Abstract
The SLOMYCO Sensititre panel and the custom JustOne strip (both from TREK Diagnostic Systems, Cleveland, OH) were evaluated for susceptibility testing of Mycobacterium avium complex isolates against clarithromycin. Seventy-one archived and prospectively collected isolates were tested using both the SLOMYCO panel and the JustOne strip, and the results were compared to those obtained using the BACTEC 460 (BD, Sparks, MD) radiometric method and a broth microdilution reference method. Results obtained by the SLOMYCO panel and the JustOne strip agreed with the BACTEC 460 method for 64/71 isolates (90%). Similarly, concordance with the broth microdilution method was 40/43 isolates (93%) for both test systems. The effect of the source medium on inoculum preparation was evaluated, and there were no differences noted in MICs, regardless of whether the inoculum was prepared from isolates grown in Middlebrook 7H9 medium, on Middlebrook 7H10 agar, or in VersaTREK broth culture bottles (Trek Diagnostics). Clarithromycin susceptibility testing of MAC using the SLOMYCO panel and the JustOne strip methods is easy to set up and simple to read and is readily incorporate into the clinical laboratory. These systems offer advantages over the BACTEC 460 system including the lack of a need for radioactive substrates, sharps, or costly instrumentation.
Nontuberculous mycobacteria (NTM) are increasingly recognized as a cause of pulmonary disease (1), and some of the most common NTM species isolated in the clinical laboratory are members of the Mycobacterium avium complex (MAC). In addition to pulmonary disease, MAC infection can result in lymphadenitis and disseminated disease in both immunocompromised and immunocompetent patients (9). The American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) recommend that a three-drug regimen be utilized for the treatment of MAC infection, including a macrolide (clarithromycin or azithromycin), ethambutol, and a rifamycin such as rifampin (4).
The ATS and the IDSA recommend performing susceptibility testing of MAC isolates only against the macrolides based on the results of the limited number of available, well-controlled clinical trials correlating in vitro susceptibility data and clinical outcomes (2, 3, 6). The ATS, the IDSA, and the Clinical and Laboratory Standards Institute (CLSI) all recommend that susceptibility testing of MAC be performed for the initial clinical isolate, for isolates from patients previously on macrolide therapy, for isolates from patients failing macrolide therapy, for isolates from AIDS patients who develop bacteremia while on macrolide prophylaxis, and for isolates from patients with positive blood cultures after 3 months of therapy for disseminated MAC infection (4, 5). The CLSI guideline also suggests that clarithromycin can serve as a class drug for azithromycin and that therefore only clarithromycin needs to be routinely tested. Although some experts disagree, testing of drugs other than clarithromycin is not recommended at this time since no correlation has been established between in vitro susceptibility testing and clinical response to agents other than the macrolides. The CLSI recommends the use of the radiometric BACTEC 460, the broth-based macrodilution, or the broth microdilution method for the testing of MAC susceptibility to clarithromycin (5).
Recently, two products, the SLOMYCO Sensititre panel (formerly called the MAISLOW Sensititre panel) and the custom JustOne clarithromycin strip, were introduced by TREK Diagnostic Systems (Cleveland, OH). Both products are labeled as research use only products. The SLOMYCO panel is a standard-order broth microdilution panel which can be used for the testing of slowly growing mycobacteria against amikacin, ciprofloxacin, clarithromycin, doxycycline, ethambutol, ethionamide, isoniazid, linezolid, moxifloxacin, rifabutin, rifampin, streptomycin, and trimethoprim-sulfamethoxazole. In this study, we evaluated the performance of the panel for predicting MAC resistance to only clarithromycin since this is the single drug recommended for in vitro testing, as discussed earlier. Similarly, we also evaluated the performance of the JustOne clarithromycin strip, a custom-manufactured broth microdilution strip containing only clarithromycin. Both the SLOMYCO panel and the JustOne strip contain antimicrobial agents lyophilized in microtiter plate wells with each well containing a different concentration of drug. Drug-free control wells are also provided for each plate or strip.
(This study was presented in part at the 108th General Meeting of the American Society for Microbiology in Boston, MA, 2008 [abstract C-202].)
