Abstract
Background and objectives: Although left ventricular hypertrophy (LVH) is a characteristic finding in hemodialysis (HD) populations, few risk factors for progressive LVH have been identified.
Design, setting, participants, & measurements: As part of a multinational, blinded, randomized, controlled trial that demonstrated no effect of hemoglobin targets on LV size, 596 incident HD patients, without symptomatic cardiac disease or cardiac dilation, had baseline echocardiograms within 18 months of starting dialysis and subsequently at 24, 48, and 96 weeks later. A wide array of baseline risk factors were assessed, as were BP and hemoglobin levels during the trial.
Results: The median age and duration of dialysis were 51.5 years and 9 months, respectively. LV mass index (LVMI) rose substantially during follow-up (114.2 g/m2 at baseline, 121 at week 48, 123.4 at week 48, and 128.3 at week 96), as did fractional shortening, whereas LV volume (68.7, 70.1, 68.7, and 68.1 ml/m2) and E/A ratio remained unchanged. At baseline, the only multivariate associations of LVMI were gender and N terminal pro–B type natriuretic peptide. Comparing first and last echocardiograms in those without LVH at baseline, independent predictors of increase in LVMI were higher time-integrated systolic BP and cause of ESRD. An unadjusted association between baseline LVMI and subsequent cardiovascular events or death was eliminated by adjusting for age, diabetes, systolic BP, and N terminal pro–B type natriuretic peptide.
Conclusions: Progressive concentric LVH and hyperkinesis occur in HD patients, which is partly explained by hypertension but not by a wide array of potential risk factors, including anemia.
Seventy-five percent of patients who start dialysis have left ventricular hypertrophy (LVH) (1). Both concentric hypertrophy, a result of LV pressure overload, and eccentric hypertrophy, a result of LV volume overload, may occur (2). Cardiomyopathy at baseline predisposes to subsequent heart failure and to earlier death (2). Progressive LVH is characteristic of the dialysis state (3,4) and has been associated with the development of de novo heart failure (5). A reduction in LV mass index (LVMI) has been linked with an improvement in all-cause and cardiovascular survival (6). In addition, LVH is associated with QT prolongation, which may predispose to sudden death (7).
Hypertension and anemia therapy have been identified as risk factors for LVH (8–11). Nonetheless, incident hemodialysis (HD) patients without symptomatic cardiac disease exhibited progressive LVH regardless of whether moderate anemia was treated (4). Other potential risk factors for progressive LVH in HD patients include age, gender, race, diabetes, primary renal disease, body mass index (BMI), dialysis vintage, hypertension, interdialytic weight gain, LV volume overload, type of vascular access (12), and perhaps inflammation markers, some of which predict sudden death in incident HD patients (13). These factors were assessed in 596 incident HD patients, without symptomatic cardiac disease or overt cardiac dilation, who had serial echocardiograms during 2 years of follow-up in a multinational, blinded, randomized, controlled trial (4). The objectives of this study were to quantify (1) the associations between traditional and nontraditional candidate variables and change in LV size (the main objective) and (2) the associations between LV size and cardiovascular outcomes.
Materials and Methods
The design, methods, randomization, and epoetin dosing protocols have been reported previously (4). Patients were randomly assigned to one of the following hemoglobin (Hb) targets: 9.5 to 11.5 (low target) or 13.5 to 14.5 g/dl (high target). Patients were masked to treatment assignment, as were their doctors; however, doctors had access to clinic Hb values when treating the patients. Mean Hb levels at the end of the initial 24-week titration phase were 13.3 and 10.9 g/dl, respectively. During the maintenance phase, from weeks 24 through 96, corresponding mean Hb levels were 13.1 and 10.8 g/dl. