the incorporation of [3H]hypoxanthine or [3H]ethanolamine (GE Healthcare) into nucleic acids or phospholipids, respectively. [3H]hypoxanthine (0.5 μCi/well) or [3H]ethanolamine (1 μCi/well) in 30 μl of complete medium was added in each well at the indicated time. Hypoxanthine incorporations were pursued for 18 h when added at 48 or for 24 h when added at 72 or 96 h. The plates were frozen at −80°C to stop the incorporation. Cells were lysed by thawing and the parasite DNA and membrane phospholipids were recovered by harvesting the lysate on glass-fiber filter plates (Unifilter 96 GF/C; Perkin-Elmer) using a FilterMate cell harvester (Packard Instruments), and the radioactivity was counted on a TopCount microplate scintillation counter (Packard Instruments).
In vitro drug sensitivity using SYBR green I-based fluorescence assay.
Parasite growth was determined by measuring the fluorescence of SYBR green I bound to malarial nucleic acids. The frozen multiwell plates were thawed, and 100 μl from each well was transferred into black 96-well plates for fluorescence analysis. Then, 100 μl of lysis buffer (20 mM Tris, 5 mM EDTA, 0.008% saponin, and 0.08% Triton X-100 [pH 7.5]) containing 0.2 μl of SYBR green I (10,000× in DMSO; Molecular Probes/Invitrogen)/ml were added to each well. After 15 min to 1 h of incubation at room temperature in the dark, the fluorescence was assessed with a Twinkle LB970 fluorometer (Berthold) with excitation and emission wavelengths of 485 and 535 nm, respectively (4, 37).
In vitro drug sensitivity using the pLDH assay.
The double-site enzyme-linked pLDH immunodetection assay was performed as described in Barends et al. (5). Briefly, the culture plates were thawed and frozen twice to obtain complete hemolysis. Then, 100 μl from each well was transferred into 96-well plates (Nunc-Immuno plate; Maxisorb; Nalgene) precoated with monoclonal antibody E14 (a gift from M. Barends) that specifically recognizes pLDH. After 1 h of incubation at 37°C, the plates were washed three times with phosphate-buffered saline (PBS; 116 mM NaCl, 8.3 mM Na2HPO4, 3.2 mM KH2PO4 [pH 7.4]) supplemented with 1% bovine serum albumin (BSA; fraction V; Boehringer Mannheim), hereafter referred to as PBS-1% BSA. A second biotinylated anti-pLDH monoclonal antibody 19G7 (100 μl/well of a 1/4,000 dilution in PBS-1% BSA) was added, and the plates were incubated for 1 h at 37°C and washed then three times with PBS-1% BSA. The plates were then incubated for 30 min at room temperature with a 1/10,000 solution of streptavidin-POD conjugate and washed three times with PBS-1% BSA. A total of 100 μl of a peroxidase substrate solution was added for up to 20 min at room temperature in the dark. The reaction was stopped with 1 M phosphoric acid. Spectrophotometric analysis was performed at 450 nm with a reference filter at 630 nm using an ELISA plate reader (Dynatech).
In vitro drug sensitivity using an HRP2 assay.
The frozen plates were thawed-frozen twice to obtain complete hemolysis. Then, 100 μl of culture/well was transferred into coated 96-well ELISA plates (Nunc-Immuno plate) coated with MPFM-55A antibody (Gentaur) for 1 h at room temperature in a humid chamber. The plates were then washed three times with PBS-Tween washing solution (0.05% Tween 20 in PBS). After the addition of 100 μl of the diluted antibody conjugate (MPFG-55P; Gentaur) (0.05 μg/ml in 2% BSA and 1% Tween 20) to each well, the plates were incubated for another hour at room temperature and washed three times with PBS-Tween washing solution.
Finally, 100 μl of TMB (3-3′,5,5′-tetramethylbenzidine) single solution chromogen (Invitrogen) was added to each well, and the plates were incubated in the dark for 5 to 10 min. Reactions were stopped with 1 M sulfuric acid. Spectrophotometric analyses were performed at 450 nm using an ELISA plate reader (Dynatech) (27).
Microscopy.
For light microscopy, thin blood smears were fixed in methanol and stained with Diff Quick stain (Medion Diagnostic). Parasitemia was determined by counting at least 1,000 cells.
Effect of adding radiolabeled precursors early on in vitro drug sensitivity tests.
These assays were based on the isotopic test used above. Drugs were added to the erythrocyte suspension at time zero. [3H]hypoxanthine (0.5 μCi/well) or [3H]ethanolamine (1 μCi/well) in 30 μl of complete medium were added to the culture at time zero for 48 h (measured during a full parasite cycle), at 24 h for 24 h, and at 48 h for 18 h (classic test), and then the assay plates were frozen to stop the reaction.
Time course of P. falciparum growth inhibition by the bis-thiazolium T3/SAR97276.
The choline analog T3/SAR97276 was added at 40 or 80 nM to synchronized cultures at the ring (6 h), trophozoite (24 h), or schizont (36 h) stages. After incubation of each parasite stage for various times, cells were washed twice and resuspended in fresh complete medium without drug. For all conditions and stages, drug effects were measured by adding [3H]hypoxanthine (0.5 μCi per well) at 52 h to monitor parasite viability, and the reactions were stopped at 76 h by freezing the plates at −80°C. Cells were lysed by thawing, and the parasite DNA was recovered by harvesting the lysate on glass-fiber filter plates, as described for the isotopic test.
Morphological effects of bis-thiazolium T3/SAR97276 on P. falciparum growth.
T3/SAR97276 was added at 80 nM to synchronized cultures at the ring stage (5% hematocrit and 0.5% parasitemia). Thin blood smears were performed at the indicated times. As already indicated, beyond 48 h of incubation, 50% of the medium was replaced every 24 h by fresh medium containing or not containing 80 nM T3/SAR97276.
Analysis of in vitro experiments.
