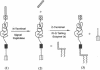Abstract
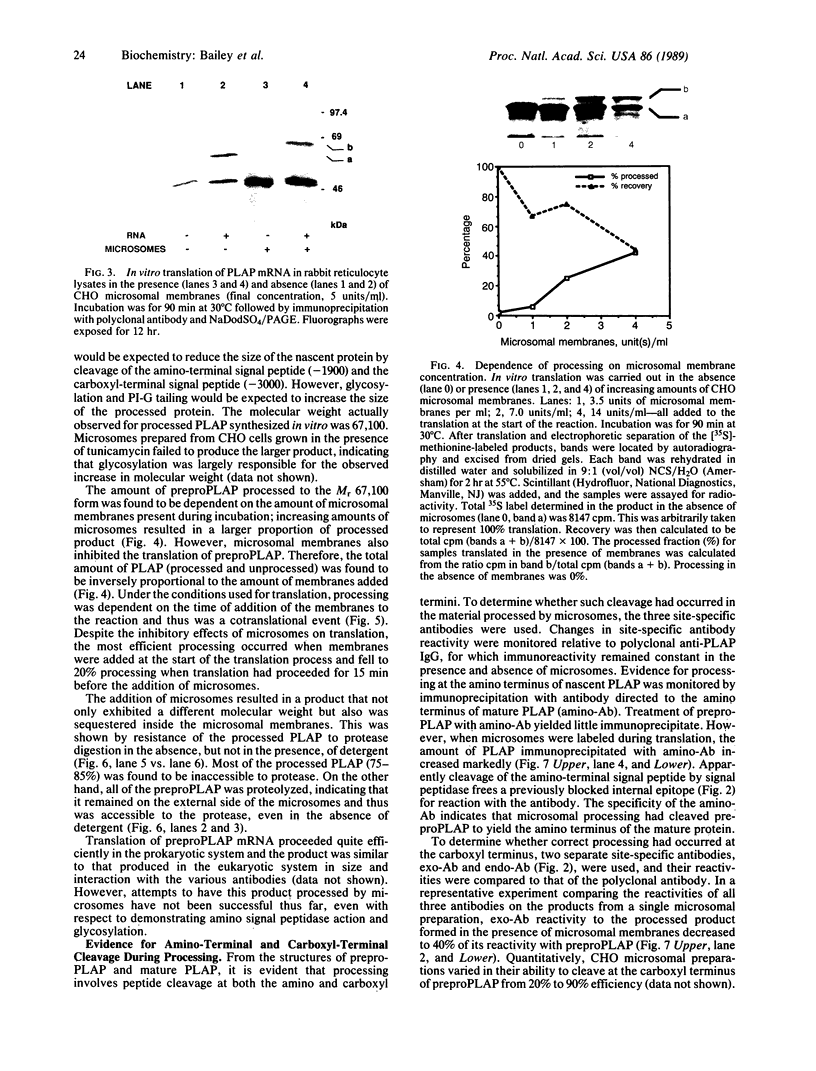
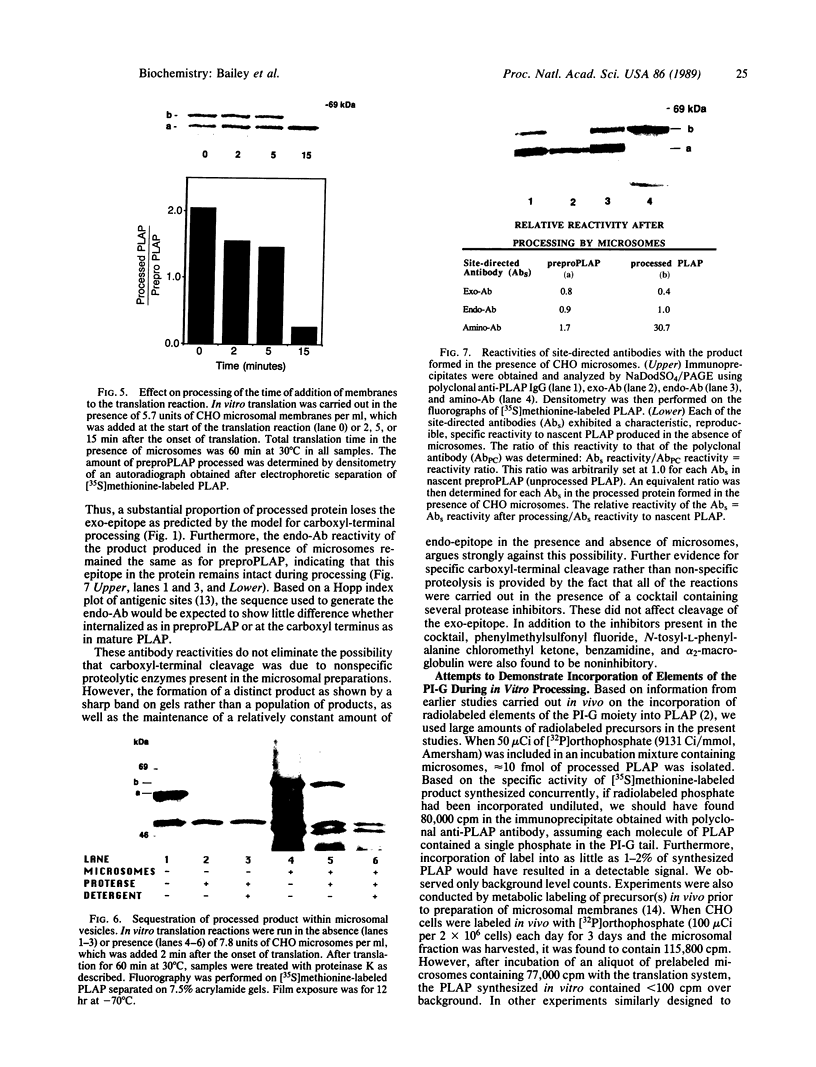
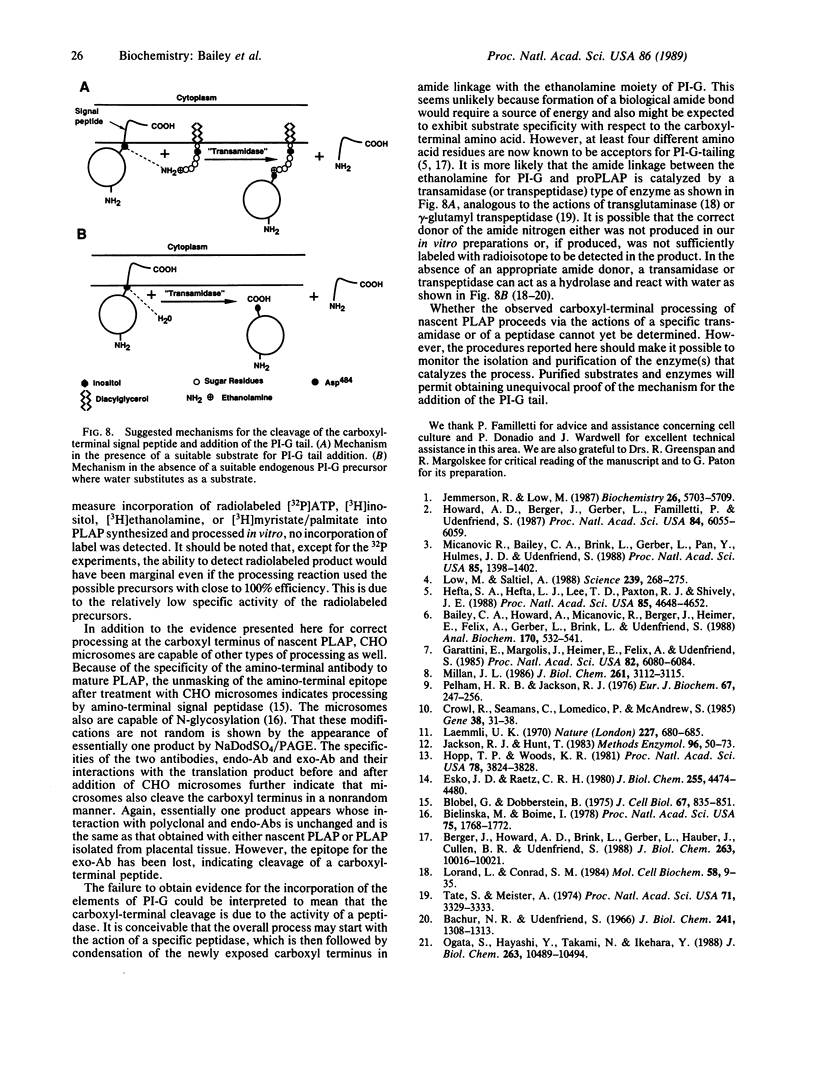
Alkaline phosphatase is anchored to the plasma membrane by a carboxyl-terminal phosphatidylinositol glycan moiety. To investigate the biosynthesis of mature alkaline phosphatase, nascent human placental alkaline phosphatase was expressed in a cell-free system and used as substrate for in vitro processing by microsomal extracts. By monitoring the processed product with three site-directed antibodies, it was shown that microsomal extracts from CHO cells that contain other recognized processing activities also remove the carboxyl-terminal signal peptide from the preproenzyme in an apparently selective manner. This peptidase-like cleavage may be brought about by the action of a specific transamidase acting on the nascent protein in the absence of an appropriate phosphatidylinositol glycan cosubstrate.
