Abstract
In a recent report, ATP, which was possibly secreted by some intestinal bacteria, was shown to cause colitis in mice via Th17 cell differentiation. However, the ATP-secreting bacteria have not been isolated and identified. In the present study, we report that Enterococcus gallinarum, which is a vancomycin-resistant Gram-positive coccus isolated from mice and humans, secretes ATP.
ATP has been shown to modulate immune cell functions by activating the ATP-sensing P2X and P2Y receptors (6, 7, 10, 12). Furthermore, Atarashi et al. have reported that ATP regulates Th17 cell differentiation in the lamina propria and is involved in colitis-related Th17 cell differentiation in mice (1), thereby suggesting that ATP causes colitis in mice via Th17 cell differentiation. Although their report suggested that some intestinal bacteria play an important role in the high concentrations of ATP in the intestine, the ATP-secreting bacteria have not been isolated and identified. In the present study, we report the isolation and identification of ATP-secreting bacteria from murine and human specimens.
In the present study, the following bacterial strains isolated from human feces, urine, and skin were used as the reference strains: 8 Enterococcus strains (3 Enterococcus faecalis and 3 Enterococcus faecium strains, which are the major enterococci found in humans [9], and 2 Enterococcus gallinarum strains [2, 4, 11, 13]), 3 Escherichia coli strains, and 3 Staphylococcus aureus strains, which are the common Gram-negative and Gram-positive bacteria found in humans. Three fecal samples (weighing 12.2 mg, 9.4 mg, and 9.1 mg, respectively; total weight, 30.7 mg) obtained from specific-pathogen-free (SPF) mice (BALB/c; Charles River Japan, Tokyo, Japan) were resolved in 1 ml of physiological saline. The sample solutions were appropriately diluted (100 to 200 CFU on each agar plate), spread on tryptic soy agar (TSA; Difco, MD), and incubated under aerobic conditions at 37°C for 16 to 72 h. After incubation, the obtained colonies were isolated by streak culture. The isolated bacteria were identified by Gram staining, biochemical tests, 16S rRNA sequence analysis, and PCR analysis.
Gram staining was used to identify the potential candidates. Hemolytic reactions were assayed using sheep or rabbit blood agar. Glucose fermentation, gas production, and catalase reaction were examined to assess the biological characteristics. The 16S rRNA gene was amplified using universal PCR primers (forward, GTT TGA TCC TGG CTC A; reverse, TAC CAG GGT ATC TAA TCC). The 16S rRNA sequences were determined using a capillary sequencer (ABI Prism 3100; Applied Biosystems, CA) after performing reactions with the BigDye Terminator version 3.0 cycle sequencing kit (ABI). The obtained DNA sequences were compared to those in the database using the basic local alignment search tool (BLAST). The PCR analysis for Enterococcus gallinarum was performed using the vanC-1 gene, which is a vancomycin-resistant gene in Enterococcus gallinarum (3, 8). The vanC-1 gene was amplified using the relevant PCR primers (forward, GAA AGA CAA CAG GAA GAC CGC; reverse, ATC GCA TCA CAA GCA CCA ATC) (3).
The isolates were cultured in serum-free RPMI medium (Gibco, CA) and incubated at 37°C for 16 h under aerobic conditions with shaking (1). The culture media were centrifuged (15,000 × g), and the supernatants were collected. Then, to remove the bacterial cells, the culture supernatants were filtered using a 0.22-μm membrane (Millipore Japan, Tokyo, Japan). The extracellular ATP levels in the culture supernatants were measured directly by using the ATP assay kit (Promega KK, Tokyo, Japan), in which the luciferase reaction with ATP generates bioluminescence. We measured the extracellular ATP concentration after 16 h and determined the number of CFU/ml. This bioluminescence was detected (500 ms) using a luminometer (Luminoskan Ascent; Thermo Fisher Scientific KK, Tokyo, Japan) after mixing 100 μl of the culture supernatant with 100 μl of the reagent for the ATP assay in each well of white opaque 96-well microtiter plates (Nunc Japan, Tokyo, Japan). The standard curve for ATP (Sigma-Aldrich Japan, Tokyo, Japan) concentrations was plotted using the values obtained for the following 5 concentrations of ATP in the RPMI medium: 0 M, 100 nM, 1 μM, 10 μM, and 100 μM. RPMI medium without bacterial culture was used as the negative control. The procedure was performed according to the manufacturer's protocols. Light measurements for each sample were obtained in triplicate.