In all experiments, background values from incubation of equal amounts of noninfected erythrocytes were subtracted from the values measured from the infected suspension. Radiolabeled precursor incorporation, the quantity of pLDH or HRP2, and the quantity of DNA in the presence of drugs are expressed as a percentage of the positive controls of infected erythrocytes incubated without drugs. A nonlinear regression model (sigmoidal dose-response/variable slope; GraphPad Prism4) was used to calculate the IC50s. Experiments were performed at least twice each at least in triplicate. Geometric means and 95% confidence intervals are indicated. The results were compared using an analysis of variance. For all tests, P values of <0.05 were taken as indicative of statistically significant differences.
RESULTS
Comparative study of five widely used in vitro drug sensitivity tests.
Antimalarial compounds have distinct mechanisms of action, i.e., they have a maximum effect within a short period of time during the parasite cycle when their targets are expressed or when their target becomes essential. Beyond this short time window, the drug may not be efficient or it may be much less efficient. Thus, we first decided to incubate the malaria parasite for a full 48-h P. falciparum cycle before applying in vitro tests to measure the parasite viability and thereby the drug effect on the parasite. This first set of experiments was carried out with both the antiphospholipid compound T3/SAR97276 and artesunate by adding the compounds to P. falciparum cultures synchronized at the ring stage.
We compared the [3H]hypoxanthine isotopic test with three other assays, with each of them being applied after a similar 48 h of incubation period with the drugs. The antimalarial activity of T3/SAR97276 determined by [3H]hypoxanthine incorporation into nucleic acids was 4.6 nM (Table 1). Microscopy counts of Giemsa-stained parasites indicated an IC50 of 5.6 nM, thus confirming the hypoxanthine assay results. Note that removing the drug after 48 h during the 18-h [3H]hypoxanthine incorporation led to similar antimalarial activities, with an IC50 of 3.5 nM (95% confidence interval [CI] = 3.2 to 4 nM) and 1.1 nM (95% CI = 0.8 to 1.4 nM) for T3/SAR97276 and artesunate, respectively, indicating that the drug had an effect during the first 48-h period. The HRP2 dosage led to a relatively close but significantly different IC50 of 18.5 nM (P < 0.05). Conversely, the IC50 resulting from the SYBR green or pLDH assays differed markedly, with values of 77 and >1,000 nM, respectively. On the contrary, there was no discrepancy in the IC50 for artesunate, with an IC50 of 1.1 nM with the isotopic test and in the 1.5 to 2.4 nM range with the fluorescent SYBR green or the HRP2 and pLDH ELISA tests (Table 1). These values are close to microscopy counting (IC50 of 3.7 nM) considering the CIs.
TABLE 1.
In vitro drug testing of antimalarial activity of T3/SAR97276 and artesunate using different drug sensitivity tests
| Drug | Incubation period (h) | Geometric mean IC50 in nM against P. falciparum 3D7 (95% CI)a as determined by using: |
||||
|---|---|---|---|---|---|---|
| MIC | HX assay | SYBR green | pLDH ELISA | HRP2 ELISA | ||
| T3/SAR97276 | 48 | 5.6 | 4.6 (4-5.2) | 77.3 (25.7-232) | >1,000 | 18.5 (14.1-24.3) |
| 72 | 2 | 4.5 (3.7-5.6) | 2.3 (0.6-9.9) | 19.5 (15.3-24.7) | 99.3 (50.3-19.6) | |
| 72 (w/o drug)b | 7.2 | 8.1 (6.9-9.5) | 7.4 (5.9-9.2) | |||
| 96 h | 1.9 | 1.3 (1.2-1.4) | 3.9 (3.6-4.4) | |||
| 96 (w/o drug) | 7.4 | 5.3 (4.6-6.3) | 6.3 (5.7-7) | |||
| Artesunate | 48 | 3.7 | 1.1 (0.8-1.4) | 1.9 (1.2-2.8) | 2.4 (2.2-2.6) | 1.5 (1.4-1.7) |
| 72 | 1.6 | 1.2 (0.8-1.6) | 1.5 (1.3-1.8) | 2.0 (1.1-3.4) | 2.5 (1.9-3.3) | |
| 72 (w/o drug) | 1.8 | 1.4 (1-2.1) | 1.5 (1.4-1.7) | |||
| 96 | 1.8 | 1.1 (0.9-1.5) | 1.3 (1.1-1.5) | |||
| 96 (w/o drug) | 1.4 | 1.4 (1.1-1.8) | 1.6 (1.5-1.8) | |||
MIC, microscopy; HX, hypoxanthine. Values in boldface do not match the real IC50 (see the text).
w/o drug, without drug. In these cases, after 48 h of contact with the drug, infected erythrocytes were washed for drug clearance and then incubated in fresh drug-free culture medium.
HRP2 assays are usually carried out over a 72-h incubation period with drugs (26). We thus carried out comparative studies by increasing the time of incubation with the drugs to 72 h before applying the in vitro drug sensitivity tests. In these conditions, the hypoxanthine IC50 of T3/SAR97276 was unchanged, and the SYBR green evaluation gave a similar IC50 of 2.3 nM, indicating that the SYBR green test requires more than 48 h of incubation to be in agreement with the hypoxanthine and microscopy test findings. In comparison with the 48-h test, pLDH indicated a much lower IC50 of 19.5 nM, but the IC50 of T3/SAR97276 remained above 10 nM. Surprisingly, the IC50 obtained in the HRP2 tests increased regularly from 18.5 to 100 nM. For artesunate, e.g., after 48 h of incubation, the four tests gave similar and unchanged IC50s (P > 0.05) (see Table 1).
For compound T3/SAR97276, the pLDH and HRP2 tests gave erroneous IC50 values when applied at 48 and 72 h. These ELISA tests, which measured the parasite-specific antigen content in the culture lysate, did not appear to be reliable for this class of antimalarial drugs, likely due to the peculiarities in its mechanism of action (43) (see below). Noedl et al. (29) has already shown that the mechanism of action of the drug influences HRP2 test results because the HRP2 protein has a long biological half-life and stability (29). The measured HRP2 levels provided a cumulative picture of the antigen production over time, which may not have been stopped immediately by the drug. These properties may be applicable to the pLDH test, suggesting that when a drug did not stop all parasite activities immediately (see below and reference 9), the continued production of HRP2 or pLDH by the parasites biased the findings.
Effect of T3/SAR97276 and artesunate on the second parasite cycle.
