Abstract
An immunocompromised patient presented with febrile episodes, an erysipelas-like rash, and thromboembolic complications. Amplification of 16S rRNA gene sequences from blood and sequence analysis revealed “Candidatus Neoehrlichia mikurensis.” We report the first case of human disease caused by “Ca. Neoehrlichia mikurensis.”
CASE REPORT
A 77-year-old man with B-cell chronic lymphocytic leukemia developed autoimmune anemia in 2007 and started long-term treatment with corticosteroids. In September of the same year, he had a transitory ischemic attack. Since his hemolytic anemia worsened despite treatment with corticosteroids, he was given courses of cyclophosphamide during the second half of 2008. The patient developed autoimmune thrombocytopenia (platelet count, 38 × 109/liter; reference range for healthy adults, 145 × 109 to 355 × 109/liter) and was splenectomized laparoscopically on 4 June 2009, with subsequent normalization of platelet counts.
While kayaking on 3 July 2009, the patient developed acute diarrhea, which was followed by fever and chills and a short episode of loss of consciousness the same night. When admitted to Kungälv Hospital, Kungälv, Sweden, the next day under suspicion of sepsis, he was hypotensive (blood pressure [BP], 85/60 mm Hg) and febrile (temperature, 38.5°C; reference, <38.0°C). Deep vein thrombosis in the left lower extremity encompassing the groin and pulmonary embolism were also discovered. The patient was treated intravenously (i.v.) with ceftazidime for 1 week, but no microbe was identified. The patient's systemic inflammatory reaction (C-reactive protein level of 92 mg/liter [reference, <5 mg/liter] and fever) was judged to result from widespread thromboembolism, and the patient was discharged on 10 July with low-molecular-weight heparin medication.
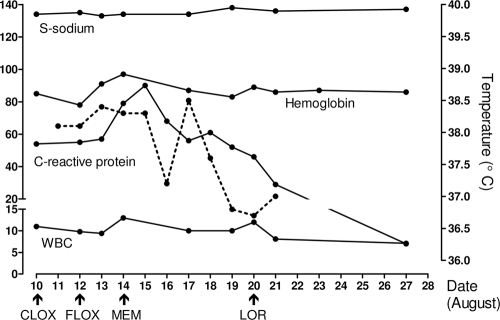
A month later, the patient was readmitted to Sahlgrenska University hospital with a fever of 39.5°C, BP of 105/55 mm Hg, and an erysipelas-like rash on the inside of the left leg. The patient was anemic (hemoglobin [Hb], 85 g/liter; reference range, 134 to 170 g/liter) and had leukocytosis (white blood cell [WBC] count, 11 × 109/liter; reference range, 3.5 × 109 to 8.8 × 109/liter) with a pronounced left shift, a normal platelet count, and a C-reactive protein level of 54 mg/liter (reference, <5 mg/liter). Hyponatremia was present (sodium level, 134 mmol/liter; reference range, 137 to 145 mmol/liter). The patient was taking warfarin, oral prednisolone, omeprazole, and vitamin B tablets. He was treated with i.v. cloxacillin for 2 days, followed by oral floxacillin (flucloxacillin) for 2 days and, finally, i.v. meropenem for 7 days (Fig. 1). Fever, elevated levels of C-reactive protein, and hyponatremia resolved within 1 week, apparently after the institution of meropenem (Fig. 1). All cultures (three blood cultures, two urinary cultures, and one oral swab culture) were negative. A chest X ray revealed scant infiltrates around the hili and in the basal part of the right lung, but computed tomography (CT) scans of the thorax and abdomen 1 week later were normal. No evidence of endocarditis was seen on an echocardiogram. The patient was discharged from the hospital on 21 August with oral loracarbef (Fig. 1).
FIG. 1.