MATERIALS AND METHODS
M. avium/intracellulare complex isolates.
Forty-six archived MAC isolates with known clarithromycin MICs, as determined previously by us using the radiometric BACTEC 460 method, were used in this study. Twenty-one of the 46 isolates were resistant to clarithromycin (MIC, ≥32 μg/ml). These isolates consisted of clinical, proficiency, and reference strains that were originally identified by 16S rRNA genes sequencing, MAC AccuProbe nucleic acid hybridization probe analysis (Gen-Probe, San Diego, CA), or PCR-restriction endonuclease analysis of the 65-kDa hsp gene (7, 8). The archived isolates were stored as frozen stocks at −70°C. In addition to the archived specimens, 25 consecutive, recent MAC clinical isolates were collected prospectively for this study. A random subset of 43 isolates (archived and recent) was also tested using a second comparison method, broth microdilution. An Institutional Review Board of the Mayo Clinic approved the use of all isolates.
Inoculum preparation.
Inocula for this study were prepared from three different medium sources (Middlebrook 7H9 medium, Middlebrook 7H10 agar, and VersaTREK broth) to determine if there was any effect of the source medium on test performance. Archived MAC isolates from frozen stocks were subcultured to a Middlebrook 7H9 broth bottle and a Middlebrook 7H10 agar plate, and the inoculum for the SLOMYCO panel and the JustOne strips was prepared according to CLSI M24-A and the manufacturer's package insert guidelines (5). Briefly, confluent growth from the 7H10 agar plate was swept with a loop and emulsified in sterile water and the concentration was adjusted to a 0.5 McFarland standard. Fifty microliters of the emulsified suspension from either the agar plate or the diluted broth was added to 10 ml of Mueller-Hinton broth with oleic acid-albumin-dextrose-catalase (TREK Diagnostics, Cleveland, OH). In addition, isolates were subcultured to VersaTREK MYCO broth bottles containing Middlebrook medium, growth supplement, and 0.5 ml of a 0.5 McFarland standard suspension of the MAC isolates. The inoculated VersaTREK bottles were placed on the VersaTREK 528 instrument, an FDA-approved platform for mycobacterial culture, and the cultures were incubated at 35°C until they signaled positive (usually 2.5 days). Susceptibility testing was performed within 3 days of the positive MYCO bottle signal. Inoculum from the VersaTREK bottles was prepared as described for the 7H9 broth.
Susceptibility testing.
The original MAISLOW panel contained clarithromycin concentrations ranging from 0.5 to 64 μg/ml, and this was the concentration range that we tested in our study (Fig. 1). Upon the introduction of the SLOMYCO name, the concentration range of clarithromycin was expanded to 0.06 to 64 μg/ml. The concentration range for clarithromycin tested in the JustOne strip was 0.12 to 128 μg/ml. The SLOMYCO panel and the JustOne Strip wells were inoculated with 100 μl of the Mueller-Hinton broth suspension described above and incubated in a non-CO2 incubator at 35 to 37°C until the growth control showed sufficient growth (7 to 14 days). The MICs were determined visually using an inverted mirror and read as the lowest concentration of clarithromycin showing 100% inhibition of growth. MICs obtained using the SLOMYCO panel and the JustOne strip were compared to results obtained using either the BACTEC 460 radiometric method or the broth microdilution reference method (3). Isolates with discordant results were retested with the same methods as used for the first MIC determination. Those isolates with repeatedly discordant results were classified as either very major errors (defined as an isolate resistant [R] by the reference method but susceptible [S] by the test method), major errors (S by the reference method and R by the test method), or minor errors (intermediate [I] by one method but S or R by the other method). Interpretative criteria were in accordance with CLSI guidelines, with an MIC of ≤4 μg/ml deemed S, an MIC of 8 to 16 μg/ml deemed I, and an MIC of ≥32 μg/ml deemed R for the BACTEC 460 method at pH 7.3 to 7.4. For the broth microdilution method, an MIC of ≤8 μg/ml is S, an MIC of 16 μg/ml is I, and an MIC of ≥32 μg/ml is R. The agreement between the various methods was expressed as percent concordance, and the strength of the agreement between methods was determined using kappa scores.
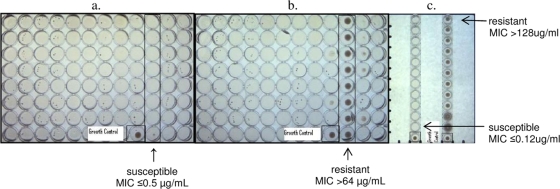
FIG. 1.
Photographs of the SLOMYCO panel and JustOne strip showing susceptible and resistant MAC isolates. Growth controls are labeled and shown in the single boxed well for each panel or strip. (a) SLOMYCO panel containing 0.5 to 64 μg/ml clarithromycin in doubling dilutions in the boxed row. The isolate is fully susceptible to clarithromycin, with an MIC of ≤0.5 μg/ml. (b) SLOMYCO panel containing 0.5 to 64 μg/ml clarithromycin in doubling dilutions in the boxed row. The isolate is fully resistant to clarithromycin, with an MIC of >64 μg/ml. (c) JustOne strips containing 0.125 to 128 μg/ml clarithromycin in doubling dilutions in each individual strip. The isolate on the left is fully susceptible to clarithromycin, with an MIC of ≤0.125 μg/ml. The isolate on the right is fully resistant to clarithromycin, with an MIC of >128 μg/ml.
