Abstract
Healthy carriers of Staphylococcus aureus strains have an important role in the dissemination of this bacterium. To investigate the presence of S. aureus in the throat and anterior nares, samples from 1,243 healthy volunteers in a Mexican community were examined. The percentage of healthy carriers was 59.8%. Results showed that colonization of the throat occurred more frequently than that of the nares (46.5% versus 37.1%, P < 0.0001). Of the S. aureus carriers, 22.2% were exclusive nasal carriers and 38% were exclusive throat carriers. A total of 1,039 strains were isolated; 12.6% were shown to be methicillin-resistant S. aureus (MRSA). Of MRSA strains, 32.1% were isolated from exclusive throat carriers. Most of the strains isolated from the anterior nares and throat of the same carriers were the same or related; however, some were different. Pulsed-field gel electrophoresis (PFGE) pattern analysis of the MRSA strains isolated from the exclusive nasal carriers or exclusive throat carriers showed that they belong to different clusters. A 6-year prospective study was performed to investigate the persistence of S. aureus in the throat. Results showed that 13% of subjects were persistent carriers. Most of them were colonized with the same clone of S. aureus throughout the time of the study, and just three had different clones. Antimicrobial susceptibility testing showed that 91.1% of the strains were penicillin resistant. The presence of mecA and nucA genes (in order to confirm methicillin resistance) and of thermostable nuclease of S. aureus was examined. This study showed that some strains of S. aureus regularly colonized the throats of healthy people and could persist for years.
Staphylococcus aureus remains one of the most frequently occurring community-acquired as well as hospital-acquired pathogens, with high rates of hospital-acquired infections (11).
Recently, the epidemiology of S. aureus, in particular methicillin-resistant S. aureus (MRSA), has changed with the emergence of community-acquired MRSA (CA-MRSA) (6, 10). Reports from different parts of the world indicate that CA-MRSA has emerged as a new pathogen (3, 26, 32).
S. aureus is a transient or persistent part of the resident flora in the anterior nares of the population (15). The anterior nares are considered to be the primary colonization site, and approximately 30% of healthy people carry this bacterium (15).
The human throat is less well studied than the nares as a carriage site; nevertheless, some isolations have been reported (19, 22, 25). Colonization of the throat but not of the nares may be more common than is currently acknowledged. Recent studies confirmed the observation that the throat may be selectively colonized and escape current routine screening programs (1, 16, 18, 27, 33). Unrecognized carriers may spread MRSA and render infection control programs futile (18, 19, 27).
Three main carriage patterns have been described when individuals are repeatedly sampled in the anterior nares for S. aureus over longer periods of time: the so-called noncarriers, who were never reported to carry the organism; the persistent carriers, who repeatedly cultured positive; and the occasional or intermittent carriers, who from time to time yielded positive cultures (31). The persistence of single S. aureus clones in some of the carriers confirms previous reports on the exchange of S. aureus strains over time in nasal carriers (5, 13, 24). It is unclear, therefore, whether throat carriers are a subgroup of the population who can maintain S. aureus in this anatomical site or whether the microorganisms are temporary residents.
The aim of this work was to determine the frequency and persistence of carriage of S. aureus, especially the MRSA strains, in the throat in relation to anterior nares in a Mexican open population and also to determine the clonal relationships of nasal and throat strains by pulsed-field gel electrophoresis (PFGE) analysis as well as the persistence of carriage over the years, which has not been documented.
MATERIALS AND METHODS
Population study.
A prospective observational study from 1998 to 2006 was carried out. Volunteers were selected from locations where we could follow them for a long period of time, such as schools or factories; we explained the reason for the study, and we asked them for consent. Volunteers were enrolled at equal time intervals over the 6-year period. All volunteers were not recently hospitalized and claimed not to have chronic diseases. After providing informed consent, 1,243 volunteers who were not aware of any ailment at the time of the sampling were screened for nasal and throat carriage. If isolates of S. aureus were from only one of these two parts of the body, we defined the carriage as exclusive nasal or exclusive throat carriage. Age ranges were from 1 to 96 years, with a mean age of 21 years. Fifty-four percent were females, and 46% were males. The entire population was divided into six groups: the first five each included a 10-year age range (1 to 50 years total) and the last one consisted of individuals over 50 years old. Among all volunteers, a group of 108 were annually sampled for throat strains for up to 6 years (1998 to 2003). We defined persistent carriage as carriage by the volunteer of the S. aureus strains for all 6 years of the study and defined intermittent carriage as evidence of the bacteria on at least two occasions but not consequently during the 6 years in which we sampled the volunteer.