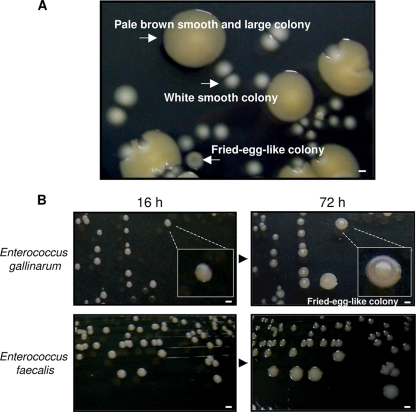
The extracellular ATP was detected in the supernatant of in vitro-cultured intestinal commensal bacteria derived from feces of SPF mice in our study and in a past report (1). To isolate the ATP-secreting bacteria, we first performed colony isolations. We obtained approximately 100 to 200 colonies per plate when 0.1-μl aliquots of 1-ml fecal solutions were spread on the TSA plates and incubated under aerobic conditions for 16 h at 37°C. We obtained the following two types of colonies: white, smooth colonies (approximately 85%; major population) and pale brown, smooth, and large colonies (approximately 15%; minor population).
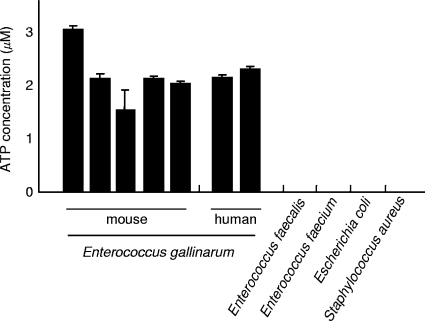
Among these, we randomly chose 96 colonies (86 white, smooth colonies and 10 pale brown, smooth, and large colonies) from one plate and measured the extracellular ATP secretion of these isolates. However, no ATP-secreting bacteria were isolated from among these colonies. Then, we assumed the presence of other ATP-secreting bacteria on the plate, and to distinguish the colony morphology of these bacteria, we further cultured the colonies at 37°C for up to 72 h. After the culturing period, we observed a third type of colony on the plate, as follows: 1 in every 100 to 300 white, smooth colonies changed to a white fried-egg-like colony (Fig. 1). Although the white fried-egg-like colonies could not be easily detected on the plate because of their very small numbers, we finally isolated 5 colonies from 10 plates. The ATP assays revealed that all 5 strains that formed white fried-egg-like colonies showed extracellular ATP secretion (Fig. 2) and that the strains that formed the other types of colonies did not secrete ATP. The results obtained were not affected by the number of CFU/ml; bacteria can be either the ATP-secreting type or the nonsecreting type. The concentration of extracellular ATP in the bacterial culture supernatant increased with the bacterial growth.
FIG. 1.
Culture images. (A) Culture image of the bacteria isolated from the fecal samples obtained from SPF mice. The feces were resolved in 1 ml of physiological saline, and the sample solutions (0.1 μl) were spread on TSA plates and incubated under aerobic conditions at 37°C for 72 h. Under our study conditions, the microbial flora of the SPF mice comprised approximately 3 bacterial species, as follows: the organism that formed white, smooth colonies, which was the major population, was Enterococcus faecalis; the organism that formed pale brown, smooth, and large colonies, which was the second major population, was Escherichia coli; and the organism that formed white fried-egg-like colonies, which was the minor population, was Enterococcus gallinarum. Scale bars indicate 1 mm. (B) Culture images of Enterococcus gallinarum and Enterococcus faecalis at 16 h and 72 h of culture. The Enterococcus gallinarum colonies changed from smooth to fried-egg-like colonies after 72 h of incubation. Scale bars indicate 1 mm.
FIG. 2.
ATP concentrations in the culture supernatants of isolates. After 16 h of culture, the ATP concentrations of each filtered culture supernatant were measured using a luciferase reaction method. Five and two strains of Enterococcus gallinarum isolated from mice and humans, respectively, were examined. Three Escherichia coli strains, three Staphylococcus aureus strains, three Enterococcus faecalis strains, and three Enterococcus faecium strains were also examined, and the mean value(s) obtained with each species is shown. Light measurements for each sample were obtained in triplicate. The error bars indicate standard deviations. Enterococcus gallinarum isolated from mice and humans showed extracellular ATP secretion. To compare the results of each group (ATP-secreting type bacteria and non-ATP-secreting type bacteria), the t test was used (P < 0.0001).