Precision studies.
Reproducibility studies evaluating intraday (10 replicates in a single day) and interday (20 replicates over 10 days) precision were performed using M. avium quality control strains ATCC 700898 (clarithromycin susceptible) and ATCC 700897 (clarithromycin resistant). Four technologists performed the reproducibility studies to test interoperator variability.
RESULTS
Comparison of the SLOMYCO panel and JustOne strip MICs to the radiometric BACTEC 460 method.
The overall agreement between the SLOMYCO panel and the BACTEC 460 method was 90% (64/71 isolates), with a kappa score of 0.79 indicating good agreement (Table 1). The very major error rate was 4.8% (1/21 isolates), and the major error rate was 0%. The most numerous discordant results were observed primarily in the I category, where 5/71 isolates (7%) that were I by the BACTEC 460 method were called S by the SLOMYCO panel and 1/71 isolates (1%) was R by the BACTEC 460 method and I by the SLOMYCO panel. Identical results were obtained regardless of the culture medium used for inoculum preparation (Middlebrook 7H9 broth, Middlebrook 7H10 agar, or VersaTREK broth). The agreement between the JustOne strip and the BACTEC 460 method was identical to that found for the SLOMYCO panel.
TABLE 1.
Comparison of clarithromycin susceptibility results from the SLOMYCO panel and the JustOne strip with those obtained by the BACTEC-460 method
| Test and category | No. of isolates found by BACTEC 460 (pH 7.3-7.4) to be: |
% Agreement (kappa) | ||
|---|---|---|---|---|
| Susceptible, ≤4.0 μg/ml | Intermediate, 8-16 μg/ml | Resistant, ≥32 μg/ml | ||
| SLOMYCO panel | ||||
| Susceptible, ≤8 μg/ml | 45 | 5 | 1 | 90 (0.79) |
| Intermediate, 16 μg/ml | 0 | 0 | 1 | |
| Resistant, ≥32 μg/ml | 0 | 0 | 19 | |
| JustOne strip | ||||
| Susceptible, ≤8 μg/ml | 45 | 5 | 1 | 90 (0.79) |
| Intermediate, 16 μg/ml | 0 | 0 | 1 | |
| Resistant, ≥32 μg/ml | 0 | 0 | 19 | |
Comparison of the SLOMYCO panel and the JustOne strip and to the broth microdilution method.
The overall agreement between the SLOMYCO panel and JustOne strip results with the broth microdilution method results was 93% (40/43 isolates), with a kappa score of 0.87 indicating very good agreement (Table 2). A single isolate was called R by the broth microdilution reference method and S by both the SLOMYCO panel and the JustOne strip, giving a very major error rate of 5%. The major error rate was 0%. As with the BACTEC 460 comparison, discordance occurred most often in the intermediate category, with two isolates (5%) deemed I by the broth microdilution reference method and S by the SLOMYCO panel and the JustOne strip. Again, the culture medium used for inoculum preparation (7H9 broth, 7H10 agar, or VersaTREK broth) had no effect on the results.
TABLE 2.
Comparison of clarithromycin susceptibility results from the SLOMYCO panel and the JustOne strip with those obtained by the broth microdilution method
| Test and category | No. of isolates found by broth microdilution to be: |
% Agreement (kappa) | ||
|---|---|---|---|---|
| Susceptible, ≤8.0 μg/ml | Intermediate, 16 μg/ml | Resistant, ≥32 μg/ml | ||
| SLOMYCO panel | ||||
| Susceptible, ≤8 μg/ml | 20 | 2 | 1 | 93 (0.87) |
| Intermediate, 16 μg/ml | 0 | 0 | 0 | |
| Resistant, ≥32 μg/ml | 0 | 0 | 20 | |
| JustOne strip | ||||
| Susceptible, ≤8 μg/ml | 20 | 2 | 1 | 93 (0.87) |
| Intermediate, 16 μg/ml | 0 | 0 | 0 | |
| Resistant, ≥32 μg/ml | 0 | 0 | 20 | |
Precision.
Reproducibility studies evaluating intraday (10 replicates in a single day) and interday (two replicates for 10 days) precision using M. avium quality control strains ATCC 700898 (clarithromycin susceptible) and ATCC 700897 (clarithromycin resistant) had a 100% correlation with the expected results of both the JustOne strip and the SLOMYCO panel. No interoperator variability was noted.