Screening for nasal and throat carriage.
Specimens were obtained with sterile cotton swabs moistened with sterile saline; samples were taken at the same time from the anterior nares and from the posterior wall of the pharynx—first the throat and then (using a different swab) the nares, always in that order.
Soy Trypticase broth (Bioxon) was inoculated with the swab samples; after overnight incubation at 35 to 37°C, the broth was subcultured onto 5% blood agar plates and phenol red mannitol salt agar (Oxoid) and incubated at 35 to 37°C for 24 h. Mannitol fermentation-positive isolates were further analyzed. Hemolysis was scored as positive if a clear zone of beta-hemolysis was observed on blood agar.
Biochemical identification.
Using standard microbial protocols, five colonies from pure cultures were subjected to Gram staining reaction, catalase test, and tube coagulase test. The biochemical properties were determined using the API Staph system (bioMérieux, France). S. aureus ATCC 29213 and Staphylococcus epidermidis ATCC 14990 were used as positive and negative controls, respectively.
Antimicrobial susceptibility testing.
All isolates were screened for resistance to different antibiotics: penicillin (10 U), erythromycin (15 μg), gentamicin (10 μg), cephalothin (30 μg), trimethoprim-sulfamethoxazole (25 μg), fosfomycin (50 μg), ciprofloxacin (5 μg), tetracycline (30 μg), clindamycin (2 μg), oxacillin (1 μg), and vancomycin (30 μg) (BD BBL Sensi-Disc; Becton Dickinson and Company, Sparks, MD). Susceptibility was determined by the disk diffusion method as recommended by the CLSI publication M2-A9 (7), and for oxacillin, MIC was determined according to the CLSI publication M7-A7 (8); a homemade broth microdilution tray was used, and oxacillin was supplied by Sigma. S. aureus ATCC 43300 was the positive control, and S. aureus ATCC 29213 was the negative control.
PFGE typing.
Methicillin-sensitive S. aureus (MSSA) and MRSA isolates were typed by PFGE. Preparation of DNA and resolution of the SmaI-digested fragments were performed as described by Mulvey et al. (21). Samples were run on a CHEF-DR II system (Bio-Rad). Gels were photographed and digitized using a Bio-Rad Gel Doc. The PFGE patterns were compared following the criteria of Tenover et al. (28) for bacterial strain typing.
Data analysis.
PFGE patterns were analyzed with Gene Tool software and Gene Directory software (Syngene, United Kingdom). The reference standard S. aureus NCTC 8325 was included in each gel for band normalization. Percent similarities were obtained from the unweighted pair group with mathematical average (UPMGA) based on Dice coefficients. Band position tolerance was set at 1.25%. A similarity coefficient of 80% was selected to define the pulsed-field type clusters (17).
Detection of mecA and nucA genes by PCR amplification.
Genomic DNA from staphylococcal cultures was extracted with the Wizard genomic DNA purification kit (Promega, Madison, WI), following the supplier's instructions. PCR assays were performed for mecA and nucA genes using a PCR kit (Qiagen); primers and conditions for mecA were as reported by Oliveira and de Lencastre (23), and those for nucA were as reported by Brakstad et al. (2). Using a T Personal Thermocycler (Biometra), PCR products were analyzed using a 1% ethidium bromide-stained agarose gel.
Statistical analysis.
Results were analyzed using statistical software JMP version 7 (SAS Institute Inc.), using the chi-square independent test, likelihood ratio, for P < 0.05.
RESULTS
S. aureus carriers.
Of the 1,243 volunteers analyzed, the percentage of healthy S. aureus carriers considering the nares, the throat, and both sites was 59.8% (743 volunteers). S. aureus was found mainly colonizing the throat (46.5%), while the anterior nares colonization was 37.1% (P < 0.001). Of the 743 S. aureus carriers, 296 (39.8%) were nasal and throat carriers, 165 (22.2%) were exclusive nasal carriers, and 282 (38%) were exclusive throat carriers (Table 1). The presence of S. aureus in the nares was greater in male carriers (40.9%) than in female carriers (33.9%; P = 0.011), and it was greater in the age group 1 to 10 years (45.9%) than in the other age groups (31 to 37%; P = 0.0077). The same analysis was made for the throat carriers, and percentages of 50.82% for ages 1 to 10 years and 50.39% for ages 11 to 20 years were obtained. The other age groups had percentages between 33 and 43% (P = 0.0077).