Laboratory-determined parameters (solid lines; left y axis) and body temperature (dotted line; right y axis) in relation to administration of antibiotics during the second period of hospitalization, 10 to 21 August, and several days following discharge. Antibiotics: CLOX, cloxacillin administered i.v.; FLOX, oral floxacillin; MEM, meropenem administered i.v.; and LOR, oral loracarbef. Units of measurements for parameters are as follows: serum sodium level (S-sodium), millimoles per liter; hemoglobin, grams per liter; C-reactive protein, milligrams per liter; and WBC count, cells (109) per liter.
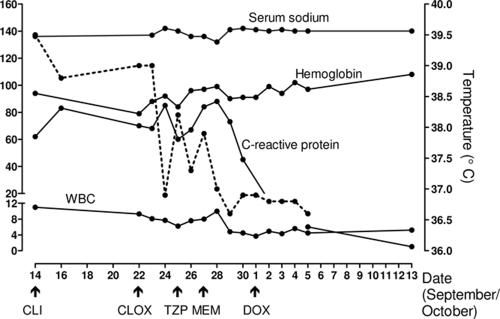
The patient developed a fever (temperature, 39.5°C; reference, <38.0°C) and chills again on 11 September and had increased mucus production in the airways. He was treated at home with oral loracarbef (Fig. 2). On 14 September, the patient presented with classical erysipelas involving the right leg and treatment was switched to oral clindamycin (Fig. 2). The patient was readmitted to the hospital on 22 September due to persistent fever (temperature, 39°C). He was again anemic (Hb, 79 g/liter; reference range, 134 to 170 g/liter) and had an elevated WBC count (9.3 × 109/liter; reference range, 3.5 × 109 to 8.8 × 109/liter) with a left shift, a normal platelet count, and increased levels of both C-reactive protein (70 mg/liter; reference, <5 mg/liter) and procalcitonin (0.6 μg/liter; reference, <0.05 μg/liter). He was given cloxacillin i.v. to cover erysipelas pathogens, e.g., Staphylococcus aureus and streptococci (Fig. 2). The patient's medication was subsequently switched first to piperacillin-tazobactam and then to meropenem (Fig. 2). Hyponatremia developed on 26 September (sodium nadir, 132 mmol/liter; reference range, 137 to 145 mmol/liter) and resolved 3 days later (Fig. 2). On 28 September, the fever disappeared but the patient developed transitory weakness of the left side of the face and arm. A CT scan of the brain did not reveal evidence of bleeding or of an infectious process. A transesophageal echocardiogram showed no embolus in the heart valves. Repeated blood cultures and other cultures (of urine, feces, and oral swab samples) were negative.
FIG. 2.
Laboratory-determined parameters (solid lines; left y axis) and body temperature (dotted line; right y axis) in relation to antibiotic treatment taken by the patient at home and administered during the third period of hospitalization (22 September to 5 October) and during subsequent polyclinical visits. Antibiotics: CLI, oral clindamycin; CLOX, cloxacillin administered i.v.; TZP, piperacillin-tazobactam administered i.v.; MEM, meropenem administered i.v.; and DOX, oral doxycycline. Units of measurement for parameters are as given in the legend to Fig. 1.
As suspicion of sepsis remained, blood culture samples in eight bottles from the BacT/Alert system (bioMérieux), including four aerobic and four anaerobic flasks, were analyzed by a PCR assay targeting the panbacterial 16S rRNA gene as described previously (12). These blood samples had been collected on two separate occasions on 23 September and had not revealed any signs of bacterial growth after incubation in the automated thermostat for 6 days. All eight blood culture bottles were positive by PCR, indicating the presence of bacterial DNA. Amplified DNA from all four aerobic bottles and one anaerobic bottle was sequenced and gave a 100% similarity score for “Candidatus Neoehrlichia mikurensis” according to the GenBank BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). To confirm the finding of “Ca. Neoehrlichia mikurensis,” EDTA blood was collected from the patient on 1 October and similarly analyzed by a panbacterial PCR assay, which again yielded the identification of “Ca. Neoehrlichia mikurensis.” In addition, a stored serum sample from 11 August was found to contain “Ca. Neoehrlichia mikurensis” DNA. These results were communicated to the hospital ward on 1 October, and the patient was started on oral doxycycline at 100 mg twice daily for 2 weeks, the treatment of choice for human ehrlichiosis (Fig. 2). Clinical improvement and a near-normal C-reactive protein level (6 mg/liter; reference, <5 mg/liter) led the patient to be discharged on 5 October (Fig. 2).
