Abstract
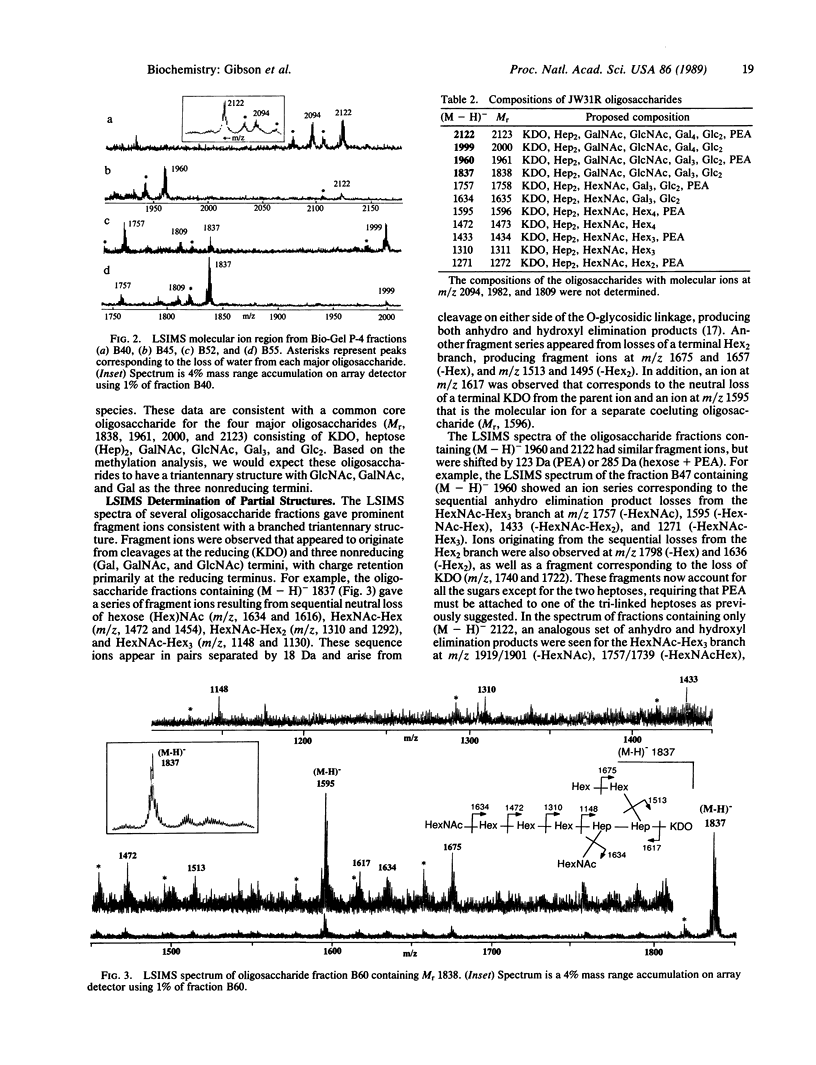
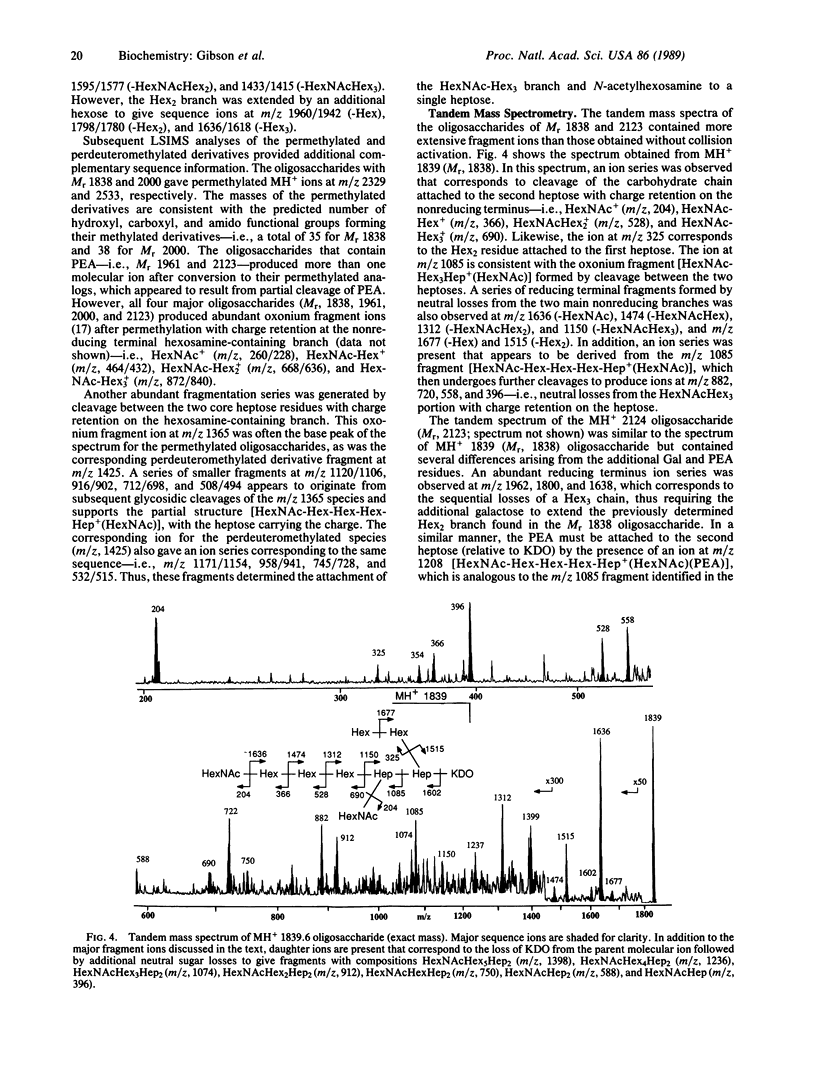
The compositions and partial structures of the oligosaccharides from the lipopolysaccharides (LPS) of a pyocin-resistant Neisseria gonorrhoeae (strain JW31R) have been determined by liquid secondary ion mass spectrometry (LSIMS), tandem mass spectrometry, and methylation analysis. Four major structures were identified with Mr 2123, 2000, 1961, and 1838, as well as seven species of lower abundance of Mr 1758-1272. The largest of the major oligosaccharides (Mr, 2122) consists of 3-deoxymanno-2-ketooctulosonic acid (KDO)-Hep2GalNAcGlcNAcGal4Glc2 (Hep, heptose) and phosphoethanolamine (PEA). The smaller oligosaccharides are truncated versions of this larger oligosaccharide. The oligosaccharides consist of a common triantennary structure containing KDO at the reducing terminus attached to a heptose disaccharide. A hexose (Hex)2-3 branch is attached to the heptose linked directly to KDO and a GalNAc-Hex3, GlcNAc, and PEA are separately attached to the second heptose. These oligosaccharides are the first structures to be determined for a gonococcal LPS and should further our understanding of the structural and antigenic diversity of these glycolipids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr S. A., Reinhold V. N., Green B. N., Hass J. R. Enhancement of structural information in FAB ionized carbohydrate samples by neutral gas collision. Biomed Mass Spectrom. 1985 Jun;12(6):288–295. doi: 10.1002/bms.1200120607. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Allen P. Z. Chemical and immunochemical studies on lipopolysaccharides from pyocin 103-sensitive and -resistant Neisseria gonorrhoeae. Carbohydr Res. 1983 Aug 16;120:171–186. doi: 10.1016/0008-6215(83)88015-x. [DOI] [PubMed] [Google Scholar]
- Crabb J. W., Armes L. G., Carr S. A., Johnson C. M., Roberts G. D., Bordoli R. S., McKeehan W. L. Complete primary structure of prostatropin, a prostate epithelial cell growth factor. Biochemistry. 1986 Sep 9;25(18):4988–4993. doi: 10.1021/bi00366a003. [DOI] [PubMed] [Google Scholar]
- Dudas K. C., Apicella M. A. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect Immun. 1988 Feb;56(2):499–504. doi: 10.1128/iai.56.2.499-504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falick A. M., Wang G. H., Walls F. C. Ion source for liquid matrix secondary ionization mass spectrometry. Anal Chem. 1986 Jun;58(7):1308–1311. doi: 10.1021/ac00298a009. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. W., Biddle J. W., Johnson S. R., Wiesner P. J. Infections due to penicillinase-producing Neisseria gonorrhoeae in the United States: 1976-1980. J Infect Dis. 1981 Aug;144(2):191–197. doi: 10.1093/infdis/144.2.191. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Glasser D., Cannon R. O., Matuszak D. L., Dunning R. W., Kline R. L., Campbell C. H., Israel E., Fauci A. S., Hook E. W., 3rd Human immunodeficiency virus infection among patients attending clinics for sexually transmitted diseases. N Engl J Med. 1988 Jan 28;318(4):197–203. doi: 10.1056/NEJM198801283180401. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]