TABLE 1.
Percentages of Staphylococcus aureus carriers
| Type of carrier | No. of volunteers (n = 1,243) | Rate (%) of positivity among: |
|
|---|---|---|---|
| Total volunteers | Carriers | ||
| Staphylococcus aureus | 743 | 59.8 | 100 |
| Nasal | 461 | 37.1 | 62 |
| Throat | 578 | 46.5 | 77.8 |
| Nasal and throat | 296 | 23.8 | 39.8 |
| Exclusive nasal | 165 | 13.3 | 22.2 |
| Exclusive throat | 282 | 22.7 | 38 |
| MRSA | 107 | 8.6 | 14.4 |
Individuals with nasal carriage are more likely to have throat carriage also (odds ratio [OR] = 3.09, P < 0.0001). This association increases when individuals are under 19 years old (OR = 1.3, P = 0.029).
Antimicrobial susceptibility.
S. aureus isolated strains were penicillin (91.1%), erythromycin (23.1%), tetracycline (15.5%), cephalothin (7.1%), and clindamycin (6.2%) resistant. With other antimicrobial agents tested (ciprofloxacin, fosfomycin, trimethoprim-sulfamethoxazole, and gentamicin), resistance was ≤2%. No vancomycin resistance was found.
MRSA carriers.
Of 743 S. aureus carriers, 107 (14.4%) were MRSA carriers. Percent carriage of MRSA strains was similar between males and females, 52.3% and 47.7%, respectively. When analyzed by age group, 33.6% was found in the 1- to 10-year-old group, followed by the 11- to 20-year-old group (25.2%), and for the other groups, the percentage decreased as the age increased.
A total of 1,039 S. aureus strains were isolated from the studied population; 908 (87.4%) were MSSA, and 131 (12.6%) were MRSA. All MRSA isolates were positive for mecA and nucA genes as tested by PCR. Of the MRSA isolates 31.3% were isolated from exclusive nasal carriers, 32.1% were isolated from exclusive throat carriers, and 36.6% were isolated from the nares and the throat of the same carrier.
PFGE analysis.
Using PFGE pattern analysis, results showed that when 16 pairs of MSSA strains isolated from the nares and the throat of the same carriers were compared, 13 (81%) were the same strain or clonally related and only three pairs were different or not related (19%). These results show that most of the strains isolated from the nares are the same carried in the throat or clonally related; however, some carriers have different strains colonizing the throat and the nares at the same time.
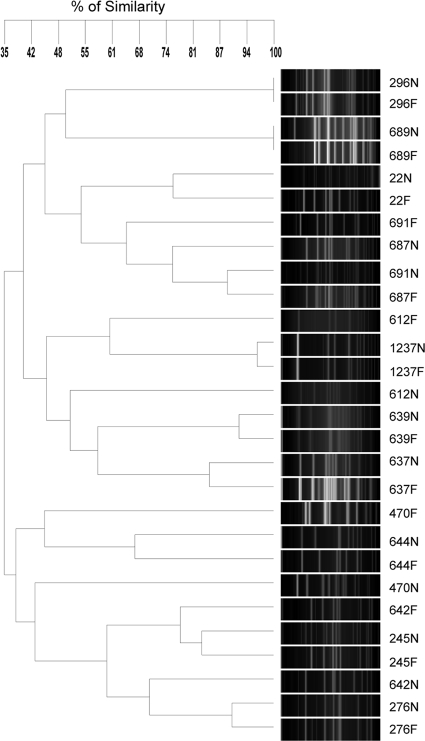
A dendrogram of PFGE patterns (Fig. 1) showed 14 MRSA pairs of strains isolated from both nares and throat of the same carrier, and it was found that 9 pairs were the same strain or related and only 5 pairs showed different band patterns and were not related, showing that MRSA strains could be different when they are isolated from different niches of the same carrier (volunteers 470, 612, 642, 687, and 691).
FIG. 1.
SmaI-PFGE dendrogram comparing pairs of MRSA strains isolated from the nares and the throat. Fourteen carriers were analyzed for S. aureus strains in the nares (N) and the throat (F). Most N and F pairs are carrying the same or related S. aureus strains.