Performing drug sensitivity tests beyond 48 h of incubation (e.g., 72 h as described above for SYBR green) might highlight a drug effect against the second generation of parasites developing from the first 48-h cycle after reinvasion. This reflects a delayed death profile that has been well described for a few apicoplast effectors (11). We pursued our characterization by incubating parasites with the drugs for 72 or 96 h before applying the hypoxanthine or SYBR green sensor test. Importantly, in control experiments, parasites were washed to clear them of the drugs (three washes reducing the drug concentration to 1/64) after the first full cycle (48 h), and incubations were continued until the tests were applied at 72 or 96 h.
Using hypoxanthine, 48 or 72 h of contact with T3/SAR97276 led to similar IC50 values (Table 1). Surprisingly, after 96 h of continuous exposure to the drug, the IC50 appeared to be significantly and reproducibly decreased to 1.3 nM. When T3/SAR97276 was removed from the incubation medium after the first 48 h of incubation, the IC50 were similar regardless of whether hypoxanthine was added at 48, 72, or 96 h (Table 1). Similar results were obtained with SYBR green, excepted at time 48 h when erroneous results were obtained, as shown above. In the same way, the IC50s of artesunate were similar when the parasites were exposed for 48, 72, or 96 h, even when the drugs were removed after the first parasite cycle (Table 1). The entire toxic effect exerted by both T3/SAR97276 and artesunate was fully visible at 48 h, with IC50s of ca. 5 and 1 nM, respectively, and there was no delayed death profile.
Effect of adding radiolabeled precursors early on in vitro drug sensitivity tests.
Many tests reported in the malaria literature involved adding the radioactive precursors much earlier than 48 h (35), sometimes simultaneously with the drug, i.e., at time zero (8, 12, 21). This is more convenient and might avoid problems of culture viability when measuring the chemosensitivity of field isolates (8). In additional experiments, parasites at the ring stage were incubated in the presence of T3/SAR97276 and artesunate for a full 48-h cycle, but the drug effect was measured after adding the radioactive precursors simultaneously with the drugs (time zero or 24 h).
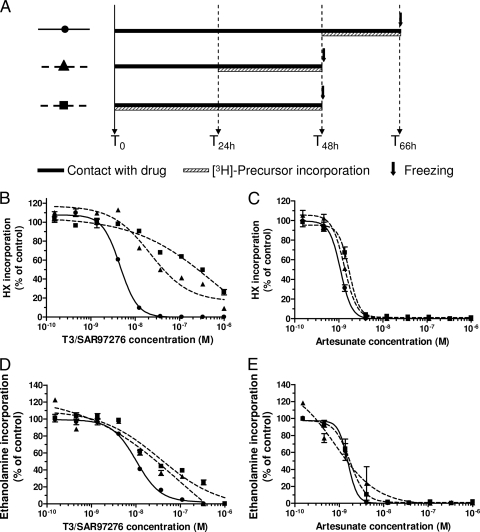
When [3H]hypoxanthine was added at time zero or 24 h, the IC50s of T3/SAR97276 were, respectively, 285.5 and 23.1 nM, i.e., much higher than the 4.6 nM obtained when added after a full parasite cycle (see Fig. 1 and Table 2).
FIG. 1.
Effect of adding [3H]hypoxanthine and [3H]ethanolamine early on in vitro T3/SAR97276 and artesunate sensitivity tests. (A) The experimental setups are summarized. T3/SAR97276 (B and D) or artesunate (C and E) were added at the indicated concentrations to synchronized P. falciparum parasites at the ring stage. [3H]hypoxanthine (B and C) or [3H]ethanolamine (D and E) was added at time zero (▪ and dashed line), 24 h (▴ and dashed line), or 48 h (•) for the indicated times, and then the reactions were stopped by freezing. Incorporations are expressed as means ± the standard error of the mean (SEM) (n > 3) and expressed as a percentage of the controls.
TABLE 2.
Effect of adding radiolabeled precursors early on in vitro drug sensitivity tests
| Time (h) of contact | Time (h) of freezing | Geometric mean IC50 in nM (95% CI)a |
|||
|---|---|---|---|---|---|
| T3/SAR97276 |
AS |
||||
| HX | Ethanolamine | HX | Ethanolamine | ||
| 0-48 | 48 | 285.5 (151.9-536.9) | 38.5 (14.3-103.5) | 1.7 (1.3-2.2) | 1.7 (1.2-2.4) |
| 24-48 | 48 | 23.1 (15.5-34.4) | 22.3 (12.8-38.7) | 1.3 (1.2-1.5) | 0.9 (0.2-6) |
| 48-66 | 66 | 4.6 (4-5.2) | 9.4 (8.7-10.3) | 1.1 (0.8-1.4) | 1.5 (1.4-1.7) |
HX, hypoxanthine.
When [3H]ethanolamine was substituted for [3H]hypoxanthine, the same profile was obtained, with increased IC50s of 38.5 and 22.3 nM compared to the 9.4 nM measured by adding ethanolamine at 48 h (Fig. 1 and Table 2). T3/SAR97276 did not immediately stop the incorporation of both precursors into the parasites' respective macromolecules even though the parasites had already lost their viability (see Fig. 2 and 3 and below).
FIG. 2.
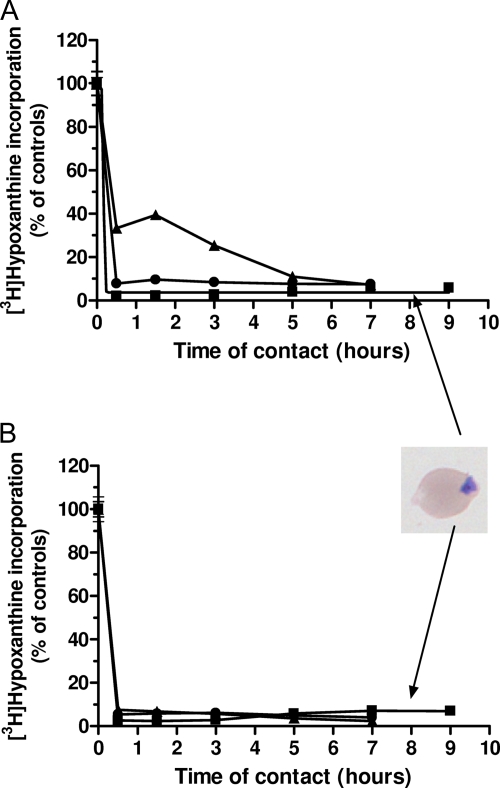
Time course of P. falciparum growth inhibition by T3/SAR97276. The drug T3/SAR97276 was added at 40 nM (A) or 80 nM (B) to synchronized cultures at the ring (6 h) (▪), trophozoite (24 h) (▴), or schizont (36 h) (•) stages. After incubation for the indicated times, cells were washed and resuspended in fresh medium. Parasite viability was monitored by adding [3H]hypoxanthine at 52 h until 76 h. Hypoxanthine incorporations are expressed as means ± the SEM (n ≥ 3) and expressed as a percentage of the controls.