Full text
PDF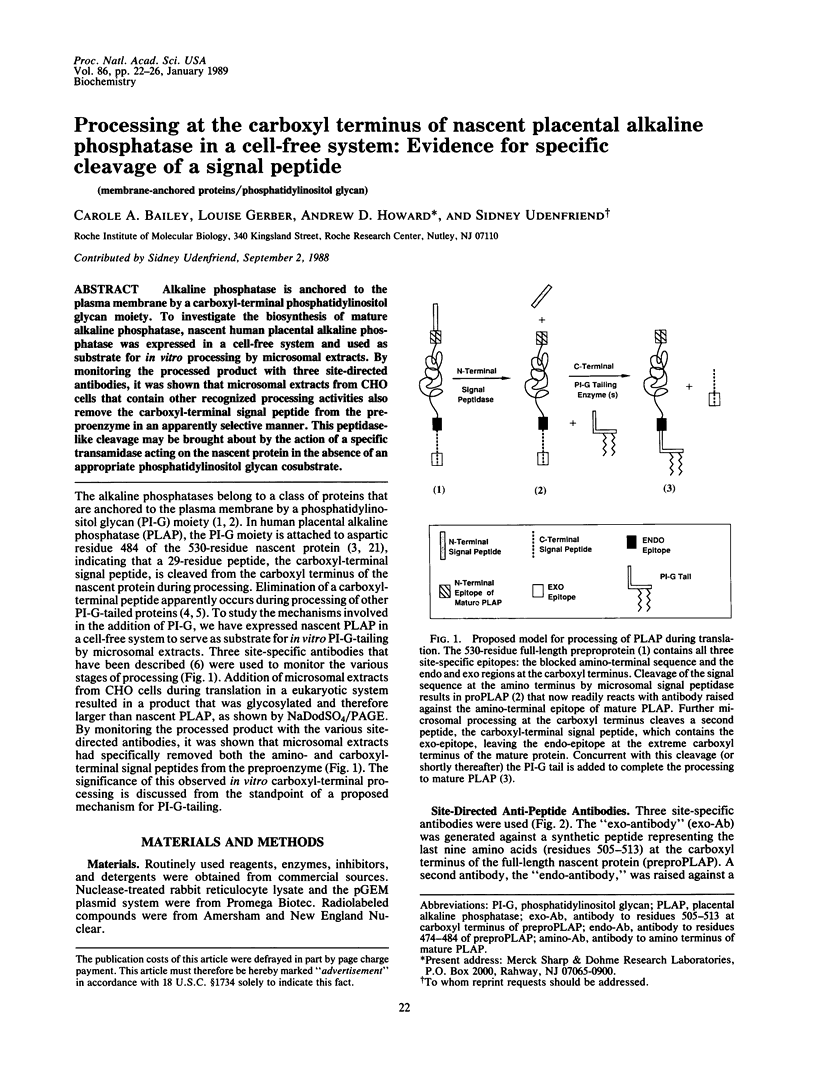

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Udenfriend S. Microsomal synthesis of fatty acid amides. J Biol Chem. 1966 Mar 25;241(6):1308–1313. [PubMed] [Google Scholar]
- Bailey C. A., Howard A., Mićanović R., Berger J., Heimer E., Felix A., Gerber L., Brink L., Udenfriend S. Site-directed antibodies for probing the structure and biogenesis of phosphatidylinositol glycan-linked membrane proteins: application to placental alkaline phosphatase. Anal Biochem. 1988 May 1;170(2):532–541. doi: 10.1016/0003-2697(88)90669-0. [DOI] [PubMed] [Google Scholar]
- Berger J., Howard A. D., Brink L., Gerber L., Hauber J., Cullen B. R., Udenfriend S. COOH-terminal requirements for the correct processing of a phosphatidylinositol-glycan anchored membrane protein. J Biol Chem. 1988 Jul 15;263(20):10016–10021. [PubMed] [Google Scholar]
- Bielinska M., Boime I. mRNA-dependent synthesis of a glycosylated subunit of human chorionic gonadotropin in cell-free extracts derived from ascites tumor cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1768–1772. doi: 10.1073/pnas.75.4.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Garattini E., Margolis J., Heimer E., Felix A., Udenfriend S. Human placental alkaline phosphatase in liver and intestine. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6080–6084. doi: 10.1073/pnas.82.18.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefta S. A., Hefta L. J., Lee T. D., Paxton R. J., Shively J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. D., Berger J., Gerber L., Familletti P., Udenfriend S. Characterization of the phosphatidylinositol-glycan membrane anchor of human placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6055–6059. doi: 10.1073/pnas.84.17.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jemmerson R., Low M. G. Phosphatidylinositol anchor of HeLa cell alkaline phosphatase. Biochemistry. 1987 Sep 8;26(18):5703–5709. doi: 10.1021/bi00392a019. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Bailey C. A., Brink L., Gerber L., Pan Y. C., Hulmes J. D., Udenfriend S. Aspartic acid-484 of nascent placental alkaline phosphatase condenses with a phosphatidylinositol glycan to become the carboxyl terminus of the mature enzyme. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1398–1402. doi: 10.1073/pnas.85.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- Ogata S., Hayashi Y., Takami N., Ikehara Y. Chemical characterization of the membrane-anchoring domain of human placental alkaline phosphatase. J Biol Chem. 1988 Jul 25;263(21):10489–10494. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of gamma-glutamyl transpeptidase by maleate; identity of a rat kidney maleate-stimulated glutaminase and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3329–3333. doi: 10.1073/pnas.71.9.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]