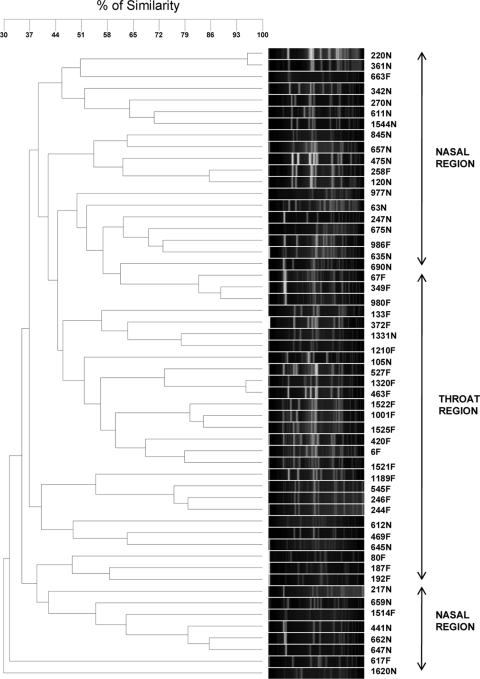
When 54 MRSA strains isolated from 28 exclusive throat carriers or 26 exclusive nares carriers were analyzed, results from the PFGE dendrogram showed three regions (Fig. 2): one in which most of the strains were isolated from the nares, a second in which most of the strains were from the throat, and a smaller, third one where nares strains predominated. These results may indicate that some special features make these MRSA strains prefer to colonize either the nares or the throat.
FIG. 2.
SmaI-PFGE dendrogram comparing MRSA strains of exclusive nasal (N) or exclusive throat (F) carriers. Two major regions are observed with mainly throat or nasal strains.
Persistence of S. aureus in the throat.
To determine the persistence of throat carriage, a group of 108 volunteers were screened for 6 years (1998 to 2003); 14 (13%) never carried S. aureus, 80 (74%) were intermittent carriers, and 14 (13%) were persistent carriers.
When S. aureus strains isolated from 12 persistent throat carriers were analyzed using PFGE, nine carriers showed the same or clonally related persistent strains during the period of the study. When the dendrogram obtained was analyzed, results showed that some carriers held the same strains for long periods of time, while others were colonized with different clones (data not shown).
DISCUSSION
The emergence of MRSA in the community has increased the importance of screening for this pathogen among the healthy population. Screening to search for such strains has been traditionally restricted to the nares (9, 15, 24, 34); however, recent studies have demonstrated the importance of the throat as a very common site of colonization (19, 22, 25).
Previous investigations had found a variable frequency of S. aureus throat carriage ranging from 4% up to 64% (34). Several studies had shown that individuals may have colonization exclusively in the throat that would be missed on screening limited to the anterior nares (18, 20, 27, 33).
As in a previous study by Nilsson and Ripa (22), our results also suggest that the presence of S. aureus is more frequent in the throat than in the nares. Percentages reported by them (40% for the throat and 31% for the nares) were similar to those found in this study (Table 1). This is important because social and economic circumstances are different for Sweden and Mexico.
If only the nares were tested, 38% (282) of the total of S. aureus carriers would be missed, and also the 32.1% of the MRSA strains isolated from the exclusive throat carriers, suggesting that a great percentage of them are excluded from the screenings, and as Mertz et al. (19) reported, we also think that an additional throat screening is important during the investigation of carriers within a community.
An interesting result was that the presence of S. aureus was greater in the nares of men than in the nares of studied women (P = 0.011), which suggests that gender is relevant as a colonization feature, as has been reported previously (14). However, when throat colonization was compared between men and women, no statistical significance was observed.
Gorwitz et al. (14) reported that carriers less than 20 years of age are more frequently colonized than the older ones; our results showed the same frequency, with 63.3% (471) of carriers being 20 years or younger. Results showed that colonization with S. aureus decreases as people grow older, as Mertz et al. (19) also found.
As expected, most of the S. aureus strains (91.1%) were penicillin resistant due to the extended use of this antibiotic worldwide in the past.
PFGE of most of the MSSA strains isolated from the nares and the throat of the same carrier showed identical or related profiles; however, a few were different. Our data confirm the existence of different clones of S. aureus carried in the nares and the throat, a finding which supports the idea of Uemura et al. (29) that staphylococcal flora in the nose and the throat were independently formed and that attention should also be directed to the carriers of S. aureus in the throat for the control of nosocomial infection.
Like the MSSA strains, the MRSA strains isolated in this study from the nares and the throat of the same carrier have the same PFGE profile or a related one, a finding which is in agreement with the results reported by Small et al. (27); however, we also found different MRSA strains in the throat and the nares, and these data support the fact that S. aureus has to be screened in both the nares and the throat.