FIG. 3.
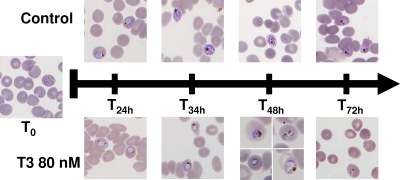
Giemsa-stained smears of T3/SAR97276-treated and untreated cultures. T3/SAR97276 was added at 80 nM at the early ring stage. The morphology of parasites in treated and untreated cultures is shown. Morphological changes are clearly observed at 30 h with an absence of schizont formation and invasion at 48 h (T48h). From 34 h, the parasites were blocked at the trophozoite/schizont stage, and then they collapsed and disappeared (T72h).
For artesunate, the IC50 were similar when hypoxanthine or ethanolamine was added simultaneously to the drugs or at 24 or 48 h. The IC50 of artesunate was between 1 and 1.7 nM when [3H]hypoxanthine was used and between 0.9 and 1.7 nM [3H]ethanolamine was used, showing that the different parasite metabolisms had rapidly and simultaneously stopped (Fig. 1 and Table 2).
Time course of T3/SAR97276 effect on parasite viability and morphology.
The results obtained in the different tests led us to explore the kinetics of T3/SAR97276 effects on biochemical reactions and morphological aspects of P. falciparum.
Synchronized cultures of infected erythrocytes were pulsed for various times with 40 (Fig. 2A) or 80 nM (Fig. 2B) of T3/SAR97276 (10- and 20-fold the IC50, respectively), and then the drug was removed. Regardless of the time of contact of the drug with the parasite, the drug effect was measured at time 52 h. Figure 2 shows that very short exposure of P. falciparum, at any of its blood stages, to 40 or 80 nM T3/SAR97276 blocked parasite development. Remarkably, 1 h of contact with 40 nM T3/SAR97276 was sufficient to obtain more than 90% irreversible growth inhibition at the ring and schizont stages, even though the drug was then removed (Fig. 2A). In the same conditions, inhibition of 90% of the parasite growth during the trophozoite stage required 5 h of contact with the drug. When T3/SAR97276 was applied at 80 nM (Fig. 2B), less than 1 h was sufficient to irreversibly inhibit more than 90% of the parasite growth. From 3 h of contact with T3/SAR97276, parasite growth was completely inhibited and the last 10% incorporation was due to residual incorporation in picnocytes (dead parasites), which appeared only on thin blood smears (Fig. 2). These results indicate a very rapid irreversible cytotoxic effect.
Figure 3 presents the drug effects on the parasite morphology when the parasites were incubated at the ring stage with 80 nM T3/SAR97276. Whereas no morphological difference was detected between untreated and drug treated parasites during the first 24 h, morphological changes and arrest of the cell cycle progression in the late trophozoite stage were noted between 24 and 48 h after the addition of T3/SAR97276. At 48 h, the untreated culture had 6.7% parasitemia and new rings had appeared. Parasitemia in the treated culture decreased to 0.6%, with a majority of unhealthy parasites and no schizogony observed. Finally, all treated parasites collapsed or disappeared before 72 h (Fig. 3).
Although the parasite morphology was not rapidly affected, T3/SAR97276 required a very short time of contact with infected erythrocytes to exert its pharmacological activity and for condemned parasites to die before the end of their cycle.
Current antimalarials and in vitro tests.
The results presented above indicate that parasite incubation with the choline analog T3/SAR97276 should last one full cycle before sensitivity tests are carried out to be able to obtain relatively reliable results. We carried out a comparative study with classes of the currently used antimalarials by incubating the drugs for at least 48 h before the sensitivity tests were performed.
When the culture was incubated with chloroquine for 48 or 72 h, the four tests gave similar results, with IC50s ranging from 9 to 15 nM (Table 3), as previously reported (15, 26, 37). For atovaquone, subnanomolar IC50s (0.06 to 0.3 nM) were recorded except in the pLDH assay after 48 h of incubation with a very different 6 nM IC50 and for HRP2 assay after 72 h of incubation, which gave a significantly increased IC50.
TABLE 3.