“Ca. Neoehrlichia mikurensis” bacteria are small, Gram-negative pleomorphic cocci 0.5 to 1.2 μm in diameter that are obligate intracellular organisms (8). They belong to the family Anaplasmataceae and lack serological cross-reactivity with other genera of the family, such as Anaplasma and Ehrlichia species (8). This novel bacterial species has been identified in blood, liver, and spleen samples from both laboratory and wild rats. The pathogen appears to reside within the cytoplasm of endothelial cells lining the venous sinuses, at least in rats (8). In humans, the pathogen's close relatives Ehrlichia chaffeensis and Anaplasma phagocytophilum selectively infect the professional phagocytes monocytes/macrophages and neutrophilic granulocytes, respectively (5), which suggests that “Ca. Neoehrlichia mikurensis” may display tropism for leukocytes also.
Until now, no one has been able to grow “Ca. Neoehrlichia mikurensis” (8), which explains why all our patient's blood cultures remained negative. E. chaffeensis and A. phagocytophilum can be cultivated only in cells such as those of human leukemia cell lines (5). In the acute phase of human ehrlichial infections, PCR analysis of EDTA- or citrate-anticoagulated whole blood is the preferred diagnostic method, followed by examination of blood films for cytoplasmic bacterial inclusions (called ehrlichial morulae) and IgM serology (5).
In the 1990s, Ehrlichia and Anaplasma species were recognized as emerging tick-borne human pathogens, although the first case of human infection had been described already in Japan in 1954 (7). Hitherto, all cases of human ehrlichiosis in Europe have been due to A. phagocytophilum, never E. chaffeensis (4). “Ca. Neoehrlichia mikurensis” has not been detected in Sweden, either in ticks or in humans or other mammals. Its name derives from its first isolation from rats captured on the Japanese island of Mikura (8). Since it has been isolated from up to 20% of Ixodes ricinus ticks in the Netherlands (15), it is not far-fetched to assume that it may also infest ticks in Sweden. If “Ca. Neoehrlichia mikurensis” resembles the other ehrlichial agents, transmission probably occurs from the reservoir of wild mammals that infect blood-sucking ticks, which in turn accidentally infect humans, who are likely to be dead-end hosts (2). The incubation period for A. phagocytophilum infection has been estimated to be 7 to 30 days after the tick bite (4). Our patient pursued outdoor activities in an area where borreliosis is endemic, and he is likely to have contracted the infection in June, during the peak season for tick-borne ehrlichial infections in Europe (June to August) (4).
A meta-analysis of the symptoms, signs, and laboratory findings for patients infected with the previously described agents of human ehrlichiosis reveals a clinical picture that resembles influenza (5). Most patients have high fever, headache, malaise, and myalgia. Fewer patients experience arthralgia, cough, nausea, vomiting, or diarrhea. Rashes were reported by a third of the patients with human monocytic ehrlichiosis but seldom by those with human granulocytic anaplasmosis. Possibly, the episodes of reversible ischemic neurological deficit, deep vein thrombosis, and pulmonary embolism experienced by our patient were caused by the infection rather than the uneventful splenectomy that took place 1 month earlier. Coagulopathy has been reported previously to be a rare complication of E. chaffeensis infections (5). Severe symptoms of ehrlichial disease develop mainly in individuals who are elderly and/or immunocompromised (5); both characteristics fit our patient. Previously described immunosuppressed patients with ehrlichial disease include solid-organ transplant recipients (14), HIV-infected individuals (11), and a child with leukemia (1).
Our patient had altered laboratory-determined parameters characteristic of “traditional” human ehrlichiosis, such as hyponatremia and anemia (although anemia predated the infection) (5). He also had leukocytosis, which is less common than leukopenia in patients with ehrlichiosis (5). A prominent left shift of the blood differential count was noted, along with the presence of myelocytes, metamyelocytes, erythroblasts, occasional megakaryocytes, and plasma cells in peripheral blood. Our patient had more pronounced C-reactive protein elevation (10- to 15-fold) than the typical 2- to 4-fold increase associated with the other ehrlichial agents (2). No increase in hepatic enzymes (aminotransferases) was observed in our patient, in contrast to what is often seen in cases of ehrlichiosis (2, 5). Lastly, our patient displayed a lowered serum cholesterol level (3.1 mmol/liter; reference range, 3.9 to 7.8 mmol/liter), which may fit with the propensity of ehrlichial organisms to pick up cholesterol from the environment (10).
Ehrlichial organisms lack the genes that encode lipid A of lipopolysaccharide and most of the genes that code for peptidoglycan (10). Hence, the bacteria are devoid of the cell wall moieties that are important danger signals for the innate immune system. Nevertheless, strong febrile reactions are generated in response to the bacteria, even in an immunocompromised host such as ours. The patient in question was splenectomized, which has been associated with more severe forms of ehrlichiosis (11). Moreover, the patient's hemolytic anemia may have been an additional risk factor: the iron chelator deferoxamine has been shown previously to inhibit the growth of E. chaffeensis in monocytes (3) and, thus, iron overload due to hemolysis may conversely have facilitated the growth of “Ca. Neoehrlichia mikurensis.”
Elevated concentrations of the cytokines gamma interferon (IFN-γ) and interleukin-10 (IL-10) in the serum are seen in patients infected with A. phagocytophilum (6). Our patient displayed another pattern of cytokines in the serum: levels of IL-8 (100 pg/ml; reference geometric mean [GM], 7.5 pg/ml; 95% confidence interval [CI], 6.7 to 8.3 pg/ml), IL-6 (41 pg/ml; reference GM, 2.0 pg/ml; CI, 1.8 to 2.2 pg/ml), IL-10 (19 pg/ml; reference GM, 0.64 pg/ml; CI, 0.42 to 1.0 pg/ml), IL-1β (4.2 pg/ml; reference GM, 2.4 pg/ml; CI, 2.3 to 2.6 pg/ml), IFN-γ (2.6 pg/ml; reference, 1.8 ± 0 pg/ml), and tumor necrosis factor alpha (TNF-α; 1.7 pg/ml; reference GM, 1.1 pg/ml; CI, 0.90 to 1.3 pg/ml) were determined using a flow cytometry-based cytokine bead array system (Becton Dickinson). High-level expression of IL-8 may actually facilitate the spread of “Ca. Neoehrlichia mikurensis” by recruitment of target cells to the site of infection (9).
The treatment of choice for ehrlichiosis in nonpregnant adults is tetracyclines, with doxycycline being the preferred agent due to its pharmacokinetics (2). Tetracyclines are used with restriction in patients with hematological malignancies due to their potential bone marrow-inhibitory effects. Cephalosporins, β-lactam antibiotics, macrolides, clindamycin, and aminoglycosides are not believed to be active against human ehrlichial agents (5). However, it appears as if meropenem suppressed the activity of “Ca. Neoehrlichia mikurensis” (Fig. 1 and 2) but did not eradicate the infection. The infection finally cleared after doxycycline treatment, as proven by a blood sample collected over a month after the cessation of doxycycline (on 23 November) which was negative for “Ca. Neoehrlichia mikurensis” DNA by PCR.
