Abstract
We report three cases of tuberculosis in alpacas from Spain caused by Mycobacterium bovis. The animals revealed two different lesional patterns. Mycobacterial culture and PCR assay yielded positive results for M. bovis. Molecular typing of the isolates identified spoligotype SB0295 and identical variable-number tandem repeat (VNTR) allele sizes.
CASE REPORT
Herd 1.
The affected herd comprised 32 alpacas (26 adults and six juveniles) and was located in the Ronda region (southern Spain). The animals were raised in outdoor facilities and were fed with commercial pellet feed, hay, and water ad libitum. In March 2009, a 7-year-old female alpaca (alpaca 1) showed dyspnea, fever, depression, lethargy, anorexia, and weight loss. One month later, an 8-year-old male alpaca (alpaca 2) presented chronic weight loss, bruxism, and dyspnea.
Herd 2.
The second herd was located in the Antequera region (southern Spain; approximately 90 km away from herd 1) and comprised four animals reared under intensive conditions. In July 2009, a 3-year-old female alpaca (alpaca 3) showed appetite loss, recumbency, muscle weakness, dyspnea, and bruxism. This animal had been moved from herd 1 6 months before and gave birth to a healthy male cria (alpaca 4) in December 2008. Alpaca 4 died 2 months after alpaca 3 with clinical signs associated with an enteric disorder.
Alpacas 1, 2 and 3 were subjected to X-ray analysis. Alpacas 1 and 2 showed cavitary lesions with heterogeneous areas of increased density inside and severe and diffuse consolidation of pseudonodular morphology in lung parenchyma. On the other hand, diffuse consolidation with micronodular and interstitial infiltrate was observed in alpaca 3. Ultrasonographic examination revealed a hypoecogenic area with hyperecogenic areas of various sizes above the heart and parenchyma within the liver and the spleen (alpacas 1 and 2). The three animals were treated with a combination of potassium penicillin (20,000 IU/kg of body weight, administered by intramuscular injection [IM], every 12 h for 10 days), gentamicin (6.6 mg/kg, IM, every 24 h for 5 days), flunixin meglumine (0.25 mg/kg, IM, every 12 h for 10 days), and metronidazole (10 mg/kg, IM, every 12 h for 10 days). No clinical improvement was observed in any case.
One month before the first case was detected, a comparative intradermal tuberculin test (IDT) (bovine and avian tuberculins from CZV, Porriño, Spain) and gamma interferon (IFN-γ) test (Bovigam test; Prionics AG, Schlieren, Switzerland) were conducted with herd 1 by the Official Veterinary Services. All alpacas were negative to both tests. However, due to the suspicion of tuberculosis (TB) infection in alpaca 1, a program of repeated intradermal tests at 90-day intervals was again implemented for herd 1 in late April 2009. Blood samples for serological testing were also taken both on the day of tuberculin injection and 21 days later. On the other hand, 1 week after the first clinical signs were observed in alpaca 3, the same program was carried out in herd 2 in late July 2009. The analyses yielded negative results in both herds.
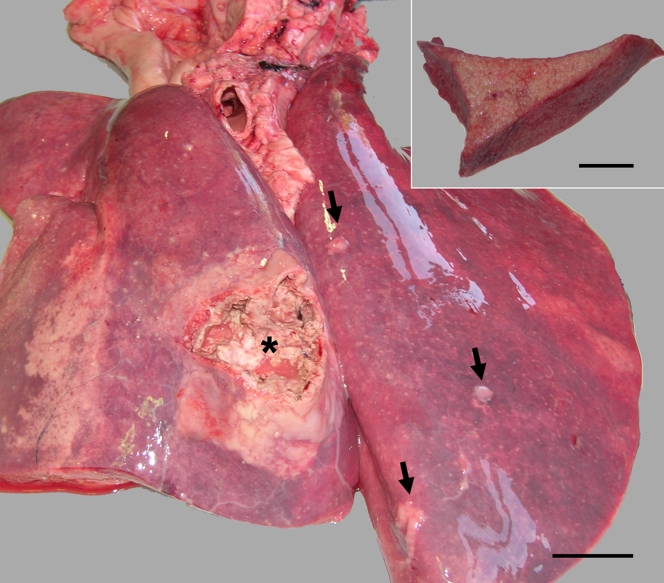
In the three alpacas (alpacas 1, 2, and 3), the disease was progressive, and despite treatment under veterinary supervision, the animals were finally euthanized and submitted to the Veterinary Medicine Faculty of the University of Cordoba at the owner's request. Postmortem examination of alpacas 1 and 2 revealed multifocal-to-coalescing, granulomatous, caseous, and calcified nodules of different diameters (from 2 mm to 10 cm) in lung, trachea, liver, and spleen and in tracheobronchial, mediastinal and mesenteric lymph nodes. At the cut sections, these nodules were yellowish and firm with a partially mineralized core. In addition to the nodular pattern, the lungs showed a diffuse pattern as well as lesions which communicated with the lumens of bronchi (Fig. 1, inset). These lesions together with the ulcerative, granulomatous lesions observed in the mucosa of the trachea are considered indicative of open TB. Alpaca 3 showed multifocal-to-coalescing, miliar, granulomatous pneumonia, pleuritis, and peritonitis covering the thoracic and abdominal cavities (miliary TB). The death of alpaca 4 was associated with an intussusception process. No gross lesions compatible with TB were observed in this animal. At the necropsy of each animal, samples from selected organs were collected for histopathological, bacteriological, and molecular studies.
FIG. 1.
Multifocal areas of granulomatous, caseous bronchopneumonia (arrows) with a large area of bronchopneumonia communicating with the bronchial lumen (asterisk). Bar = 5 cm. The inset image shows a diffuse pattern of granulomatous pneumonia observed in alpaca 1. Bar = 4.5 cm.
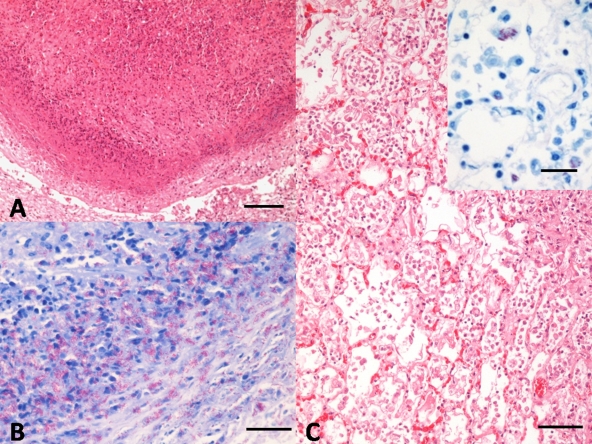
Tissue samples of different organs were fixed in 10% neutral buffered formalin, routinely processed, and stained with hematoxylin and eosin and Ziehl-Neelsen staining. Histopathology revealed granulomatous lesions composed of a central core of epithelioid macrophages and scattered lymphocytes and plasma cells at the periphery. Epithelioid macrophages showed a finely granular, foamy cytoplasm. Some granulomatous lesions showed a central core of necrosis and occasional mineralization (Fig. 2A). Granulomata were surrounded by a marked connective tissue capsule. In addition to the nodular pattern, alpacas 1 and 2 showed a diffuse pattern of granulomatous inflammation with lack of delimiting capsule in lung and trachea (Fig. 2C), which focally ulcerated the epithelium of the mucosa of bronchi and trachea. Abundant acid-fast bacteria (AFB) were identified on Ziehl-Neelsen-stained smears and tissue sections of all the tissues examined in alpacas 1 and 2 (Fig. 2B and C, inset), whereas alpaca 3 showed a paucibacillary staining with mycobacteria being identified only occasionally.
FIG. 2.
(A) Alpaca 1. Nodular granulomatous lesion composed of a central core of necrosis, surrounded by degenerated neutrophils and cell debris, epithelioid macrophages, and scattered lymphocytes and plasma cells. Note the manifest capsule of connective tissue delimiting the lesion (as shown by hematoxylin and eosin [H&E] staining). Bar = 100 μm. (B) Alpaca 1. Abundant acid-fast bacteria in the periphery of the granulomatous lesion. Ziehl-Neelsen staining. Bar = 20 μm. (C) Alpaca 3. Marked proliferation of histiocytes, with diffuse intraalveolar infiltrate of foamy macrophages and epithelioid cells. H-E. Bar = 50 μm. The inset image shows random foamy macrophages, laden with numerous acid-fast bacteria within their cytoplasm. Ziehl-Neelsen staining. Bar = 20 μm.
A pool of lung, spleen, liver, and tracheobronchial lymph node homogenates was subjected to specific mycobacterial culture by using standard procedures. The homogenates, previously decontaminated with hexadecylpyridinium chloride, were cultured on Löwenstein-Jensen medium with pyruvate and Coletsos solid selective media (Biomedics, Madrid, Spain). Growth of mycobacteria in the specific culture was obtained in the three alpacas. Identification of Mycobacterium bovis (alpacas 1, 2, 3) was performed from suspected colonies by using a multiplex PCR amplification of the fragments coding for rRNA 16S and MPB70 protein (24). Moreover, Mycobacterium tuberculosis complex (MTC) DNA was also directly identified from fresh tissue samples by PCR based on an MTC-specific IS6110 insertion sequence (13). Spoligotype SB0295 was identified using the standardized membrane with 43 spacers as previously described (12). Variable-number tandem repeat (VNTR) typing was carried out as described by Frothingham and Meeker-O'Connell (9) by using nine VNTR markers (ETR-A [VNTR2165], ETR-B [VNTR2461], ETR-D [VNTR580], ETR-E [VNTR3192, MIRU31], MIRU26 [VNTR2996], QUB11a [VNTR2163a], QUB11b [VNTR2163b], QUB26 [VNTR4052], QUB3232 [VNTR3232]). The VNTR profiles were identical for all isolates: 6-4-3-4-5-11-2-5-6 (order of markers as described above).
Alpaca 4 showed negative results to both PCR and specific mycobacterial culture, and Clostridium perfringens was isolated in large quantities in the digestive tract.
TB is an infectious disease responsible for millions of human deaths annually and significant economic losses in livestock worldwide (5, 19). In many countries, M. tuberculosis and M. bovis are the most common agents isolated in TB cases in humans and ruminant species, respectively (7). These pathogens that belong to MTC affect also a wide range of domestic and wild species (7, 15). The disease in South American camelids has recently acquired importance since alpacas and llamas are being imported and kept in increasing numbers in many European countries (2). Camelids are known to be susceptible to MTC, including M. tuberculosis, M. bovis, and/or Mycobacterium microti (8, 17, 23), and to Mycobacterium kansasii infections (11). Furthermore, TB cases have been recently reported in alpacas and llamas from different European countries (2, 14, 16, 20).
Although M. bovis was isolated in the three alpacas, two different lesional patterns were observed. Alpacas 1 and 2 showed a combination of both nodular and diffuse patterns of TB in lungs and trachea together with ulceration of the mucosa and numerous AFB. Similar lesions have been previously reported in alpacas, other camelid species, and wild ruminants (3, 14, 16, 21, 22). On the other side, alpaca 3, which was also infected by M. bovis, showed miliary TB lining the pleural and peritoneal cavities with scarce AFB. The lesional pattern found in this animal was similar to those observed in cattle (3). The diffuse pattern in contrast to the nodular pattern may point to a failure in the control of the lesion, with the host immune response not able to delimit and isolate the affected from the nonaffected parenchyma by a connective tissue capsule. Moreover, the evidence of open TB in trachea and lung suggests that this animal species may be a potential source of mycobacterial excretion.
The antemortem detection of TB in camelids presents many difficulties, with none of the currently available tests being able to detect disease with certainty (22). In our study, neither comparative IDT nor IFN-γ tests were able to identify the positive animals in the herds. The Bovigam test is a current method of diagnosis for cattle; however, it has already been reported to be a nonvalid test for the diagnosis of TB in camelids (22). The intradermal tuberculin test, which is the traditional diagnostic approach for a number of other species, is believed to produce nonspecific reactions in camelids (6, 8, 20). Serological assays may be a promising alternative, but little is known about antibody responses during TB in these species (14, 23). There have been previous attempts to develop alternative immunodiagnostic assays for TB in camelids based on in vitro lymphocyte transformation or antibody measuring by enzyme-linked immunosorbent assay (ELISA) (10), but no reliable test is currently available. Furthermore, there is little evidence that detection of specific antibodies (using methods such as ELISA) could be a useful indicator of field infection (4). Recently, multiantigen print immunoassay (MAPIA) and lateral-flow-based rapid test (RT) have been experimentally showed as useful diagnostic tools for antemortem detection of TB in multiple host species, including camelids (6, 14, 23).