Subsequently, to identify the 5 ATP-secreting strains, we performed Gram staining and biochemical assays. These bacteria were Gram-positive cocci (free cells, diplococci, or chain-like colonies). They exhibited gamma-hemolysis on sheep blood agar. They fermented glucose without gas production and did not produce a catalase reaction with hydrogen peroxide. These results suggested that the ATP-secreting bacteria were Enterococcus spp. Further, to identify the species of the bacteria, we analyzed the 16S rRNA sequences. The 16S rRNA analysis revealed that the ATP-secreting bacteria were Enterococcus gallinarum, which is a vancomycin-resistant Gram-positive coccus (2, 4, 11, 13). This finding was further confirmed by PCR for vanC-1 (3, 8), which is a vancomycin-resistant gene in Enterococcus gallinarum. We also identified the other bacteria that did not secrete ATP, as follows: the major organism (white, smooth colonies) and the second major organism (pale brown, smooth, and large colonies) recovered from murine feces under our study conditions were Enterococcus faecalis and Escherichia coli, respectively (Fig. 1).
We also investigated whether the bacteria isolated from human specimens secrete ATP. This investigation revealed that Enterococcus gallinarum isolated from humans also showed extracellular ATP secretion (Fig. 2). In contrast, Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium did not secrete ATP (Fig. 2).
In humans, assessment of the relationships between Enterococcus gallinarum and colitis will facilitate the understanding of various aspects of human colitis (5), including the pathology, development of treatment avenues, prophylaxis, and prognosis. Since ATP is essential for organisms, extracellular ATP secretion may indicate unknown symbiotic relationships with some commensal or pathogen in the microbial flora of the gut.
Acknowledgments
We thank S. Matsufuji for his support.
Part of the study was supported by The Jikei University Research Fund.
Footnotes
Published ahead of print on 19 March 2010.
REFERENCES
- 1.Atarashi, K., J. Nishimura, T. Shima, Y. Umesaki, M. Yamamoto, M. Onoue, H. Yagita, N. Ishii, R. Evans, K. Honda, and K. Takeda. 2008. ATP drives lamina propria Th17 cell differentiation. Nature 455:808-812. [DOI] [PubMed] [Google Scholar]
- 2.Choi, S. H., S. O. Lee, T. H. Kim, J. W. Chung, E. J. Choo, Y. G. Kwak, M. N. Kim, Y. S. Kim, J. H. Woo, J. Ryu, and N. J. Kim. 2004. Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin. Infect. Dis. 38:53-61. [DOI] [PubMed] [Google Scholar]
- 3.Clark, N. C., L. M. Teixeira, R. R. Facklam, and F. C. Tenover. 1998. Detection and differentiation of vanC-1, vanC-2, and vanC-3 glycopeptide resistance genes in enterococci. J. Clin. Microbiol. 36:2294-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, M. P., F. Lessa, B. Brems, R. Shoulson, S. York, A. Peterson, J. Noble-Wang, R. Duffy, and L. C. McDonald. 2008. Outbreak of Enterococcus gallinarum infections after total knee arthroplasty. Infect. Control Hosp. Epidemiol. 29:361-363. [DOI] [PubMed] [Google Scholar]
- 5.Denning, T. L., and S. V. Sitaraman. 2009. Adenosine triphosphate energizes intestinal Th17 cells. Gastroenterology 136:1107-1109. [DOI] [PubMed] [Google Scholar]
- 6.Idzko, M., H. Hammad, M. van Nimwegen, M. Kool, M. A. Willart, F. Muskens, H. C. Hoogsteden, W. Luttmann, D. Ferrari, F. Di Virgilio, J. C. Virchow, Jr., and B. N. Lambrecht. 2007. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 13:913-919. [DOI] [PubMed] [Google Scholar]
- 7.Khakh, B. S., and R. A. North. 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527-532. [DOI] [PubMed] [Google Scholar]
- 8.Leclercq, R., S. Dutka-Malen, J. Duval, and P. Courvalin. 1992. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob. Agents Chemother. 36:2005-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moellering, R. C., Jr. 1992. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 14:1173-1176. [DOI] [PubMed] [Google Scholar]
- 10.North, R. A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82:1013-1067. [DOI] [PubMed] [Google Scholar]
- 11.Reid, K. C., I. F. Cockerill, and R. Patel. 2001. Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteremia: a report of 20 cases. Clin. Infect. Dis. 32:1540-1546. [DOI] [PubMed] [Google Scholar]
- 12.Schnurr, M., T. Toy, A. Shin, M. Wagner, J. Cebon, and E. Maraskovsky. 2005. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 105:1582-1589. [DOI] [PubMed] [Google Scholar]
- 13.Vincent, S., P. Minkler, B. Bincziewski, L. Etter, and D. M. Shlaes. 1992. Vancomycin resistance in Enterococcus gallinarum. Antimicrob. Agents Chemother. 36:1392-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]