This is the first reported case of human disease caused by “Ca. Neoehrlichia mikurensis.” Previously, a study in an area in Japan where ticks are endemic failed to detect “Ca. Neoehrlichia mikurensis” in 62 patients with fevers of unknown origin by using the approach of amplifying target sequences of Anaplasmataceae groESL genes (13).
Acknowledgments
This work was performed via the AIDA (Acquired Immune Deficiency Infectious Defense Analyses) network and was funded by Västra Götaland Regional grant 93900, the Föreningen för Medicinsk Mikrobiologi, the Cancer and Allergy Foundation, and LUA-ALF grant 71580.
Footnotes
Published ahead of print on 10 March 2010.
REFERENCES
- 1.Arav-Boger, R., J. H. Knepp, J. J. Walls, and J. S. Dumler. 2000. Human monocytic ehrlichiosis in a child with leukemia. Pediatr. Infect. Dis. J. 19:173-175. [DOI] [PubMed] [Google Scholar]
- 2.Bakken, J. S., and J. S. Dumler. 2006. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann. N. Y. Acad. Sci. 1078:236-247. [DOI] [PubMed] [Google Scholar]
- 3.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron-transferrin. Infect. Immun. 62:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, J. R., and J. A. Oteo. 2002. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8:763-772. [DOI] [PubMed] [Google Scholar]
- 5.Dumler, J. S., J. E. Madigan, N. Pusterla, and J. S. Bakken. 2007. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 45(Suppl. 1):S45-S51. [DOI] [PubMed] [Google Scholar]
- 6.Dumler, J. S., E. R. Trigiani, J. S. Bakken, M. E. Aguero-Rosenfeld, and G. P. Wormser. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin. Diagn. Lab. Immunol. 7:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda, T., T. Kitao, and Y. Keida. 1954. Studies on the causative agent of “Hyuganetsu” disease. I. Isolation of the agent and its inoculation trial in human beings. Med. Biol. 32:200-209. [Google Scholar]
- 8.Kawahara, M., Y. Rikihisa, E. Isogai, M. Takahashi, H. Misumi, C. Suto, S. Shibata, C. Zhang, and M. Tsuji. 2004. Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54:1837-1843. [DOI] [PubMed] [Google Scholar]
- 9.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 10.Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 71:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddock, C. D., S. M. Folk, G. M. Shore, L. J. Machado, M. M. Huycke, L. N. Slater, A. M. Liddell, R. S. Buller, G. A. Storch, T. P. Monson, D. Rimland, J. W. Sumner, J. Singleton, K. C. Bloch, Y. W. Tang, S. M. Standaert, and J. E. Childs. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586-1594. [DOI] [PubMed] [Google Scholar]
- 12.Skovbjerg, S., C. Welinder-Olsson, N. Kondori, E. Kjellin, F. Nowrouzian, A. E. Wold, D. Stockelberg, P. Larsson, and C. Wenneras. 2009. Optimization of the detection of microbes in blood from immunocompromised patients with haematological malignancies. Clin. Microbiol. Infect. 15:680-683. [DOI] [PubMed] [Google Scholar]
- 13.Tabara, K., S. Arai, T. Kawabuchi, A. Itagaki, C. Ishihara, H. Satoh, N. Okabe, and M. Tsuji. 2007. Molecular survey of Babesia microti, Ehrlichia species and Candidatus Neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 51:359-367. [DOI] [PubMed] [Google Scholar]
- 14.Tan, H. P., J. S. Dumler, W. R. Maley, A. S. Klein, J. F. Burdick, F. F. Poordad, P. J. Thuluvath, and J. S. Markowitz. 2001. Human monocytic ehrlichiosis: an emerging pathogen in transplantation. Transplantation 71:1678-1680. [DOI] [PubMed] [Google Scholar]
- 15.van Overbeek, L., F. Gassner, C. L. van der Plas, P. Kastelein, U. Nunes-da Rocha, and W. Takken. 2008. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS Microbiol. Ecol. 66:72-84. [DOI] [PubMed] [Google Scholar]