Although this is the first record of bovine TB in alpacas from Spain, the animals affected in the present study came from Peru (alpacas 1 and 3), the United Kingdom (alpaca 2), and Spain (alpaca 4). The M. bovis isolates were confirmed as spoligotype SB0295 with the VNTR profile 6-4-3-4-5-11-2-6-6 (ETR-A, ETR-B, ETR-D, ETR-E, MIRU26, QUB11a, QUB11b, QUB26, QUB3232). Spoligotype SB0295 represents 4.1% of the strains isolated from TB cases in domestic and wildlife species in Spain (1, 18). This spoligotype has been frequently isolated in cattle (94.1%) from southern regions (40.2%) in this country. This finding indicates that the animals were probably infected in Spain. In addition, the MIRU/VNTR typing also revealed identical profiles in the three affected alpacas. Therefore, alpaca 3 was probably infected in herd 1. Further molecular studies involving neighboring farms and wildlife are in progress in order to trace back the infection. In Spanish Mediterranean ecosystems, wildlife species are able to maintain M. bovis infection in the environment in the absence of domestic livestock and are probably able to transmit the disease to other species, acting as reservoirs (1, 15).
Transmission between alpacas by direct contact has been recently suggested (21). However, although alpaca 4 remained together with alpaca 3 all the time, M. bovis transmission by direct contact or via infected milk was not detected in this animal.
The results confirm the susceptibility of alpacas to M. bovis infection and show a wide variety of consequent pathological findings. The open TB observed in alpacas 1 and 2 suggests that this species may act as a potential source of mycobacterial excretion. Therefore, given the risk of transmission, not only to other domestic or wild species but also to human beings, the infection by M. bovis should be considered in the differential diagnoses of respiratory diseases in alpacas (8), particularly in recognized regions where TB is endemic. Moreover, our study highlights the difficulty of antemortem diagnosis using the official tests currently available for the diagnosis of TB in other species. In this sense, the use of complementary immunological diagnostic methods, such as RT and MAPIA, may provide a useful screening tool to identify infected animals (6, 14, 23).
Acknowledgments
This work was partially supported by Ministry of Environment and Rural and Marine Affairs (MARM).
We thank the veterinary practitioners, Fátima García, Nacho Camps, and Aida Huertas, for their help with the fieldwork. We are also grateful to Zoraida Cervera, Maite Martín and Nuria Moya for technical assistance and F. J. Salguero for the revision of the manuscript.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Aranaz, A., L. de Juan, N. Montero, C. Sánchez, M. Galka, C. Delso, J. Álvarez, B. Romero, J. Bezos, A. I. Vela, V. Briones, A. Mateos, and L. Domínguez. 2004. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J. Clin. Microbiol. 42:2602-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow, A. M., K. A. Mitchell, and K. H. Visram. 1999. Bovine tuberculosis in llama (Lama glama) in the UK. Vet. Rec. 145:639-640. [DOI] [PubMed] [Google Scholar]
- 3.Casweel, J. L., and K. J. Williams. 2007. Respiratory system, p. 606-610. In M. G. Maxie (ed.), Jubb, Kennedy, and Palmer's pathology of domestic animals, vol. 2, 5th ed. Elsevier-Saunders, Philadelphia, PA. [Google Scholar]
- 4.Cousins, D. V., and N. Florisson. 2005. A review of tests available for use in the diagnosis of tuberculosis in non-bovine species. Rev. Sci. Tech. 24:1039-1059. [PubMed] [Google Scholar]
- 5.Cousins, D. V. 2001. Mycobacterium bovis infection and control in domestic livestock. Rev. Sci. Tech. 20:71-85. [DOI] [PubMed] [Google Scholar]
- 6.Dean, G. S., T. R. Crawshaw, R. de la Rua-Domenech, L. Farrant, R. Greenwald, R. J. Higgins, K. Lyashchenko, H. M. Vordermeier, and D. F. Twomey. 2009. Use of serological techniques for diagnosis of Mycobacterium bovis infection in a llama herd. Vet. Rec. 165:323-324. [DOI] [PubMed] [Google Scholar]
- 7.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 8.Fowler, M. E. 1998. Tuberculosis, p. 169-171. In Medicine and surgery of South American camelids (llama, alpaca, vicuña, guanaco), 2nd ed. Iowa State University Press, Ames, IA.