When PFGE profiles for exclusive nasal carriers or exclusive throat carriers were analyzed, we determined that exclusive nasal types were grouped mostly in two different regions and exclusive throat isolates were grouped mostly in one region (Fig. 2). Here we are comparing two different environments with different features that could determine the preference for colonization of each particular strain.
Isolation of MRSA strains from hospitals in Mexico has been reported (4, 12). The percentage of MRSA strains reported by Calderón-Jaimes et al. (4) isolated from hospitals in Mexico City was 14.2% between 1998 and 2000. Our results showed a smaller value, but MRSA strains are preferentially isolated from hospital environments. This is the first work that studies the presence of S. aureus in the nares and throat within a healthy Mexican community, and so it may be different from those reported for hospital environments.
Persistence of S. aureus in the anterior nares has been extensively studied (9, 15, 24, 30, 31); however, few works had studied persistence in the throat. VandenBergh et al. (31) reported persistent and intermittent carriage and noncarriage for nasal samples. The same pattern was found in our results when throat carriers were studied. Results showed that 13% of the studied population never carried S. aureus, 74% were intermittent carriers, and 13% were persistent carriers. Nilsson and Ripa (22) reported 31% persistent carriers in their study in a 2-year period; we continued sampling some carriers for a longer period of time, and we believe that this was the reason for a lower value.
Persistence of S. aureus in the throat in some cases for up to 6 years was found; few persistent carriers were colonized with different clones of S. aureus over time. However, the present data showed that most S. aureus persistent strains produce PFGE patterns that are relatively stable over the years, suggesting that these strains remain genetically unchanged during the time of colonization.
The present study investigated the clonality of S. aureus nasal and throat carriage; results demonstrated strain diversity among carriers colonized with S. aureus.
Much work has been done to identify nasal MRSA carries; however, throat carriers may contribute to spread MRSA infections and the decolonization schemes could fail (18). Our results support what was previously reported (18, 27, 33): culturing the anterior nares alone is insufficient for efficient detection of MRSA carriers.
This study showed that the throat is an important habitat of S. aureus, including MRSA, and the bacteria could persist as colonizers for many years. In conclusion, any screening for S. aureus, in particular screening for MRSA within a community, should include cultures from both the anterior nares and the throat.
Acknowledgments
A. Hamdan-Partida is a Ph.D. student at the Doctorado en Ciencias Biológicas of Universidad Autónoma Metropolitana, Unidad Xochimilco, and was supported by grant 103369 of CONACYT, Mexico. Partial support for this study was provided by Acuerdo del Rector 12/2008, UAM.
We thank Esther Irigoyen for statistical analysis support. We thank Rocio Martínez-Colula, Carmen Galicia-Galicia, and Gabriela Ramírez-Benítez for technical support.
Footnotes
Published ahead of print on 24 March 2010.
REFERENCES
- 1.Batra, R., A. C. Eziefula, D. Wyncoll, and J. Edgeworth. 2008. Throat and rectal swabs may have an important role in MRSA screening of critically ill patients. Intensive Care Med. 34:1703-1706. [DOI] [PubMed] [Google Scholar]
- 2.Brakstad, O. G., K. Aasbakk, and J. A. Maeland. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30:1654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustos-Martínez, J. A., A. Hamdan-Partida, and M. E. Gutiérrez-Cárdenas. 2006. Staphylococcus aureus: the reemergence of a pathogen in the community. Rev. Biomed. 17:287-305. [Google Scholar]
- 4.Calderón-Jaimes, E., L. E. Espinosa de los Monteros, and R. Avila-Beltrán. 2002. Epidemiology of drug resistance: the case of Staphylococcus aureus and coagulase-negative staphylococci infections. Salud Publica Mex. 44:108-112. [DOI] [PubMed] [Google Scholar]
- 5.Cespedes, C., B. Said-Salim, M. Miller, S.-A. Lo, B. N. Kreiswirth, R. J. Gordon, P. Vavagiakis, R. S. Klein, and F. D. Lowy. 2005. The clonality of Staphylococcus aureus nasal carriage. J. Infect. Dis. 191:444-452. [DOI] [PubMed] [Google Scholar]