Antimalarial activities of chloroquine, atovaquone, doxycycline, and pyrimethamine according to the different antimalarial in vitro tests
| Drug | Incubation period (h) | Geometric mean of IC50 in nM (95% CI) as determined usinga: |
||||
|---|---|---|---|---|---|---|
| MIC | [3H]HX | SYBR green | pLDH | HRP2 | ||
| Chloroquine | 48 | 14.79 | 9.56 (7.58-12.07) | 11.54 (4.74-28.12) | 14.97 (12.37-18.12) | 10.10 (8.04-12.69) |
| 72 | 4.60 | 10.04 (8.8-11.45) | 8.70 (6.17-12.28) | 9.48 (6.82-13.18) | 9.68 (7.03-13.31) | |
| Atovaquone | 48 | 0.11 | 0.06 (0.05-0.07) | 0.06 (0.02-0.18) | 6.05 (4.07-9) | 0.32 (0.12-0.82) |
| 72 | 0.13 | 0.13 (0.11-0.16) | 0.07 (0.07-0.08) | 0.30 (0.25-0.36) | 0.79 (0.39-1.58) | |
| Doxycycline | 48 | 6.58 | 2.85 (1.84-4.4) | 10.20 (8.49-12.26) | 24.39 (7.14-83.28) | 7.73 (7.56-7.9) |
| 72 | 4.20 | 0.48 (0.34-0.68) | 4.90 (3.67-6.57) | 9.68 (8.21-11.42) | 13.09 (10.27-16.7) | |
| 96 | 0.25 (0.24-0.27) | 0.35 (0.29-0.42) | 8.37 (6.48-10.81) | 21.37 (5.85-78.01) | ||
| 96 (drug removed)c | 0.23 | 0.21 (0.09-0.5) | 0.49 (0.47-0.5) | 11.38 (7.54-17.18) | 12.50 (5.74-27.18) | |
| Pyrimethamineb | 48 | 3.33 | 2.98 (2.7-3.3) | >100 | >100 | 3.29 (0.63-17.21) |
| 72 | 4.53 | 3.62 (3.2-4.14) | 3.79 (3.22-4.45) | 4.09 (3.82-4.38) | 4.63 (2.54-8.45) | |
| Pyrimethamine | 48 | 32.20 | 19.33 (17.7-21.1) | >100 | >100 | 26.57 (20.28-34.82) |
| 72 | 26.30 | 19.35 (13.96-26.81) | 23.92 (22.47-25.48) | 27.91 (24.74-31.49) | 53.85 (46.06-62.96) | |
MIC, microscopy; HX, hypoxanthine. Values in boldface do not match the real IC50 (see the text).
RPMI SP823 (with less PABA and folic acid).
That is, the drug was removed at 48 h and replaced by fresh complete medium.
Doxycycline, which induces delayed death (11), was evaluated at 48, 72, and 96 h of incubation. For 96 h, we made an essential control by washing the cells at 48 h and reincubating parasites without the drug during the second 48- to 96-h cycle. A subnanomolar IC50 in the 0.2 to 0.5 nM range reflects the real potency of this antibiotic, which exerts its antimalarial effect through a delayed death process (11). This value was obtained in the microscopic and SYBR green tests only when applied at 96 h (but not before), whereas hypoxanthine gave a correct value from 72 h. The difference in IC50 between hypoxanthine incorporation and microscopy indicates that DNA synthesis had already been arrested in the morphologically intact parasites observed at 72 h. Parasite multiplication had stopped at time 72 h; therefore, hypoxanthine incorporation reflects the real doxycycline IC50. However, the SYBR green assay based on the parasite DNA content did not give the correct IC50 at 72 h (4.9 nM) but only after 96 h of incubation (0.35 nM). At 72 h, SYBR green stained dying parasites, thus causing an underestimation of doxycycline activity. Regardless of the conditions, both pLDH and HRP2 tests gave highly erroneous results, with IC50 at two-digit nanomolar concentrations instead of subnanomolar concentrations (Table 3). Indeed, the parasites were not very affected during the first cycle since they were still synthesizing pLDH and HRP2 proteins, which induced erroneous results because the two ELISA tests provided a time course cumulative picture of protein production.
The antifolate pyrimethamine was tested in normal RPMI 1640, which contained 1 mg of p-aminobenzoic acid (PABA) and folic acid/liter, or in special RPMI with lower concentrations of both precursors (0.5 and 10 μg/liter, respectively; RPMI SP823; Invitrogen [a gift from B. Pradines, IMTSSA, Marseille, France]). The IC50s resulting from hypoxanthine testing were quite close to microscopy findings for both 48- and 72-h incubations. The IC50s measured at low PABA and folic acid concentrations were in the 3 to 5 nM range and had increased 6-fold when measured in normal RPMI. SYBR green and pLDH shared very similar IC50s, which were erroneous and correct at 48 and 72 h, respectively. In this case, in contrast to the pLDH assay, the ELISA test HRP2 gave reliable results for both times (Table 3).
DISCUSSION
In vitro drug susceptibility assays have a pivotal role in strategies aimed at discovering new antimalarial compounds or monitoring the emergence of drug resistance in field isolates. They must combine accuracy, reliability, reproducibility, rapidity, and suitability for medium- to high-throughput screening. The ability of a drug to irreversibly alter the parasite viability is commonly used as the most informative indicator of its antimalarial potential.
Most antimalarial drugs affect a target expressed only within a limited time window during the erythrocyte cycle. Consequently, one way to sidestep the pitfall of missing an antimalarial effect is to expose the parasite to the drug for at least a full cycle before measuring parameters that will best reflect the drug effect (Fig. 1). However, in some cases, the effective toxic impact during the first 48 h of exposure is only visible during the following cycle because drug-exposed parasites may give rise to a second generation of parasites that are hampered in their proliferation capacity (13). This is now defined as the delayed death profile (11). In this specific case, tests must be extended to include longer incubation periods since toxic effects that are effective during the first exposure cycle will only be fully detectable at the end of the second cycle. Removing drugs at 48 h allows verifying that the lethal target was exerted during the first cycle (11, 33).
Difficulties arise when choosing in vitro drug susceptibility assays because they are generally based on different concepts to monitor drug effects. These concepts differ in the reliability of correlation with parasite viability. For example, drugs with action on the later parasite stage will not show inhibition of processes that occur earlier in development and/or biochemical processes may continue even after the replicative ability of the parasites has been damaged.
We carried out comparative studies of standard isotopic tests (using hypoxanthine and ethanolamine) and pLDH and HRP2 ELISAs based on the detection of malaria-specific proteins and fluorescence-based SYBR green assays measuring the DNA content. They were first applied to artesunate and T3/SAR97276, which are two potent antimalarials possessing different modes of action. Artesunate is an outstanding antimalarial drug that likely acts through the production of toxic radical reactions that induce rapid destruction of intraerythrocytic P. falciparum parasites (31). The bis-thiazolium T3/SAR97276 is an original antiphospholipid effector that is currently in the development phase for severe malaria. This new type of antimalarial affects a polyspecific organic cation transporter that mediates choline entry into the intracellular parasite (6), thus preventing the synthesis of phosphatidylcholine, the major malaria lipid (43). One prominent feature of biscationic choline analogs is their ability to accumulate by several hundredfold in hematozoan-infected erythrocytes (45). Drug T16 is partially recovered in the parasite's digestive vacuole, interacting with haem, which is also critical for antiplasmodial activity (7). Figure 2 shows that the T3/SAR97276 compound inhibited parasite growth by more than 90% after less than 5 h of contact at 40 nM and less than 2 h of contact at 80 nM, irrespective of the parasite development stage. Thus, T3/SAR97276 irreversibly kills the parasites very rapidly after contact with the drug. We then tested one compound of each class of current antimalarials (chloroquine, atovaquone, pyrimethamine, and doxycycline) in order to investigate the reliability of the four assays according to the drug mechanisms of action.