DISCUSSION
Susceptibility testing of MAC isolates against clarithromycin is recommended for initial isolates from significant sources or when therapy appears to be failing (4). Methods currently recommended for MIC determination include the radiometric BACTEC 460 and broth microdilution methods (4, 5). In this study, we evaluated the performance of the SLOMYCO microtiter panel and a custom JustOne microtiter strip for susceptibility testing of MAC isolates against clarithromycin. The concordance between the SLOMYCO panel or the JustOne strip and the BACTEC 460 method was 90%, with the kappa score indicating good agreement between the methods. One isolate was deemed a very major error for both the panel and the strip compared to the radiometric method. Interestingly, this isolate was found to be susceptible by the broth microdilution reference method. The percent agreement between the panel or the strip and the broth microdilution reference method was 93%, and the kappa score indicated very good agreement between the methods, with a single very major error detected (1/20 resistant isolates). A limitation of our study is that clarithromycin-resistant MAC isolates are rare and therefore we were only able to obtain a total of 21 resistant isolates.
The radiometric BACTEC 460 method is a well-established assay for clarithromycin susceptibility testing of MAC isolates. However, the assay is laborious and uses a radioactive substrate. The JustOne strip and the SLOMYCO panel are both broth microdilution methods, and they demonstrate ≥90% correlation with both the radiometric method and a broth microdilution reference method. The SLOMYCO panel and the JustOne strip have the added advantage of being commercially available and thus standardized, and they are easy to set up and interpret. There were no significant medium-dependent effects when the isolates were set up from different source media (7H9 broth, 7H10 agar, and VersaTREK broth). Susceptibility testing can therefore be performed without additional subcultures if the source culture is less than 5 weeks old and from 7H9 broth or 7H10 agar or ≤3 days after signaling positive in the VersaTREK MYCO bottle. Finally, the susceptibility results obtained using the SLOMYCO panel or the JustOne strip are often available within approximately 7 days, which can have a positive impact on patient care since this is shorter than the typical turnaround time for the BACTEC 460 method. Therefore, the JustOne strip and SLOMYCO panels are alternatives to the radiometric method for clarithromycin susceptibility testing of MAC isolates.
Acknowledgments
This study was funded by the Mayo Clinic and the University of Texas Health Sciences Center at Tyler, TX.
No financial or other form of support was received from TREK Diagnostics, the manufacturer of the SLOMYCO plate and the JustOne Strip.
Footnotes
Published ahead of print on 24 March 2010.
REFERENCES
- 1.Arend, S. M., D. van Soolingen, and T. H. Ottenhoff. 2009. Diagnosis and treatment of lung infection with nontuberculous mycobacteria. Curr. Opin. Pulm. Med. 15:201-208. [DOI] [PubMed] [Google Scholar]
- 2.Chaisson, R. E., C. A. Benson, M. P. Dube, L. B. Heifets, J. A. Korvick, S. Elkin, T. Smith, J. C. Craft, and F. R. Sattler. 1994. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. Ann. Intern. Med. 121:905-911. [DOI] [PubMed] [Google Scholar]
- 3.Dautzenberg, B., C. Truffot, S. Legris, M. C. Meyohas, H. C. Berlie, A. Mercat, S. Chevret, and J. Grosset. 1991. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am. Rev. Respir. Dis. 144:564-569. [DOI] [PubMed] [Google Scholar]
- 4.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzaro, C. Daley, F. Gordin, S. M. Holland, R. Horsburgh, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Ruoss, C. F. von Reyn, R. J. Wallace, Jr., and K. Winthrop. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367-416. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. NCCLS document M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 6.Ruf, B., D. Schurmann, H. Mauch, G. Jautzke, F. J. Fehrenbach, and H. D. Pohle. 1992. Effectiveness of the macrolide clarithromycin in the treatment of Mycobacterium avium complex infection in HIV-infected patients. Infection 20:267-272. [DOI] [PubMed] [Google Scholar]
- 7.Steingrube, V. A., J. L. Gibson, B. A. Brown, Y. Zhang, R. W. Wilson, M. Rajagopalan, and R. J. Wallace, Jr. 1995. PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J. Clin. Microbiol. 33:149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waller, E. A., A. Roy, L. Brumble, A. Khoor, M. M. Johnson, and J. L. Garland. 2006. The expanding spectrum of Mycobacterium avium complex-associated pulmonary disease. Chest 130:1234-1241. [DOI] [PubMed] [Google Scholar]