- 6.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility test, 9th ed., vol. 26, no 1. Approved standard M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed., vol. 26, no. 2. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Cole, A. M., S. Tahk, A. Oren, D. Yoshioka, Y. Kim, A. Park, and T. Ganz. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin. Diagn. Lab. Immunol. 8:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, S. L., M. B. Perri, S. M. Donabedian, C. Manierski, A. Singh, D. Vager, N. Z. Haque, K. Speirs, R. R. Muder, B. Robinson-Dunn, M. K. Hayden, and M. J. Zervos. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 45:1705-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeo, F. R., and H. F. Chambers. 2009. Reemergence of antibiotic-resistant Sthapylococcus aureus in the genomics era. J. Clin. Invest. 119:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echániz-Aviles, G., M. E. Velázquez-Meza, M. Aires de Sousa, R. Morfín-Otero, E. Rodríguez-Noriega, N. Carnalla-Barajas, S. Esparza-Ahumada, and H. de Lencastre. 2006. Molecular characterisation of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone in a Mexican hospital (1999-2003). Clin. Microbiol. Infect. 12:22-28. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen, N. H., F. Espersen, V. T. Rosdahl, and K. Jensen. 1995. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 115:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorwitz, R. J., D. Kruszon-Moran, S. K. McAllister, G. McQuillan, L. K. McDougal, G. E. Fosheim, B. J. Jensen, G. Killgore, F. C. Tenover, and M. J. Kuehnert. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J. Infect. Dis. 197:1226-1234. [DOI] [PubMed] [Google Scholar]
- 15.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall, C., and D. Spelman. 2007. Is throat screening necessary to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit? J. Clin. Microbiol. 45:3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mertz, D., R. Frei, B. Jaussi, A. Tietz, C. Stebler, U. Flückinger, and A. F. Widmer. 2007. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 45:475-477. [DOI] [PubMed] [Google Scholar]
- 19.Mertz, D., R. Frei, N. Periat, M. Zimmerli, M. Battegay, U. Flückiger, and A. F. Widmer. 2009. Exclusive Staphylococcus aureus throat carriage at-risk populations. Arch. Intern. Med. 169:172-178. [DOI] [PubMed] [Google Scholar]
- 20.Meurman, O., M. Routamaa, and R. Peltonen. 2005. Screening for methicillin-resistant Staphylococcus aureus: which anatomical sites to culture? J. Hosp. Infect. 61:351-353. [DOI] [PubMed] [Google Scholar]
- 21.Mulvey, M. R., L. Chui, J. Ismail, L. Louie, C. Murphy, N. Chang, M. Alfa, and the Canadian Committee for the Standardization of Molecular Methods. 2001. Development of a Canadian standardized protocol for subtyping methicillin-resistant Staphylococcus aureus using pulse-field gel electrophoresis. J. Clin. Microbiol. 39:3481-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson, P., and T. Ripa. 2006. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. J. Clin. Microbiol. 44:3334-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605-610. [DOI] [PubMed] [Google Scholar]
- 25.Ringberg, H., A. C. Petersson, M. Walder, and P. J. H. Johansson. 2006. The throat: an important site for MRSA colonization. Scand. J. Infect. Dis. 38:888-893. [DOI] [PubMed] [Google Scholar]
- 26.Saïd-Salim, B., B. Mathema, and B. N. Kreiswirth. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect. Control Hosp. Epidemiol. 24:451-455. [DOI] [PubMed] [Google Scholar]
- 27.Small, H., A. L. Casey, T. S. J. Elliott, J. Rollason, and S. Ball. 2007. The oral cavity an overlooked site for MRSA screening and subsequent decolonisation therapy? J. Infect. 55:378-383. [DOI] [PubMed] [Google Scholar]
- 28.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uemura, E., S. Kakinohana, N. Higa, C. Toma, and N. Nakasone. 2004. Comparative characterization of Staphylococcus aureus isolates from throats and noses of healthy volunteers. Jpn. J. Infect. Dis. 57:21-24. [PubMed] [Google Scholar]
- 30.van Belkum, A., N. J. Verkaik, C. P. de Vogel, H. A. Boelens, J. Verveer, J. L. Nouwen, H. A. Verbrugh, and H. F. L. Wertheim. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 31.VandenBergh, M. F. Q., E. P. F. Yzerman, A. van Belkum, H. A. M. Boelens, M. Sijmons, and H. A. Verbrugh. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widmer, A. F., D. Mertz, and R. Frei. 2008. Necessity of screening of both the nose and the throat to detect methicillin-resistant Staphylococcus aureus colonization in patients upon admission to an intensive care unit. J. Clin. Microbiol. 46:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, R. E. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27:56-71. [DOI] [PMC free article] [PubMed] [Google Scholar]