The isotopic tests are based on fluxomic data (i.e., quantitative incorporation of radioactive precursors) in the nucleic acid and phospholipid malaria metabolic pathways and appear correlated with the number of parasites, i.e., parasitemia with a background of zero in the absence of parasites. Overall, the results obtained with artesunate and T3/SAR97276 (Fig. 1), as well as with the different drugs and assays (Table 3), indicated that determinations of the drug concentration inhibiting parasite growth by 50% (IC50) required 48 h of incubation with the drug before radioactive isotope addition. After this incubation period, the level of radioactive nucleic acids or phospholipids recovered only reflects the parasite metabolisms and the number of viable parasites, i.e., parasitemia (Fig. 1).
Adding the radioactive isotope during the first 48 h of the parasite cycle led to much higher IC50s, which may not reflect the antimalarial potential of the drugs. For T3/SAR97276, Fig. 1 clearly shows that nucleic acid and phosphatidylethanolamine biosynthesis were still active until at least 36 h, even though the parasites had lost their capacity to proliferate a couple of hours after drug addition, as confirmed in Fig. 2, which shows that the T3/SAR97276 compound rapidly kill P. falciparum in <2 h. On the other hand, the choline analog do not rapidly affect the parasite morphology, which appears to be normal until maturation. Visible effects are delayed until the parasite collapse during trophozoite maturation.
In other cases, toxic effects of the drugs may occur later on during the first parasite cycle (e.g., very mature stage), and the results may be biased by prior radioactive incorporation (e.g., for ciprofloxacin, rifampin, and thiostrepton [13, 17]). Since the lethal effect of a drug on the malaria parasite may be exerted very rapidly but without immediately stopping the parasite metabolic activities (e.g., T3/SAR97276 and doxycycline), exposure of the parasite to the drug for a full 48-h cycle is required before the parasite viability is measured.
The present findings also indicated that for drugs with a delayed effect, a longer incorporation period is necessary to determine the real antimalarial activity after a full cycle. The incubation time before applying the test must therefore be increased to 96 h (Table 3).
[3H]hypoxanthine incorporation, when performed after a full parasite cycle, gave reliable antimalarial activities, thus confirming the microscopy data, regardless of the compound and its mechanism of action. Moreover, the isotopic tests are sometimes more informative than the microscopy tests, reflecting the arrest of metabolic activity of the parasites before they disappear. The results obtained with the SYBR green assay depend greatly on the preassay incubation time. To obtain reliable results, i.e., in agreement with the microscopy and [3H]hypoxanthine incorporation findings, regardless of the compound, the incubation time must be longer than 48 h (Tables 1 and 3). This is out of line with the results reported by Johnson et al. (20), who claimed that the SYBR green assay could be used after 48 or 72 h of incubation, even for doxycycline and pyrimethamine. This is likely due to the comparator isotopic test that they applied at time 24 h, which give an increased IC50 for some compounds such as T3/SAR97276 (Table 2). The nonreliability of the SYBR green test when used at 48 h suggests a labeling of remnant dead parasites. This can be avoided by applying the test at 72 h, as in most recent studies (32, 34).
It is also striking that the results obtained with pLDH and HRP2 tests very significantly differ according to the drug tested. These ELISAs give correct results for chloroquine or artesunate, two drugs that quickly destroyed the parasites (36, 38). For all other molecules (atovaquone, pyrimethamine, doxycycline, and T3/SAR97276), the results obtained at 48 h are not correct, and their reliability appear to vary according to the drug (Tables 3 and 4). This might be explained by the rate of parasite alteration after contact with the drug, particularly for T3/SAR97276 and doxycycline. Indeed, the parasites' viability and proliferation are rapidly affected by T3/SAR97276, but parasites only die at the schizont stage (Fig. 2 and 3). Doxycycline exerts its effect in the first parasite cycle, but the parasites die in the second cycle (Table 3) (11).
TABLE 4.
Mechanism of action and in vitro drug sensitivity assays
| Drug | Chemical family | Mode of action | Incubation period (h) | Drug sensitivity test reliability |
|||
|---|---|---|---|---|---|---|---|
| [3H]HXa | SYBR green | pLDH | HRP2 | ||||
| Chloroquine | 4-Aminoquinoleine | Interaction with heme | 48 | Yes | Yes | Yes | Yes |
| 72 | Yes | Yes | Yes | Yes | |||
| Artesunate | Sesquiterpene lactone | Oxidative stress | 48 | Yes | Yes | Yes | Yes |
| 72 | Yes | Yes | Yes | Yes | |||
| Atovaquone | Hydroxynaphtoquinone | Inhibition of orotate biosynthesis | 48 | Yes | Yes | No | Yes |
| 72 | Yes | Yes | Yes | No | |||
| Pyrimethamine | Antifolic compound | Inhibition of pyrimidines synthesis | 48 | Yes | No | No | Yes |
| Inhibition of DHFR | 72 | Yes | Yes | Yes | Yes | ||
| T3/SAR97276 | Bis-thiazolium | Inhibition of PC biosynthesis | 48 | Yes | No | No | Yes |
| 72 | Yes | Yes | No | No | |||
| Doxycycline | Antibiotic | Inhibition of protein synthesis of apicoplast | 48 | Yes | No | No | Yes |
| 72 | Yes | No | No | No | |||
| 96 | Yes | Yes | No | No | |||
HX, hypoxanthine.