- 9.Frothingham, R., and W. A. Meeker-O'Connell. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144:1189-1196. [DOI] [PubMed] [Google Scholar]
- 10.Hesketh, J. B., C. G. Mackintosh, and J. F. T. Griffin. 1994. Development of a diagnostic blood test for tuberculosis in alpacas (Lama pacos). N. Z. Vet. J. 42:104-109. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, C. T., E. C. Winkler, E. Boughton, and J. W. Penfold. 1993. Mycobacterium kansasii infection in a llama. Vet. Rec. 133:243-244. [DOI] [PubMed] [Google Scholar]
- 12.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunshoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liébana, E., A. Aranaz, A. Mateos, M. Vilafranca, E. Gomez-Mampaso, J. Tercero, J. Alemany, G. Suarez, M. Domingo, and L. Dominguez. 1995. Simple and rapid detection of Mycobacterium tuberculosis complex organisms in bovine tissue samples by PCR. J. Clin. Microbiol. 33:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyashchenko, K. P., R. Greenwald, J. Esfandiari, M. Meylan, I. H. Burri, and P. Zanolari. 2007. Antibody responses in New World camelids with tuberculosis caused by Mycobacterium microti. Vet. Microbiol. 125:265-273. [DOI] [PubMed] [Google Scholar]
- 15.Naranjo, V., C. Gortázar, J. Vicente, and J. de la Fuente. 2008. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 127:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Oevermann, A., G. E. Pfyffer, P. Zanolari, M. Meylan, and Robert. 2004. Generalized tuberculosis in llamas (Lama lama) due to Mycobacterium microti. J. Clin. Microbiol. 42:1818-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattyn, S. R., F. A. Portaels, P. Kageruka, and P. Gigase. 1970. Mycobacterium microti infection in a zoo-llama, Lama vicugna (molina). Acta Zool. Pathol. Antverp. 51:17-24. [PubMed] [Google Scholar]
- 18.Rodríguez, S., B. Romero, J. Bezos, L. de Juan, J. Álvarez, E. Castellanos, N. Moya, F. Lozano, S. González, J. L. Sáez-Llorente, A. Mateos, L. Domínguez, and A. Aranaz. 2010. High spoligotype diversity within a Mycobacterium bovis population: clues to understanding the demography of the pathogen in Europe. Vet. Microbiol. 141:89-95. [DOI] [PubMed] [Google Scholar]
- 19.Thoen, C., P. Lobue, and I. de Kantor. 2006. The importance of Mycobacterium bovis as a zoonosis. Vet. Microbiol. 112:339-345. [DOI] [PubMed] [Google Scholar]
- 20.Twomey, D. F., T. R. Crawshaw, J. E. Anscombe, L. Farrant, L. J. Evans, W. S. McElligott, R. J. Higgins, G. Dean, M. Vordermeier, K. Jahans, and R. de la Rua-Domenech. 2007. TB in llamas caused by Mycobacterium bovis. Vet. Rec. 160:170. [DOI] [PubMed] [Google Scholar]
- 21.Twomey, D. F., T. R. Crawshaw, A. P. Foster, R. J. Higgins, N. H. Smith, L. Wilson, K. McDean, J. L. Adams, and R. de la Rua-Domenech. 2009. Suspected transmission of Mycobacterium bovis between alpacas. Vet. Rec. 25:121. [DOI] [PubMed] [Google Scholar]
- 22.Wernery, U., and O. R. Kaaden. 2002. Infectious diseases in camelids, p. 169-171. 2nd ed. Blackwell Science, Berlin, Germany.
- 23.Wernery, U., J. Kinne, K. L. Jahans, H. M. Vordermeier, J. Esfandiari, R. Greenwald, B. Johnson, A. Ul-Haq, and K. P. Lyashchenko. 2007. Tuberculosis outbreak in a dromedary racing herd and rapid serological detection of infected camels. Vet. Microbiol. 122:108-115. [DOI] [PubMed] [Google Scholar]
- 24.Wilton, S., and D. Cousins. 1992. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. PCR Methods Appl. 1:269-273. [DOI] [PubMed] [Google Scholar]