Both ELISAs measure malaria-specific proteins, i.e., pLDH or HRP2. The HRP2 protein is known to have a long biological half-life, and its persistence may last few days in the bloodstream (19, 29). pLDH also persists in the bloodstream even although its half-life is shorter than that of HRP2 (19). The HRP2 and pLDH levels measured are thus the result of the temporal accumulation of production. The unreliable results obtained with these ELISA tests suggests that, despite the lethal effect exerted, antigen secretion and some maturations are not stopped rapidly, and measurement of the production of HRP2 or pLDH still being secreted by the parasites biased the test. To overcome this problem, it has been suggested that a background value measured at 24 h could be subtracted from the overall results to avoid taking antigen secreted by the dying parasite into account (30). For molecules that induce delayed death, the per-cycle production of HRP2 or pLDH would be calculated after deducing the amount of HRP2 (9) or pLDH in the supernatant. This would be time- and labor-consuming, requiring parallel additional plates with the drug being removed at 48 h.
For drugs that do not exert their activity through a delayed effect, similar results are observed when adding the precursors at 48, 72, or 96 h. This is the case of T3/SAR97276, artesunate, chloroquine, atovaquone, and pyrimethamine (see Tables 1 and 2). The IC50 is not decreased by incubating parasites for more than one cell cycle because the drug still exerts its pharmacological effect with the same affinity with the target, thus not altering the IC50 (or the IC50 remains at similar concentration).
Malaria is a major global health problem, and the emergence of (multi)resistance to existing drugs is an increasing problem. Drug testing through in vitro evaluation tests is crucial for malaria drug research. We showed here that evaluations of antimalarial activity based on the incorporation of radiolabeled precursors ([3H]hypoxanthine or [3H]ethanolamine) are the most reliable tests for malaria drug research. These tests have to be performed after a full parasite cycle (48 h) in order to detect the entire antimalarial potential of compounds regardless of the expression time of its pharmacological target during the blood cycle. Another reason for this 48-h prior incubation is that the effects on the parasite viability may not immediately affect the measured parameters. Moreover, these tests have to be modified for molecules that induce delayed death (e.g., antibiotic) over an increasing time, up to 96 h.
The SYBR green assay, which measures the DNA level (not its biosynthesis), requires 72 h of incubation to obtain reliable results with most of the compounds and 96 h for compounds that exert delayed death. On the other hand, both ELISAs used in the present study (pLDH and HRP2), which were supposed to measure parasite maturation, gave disappointing and even erroneous results for molecules that do not share an artesunatelike profile. Clearly, the reliability of these tests is linked to the mode of action of the drug and the conditions required to get informative results are hard to predict. The isotopic test thus appears to be the best assay, but the use of radioactivity in endemic zones and for high-throughput screening remains problematic. In these cases, the use of SYBR green determination seems to be the best option to adapt to the largest number/type of antimalarial compounds, using a 72-h (or 96-h) incubation period.
Acknowledgments
We thank F. Nosten's team for assistance in the pLDH experiments.
This study was supported by the European Commission (Antimal Integrated Project LSHP-CT-2005-018834) and the Région Languedoc Roussillon/OSEO/EuroBioMed project (InnoMad).
The authors have paid a fee to allow immediate free access to this article.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bis-quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon, D. J., C. Latour, C. Lucas, O. Colina, P. Ringwald, and S. Picot. 2007. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob. Agents Chemother. 51:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barends, M., A. Jaidee, N. Khaohirun, P. Singhasivanon, and F. Nosten. 2007. In vitro activity of ferroquine (SSR 97193) against Plasmodium falciparum isolates from the Thai-Burmese border. Malaria J. 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biagini, G. A., E. M. Pasini, R. Hughes, H. P. De Koning, H. J. Vial, P. M. O'Neill, S. A. Ward, and P. G. Bray. 2004. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood 104:3372-3377. [DOI] [PubMed] [Google Scholar]
- 7.Biagini, G. A., E. Richier, P. G. Bray, M. Calas, H. Vial, and S. A. Ward. 2003. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob. Agents Chemother. 47:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briolant, S., M. Baragatti, P. Parola, F. Simon, A. Tall, C. Sokhna, P. Hovette, M. M. Mamfoumbi, J. L. Koeck, J. Delmont, A. Spiegel, J. Castello, J. P. Gardair, J. F. Trape, M. Kombila, P. Minodier, T. Fusai, C. Rogier, and B. Pradines. 2009. Multinormal in vitro distribution model suitable for the distribution of Plasmodium falciparum chemosusceptibility to doxycycline. Antimicrob. Agents Chemother. 53:688-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkhardt, D., J. Wiesner, N. Stoesser, M. Ramharter, A. C. Uhlemann, S. Issifou, H. Jomaa, S. Krishna, P. G. Kremsner, and S. Borrmann. 2007. Delayed parasite elimination in human infections treated with clindamycin parallels “delayed death” of Plasmodium falciparum in vitro. Int. J. Parasitol. 37:777-785. [DOI] [PubMed] [Google Scholar]
- 10.Carlson, J., H. Helmby, A. V. Hill, D. Brewster, B. M. Greenwood, and M. Wahlgren. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457-1460. [DOI] [PubMed] [Google Scholar]
- 11.Dahl, E. L., and P. J. Rosenthal. 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob. Agents Chemother. 51:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divo, A. A., T. G. Geary, and J. B. Jensen. 1985. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob. Agents Chemother. 27:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Druilhe, P., A. Moreno, C. Blanc, P. H. Brasseur, and P. Jacquier. 2001. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 64:233-241. [DOI] [PubMed] [Google Scholar]
- 16.Elabbadi, N., M. L. Ancelin, and H. J. Vial. 1992. Use of radioactive ethanolamine incorporation into phospholipids to assess in vitro antimalarial activity by the semiautomated microdilution technique. Antimicrob. Agents Chemother. 36:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman, C. D., V. Su, and G. I. McFadden. 2007. The effects of antibacterials on the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 152:181-191. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood, B. M., D. A. Fidock, D. E. Kyle, S. H. Kappe, P. L. Alonso, F. H. Collins, and P. E. Duffy. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal, J., A. Siddique, M. Jameel, and P. R. Hira. 2004. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J. Clin. Microbiol. 42:4237-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, J. D., R. A. Dennull, L. Gerena, M. Lopez-Sanchez, N. E. Roncal, and N. C. Waters. 2007. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob. Agents Chemother. 51:1926-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaddouri, H., A. Djimde, S. Dama, A. Kodio, M. Tekete, V. Hubert, A. Kone, H. Maiga, O. Yattara, B. Fofana, B. Sidibe, C. P. Sangare, O. Doumbo, and J. Le Bras. 2008. Baseline in vitro efficacy of ACT component drugs on Plasmodium falciparum clinical isolates from Mali. Int. J. Parasitol. 38:791-798. [DOI] [PubMed] [Google Scholar]
- 22.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 23.Lang-Unnasch, N., and A. D. Murphy. 1998. Metabolic changes of the malaria parasite during the transition from the human to the mosquito host. Annu. Rev. Microbiol. 52:561-590. [DOI] [PubMed] [Google Scholar]
- 24.Le Roch, K. G., J. R. Johnson, H. Ahiboh, D. W. Chung, J. Prudhomme, D. Plouffe, K. Henson, Y. Zhou, W. Witola, J. R. Yates, C. B. Mamoun, E. A. Winzeler, and H. Vial. 2008. A systematic approach to understand the mechanism of action of the bisthiazolium compound T4 on the human malaria parasite, Plasmodium falciparum. BMC Genomics 9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makler, M. T., J. M. Ries, J. A. Williams, J. E. Bancroft, R. C. Piper, B. L. Gibbins, and D. J. Hinrichs. 1993. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 48:739-741. [DOI] [PubMed] [Google Scholar]
- 26.Noedl, H., J. Bronnert, K. Yingyuen, B. Attlmayr, H. Kollaritsch, and M. Fukuda. 2005. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob. Agents Chemother. 49:3575-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noedl, H., S. Krudsood, W. Leowattana, N. Tangpukdee, W. Thanachartwet, S. Looareesuwan, R. S. Miller, M. Fukuda, K. Jongsakul, K. Yingyuen, S. Sriwichai, C. Ohrt, and C. Knirsch. 2007. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob. Agents Chemother. 51:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noedl, H., D. Socheat, and W. Satimai. 2009. Artemisinin-resistant malaria in Asia. N. Engl. J. Med. 361:540-541. [DOI] [PubMed] [Google Scholar]
- 29.Noedl, H., C. Wongsrichanalai, R. S. Miller, K. S. Myint, S. Looareesuwan, Y. Sukthana, V. Wongchotigul, H. Kollaritsch, G. Wiedermann, and W. H. Wernsdorfer. 2002. Plasmodium falciparum: effect of anti-malarial drugs on the production and secretion characteristics of histidine-rich protein II. Exp. Parasitol. 102:157-163. [DOI] [PubMed] [Google Scholar]
- 30.Noedl, H., C. Wongsrichanalai, and W. H. Wernsdorfer. 2003. Malaria drug-sensitivity testing: new assays, new perspectives. Trends Parasitol. 19:175-181. [DOI] [PubMed] [Google Scholar]
- 31.Olliaro, P. L., R. K. Haynes, B. Meunier, and Y. Yuthavong. 2001. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17:122-126. [DOI] [PubMed] [Google Scholar]
- 32.Plouffe, D., A. Brinker, C. McNamara, K. Henson, N. Kato, K. Kuhen, A. Nagle, F. Adrian, J. T. Matzen, P. Anderson, T. G. Nam, N. S. Gray, A. Chatterjee, J. Janes, S. F. Yan, R. Trager, J. S. Caldwell, P. G. Schultz, Y. Zhou, and E. A. Winzeler. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. U. S. A. 105:9059-9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradines, B., A. Spiegel, C. Rogier, A. Tall, J. Mosnier, T. Fusai, J. F. Trape, and D. Parzy. 2000. Antibiotics for prophylaxis of Plasmodium falciparum infections: in vitro activity of doxycycline against Senegalese isolates. Am. J. Trop. Med. Hyg. 62:82-85. [DOI] [PubMed] [Google Scholar]
- 34.Rason, M. A., T. Randriantsoa, H. Andrianantenaina, A. Ratsimbasoa, and D. Menard. 2008. Performance and reliability of the SYBR green I-based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans. R. Soc. Trop. Med. Hyg. 102:346-351. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu, A. B., Q. Sun, L. J. Nkrumah, M. W. Dunne, J. C. Sacchettini, and D. A. Fidock. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 282:2494-2504. [DOI] [PubMed] [Google Scholar]
- 36.Skinner, T. S., L. S. Manning, W. A. Johnston, and T. M. Davis. 1996. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int. J. Parasitol. 26:519-525. [DOI] [PubMed] [Google Scholar]
- 37.Smilkstein, M., N. Sriwilaijaroen, J. X. Kelly, P. Wilairat, and M. Riscoe. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terkuile, F., N. J. White, P. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. experimental parasitology 76:85-95. [DOI] [PubMed]
- 39.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 40.Vial, H. J., and M. L. Ancelin. 1992. Malarial lipids: an overview. Subcell. Biochem. 18:259-306. [PubMed] [Google Scholar]
- 41.Vial, H. J., and C. Ben Mamoun. 2005. Plasmodium lipids: metabolism and function, p. 327-352. In I. W. Sherman (ed.), Molecular approaches to malaria. ASM Press, Washington, DC.
- 42.Vial, H. J., P. Eldin, A. G. Tielens, and J. J. van Hellemond. 2003. Phospholipids in parasitic protozoa. Mol. Biochem. Parasitol. 126:143-154. [DOI] [PubMed] [Google Scholar]
- 43.Vial, H. J., S. Wein, C. Farenc, C. Kocken, O. Nicolas, M. L. Ancelin, F. Bressolle, A. Thomas, and M. Calas. 2004. Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc. Natl. Acad. Sci. U. S. A. 101:15458-15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellems, T. E., and R. J. Howard. 1986. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 83:6065-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wengelnik, K., V. Vidal, M. L. Ancelin, A. M. Cathiard, J. L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. J. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. 2008. Roll Back Malaria: the global malaria action plan for a malaria-free world. World Health Organization, Geneva, Switzerland. http://www.rollbackmalaria.org/gmap/index.html.
- 47.World Health Organization. 2008. World malaria report 2008. World Health Organization, Geneva, Switzerland. http://www.who.int/malaria/wmr2008/.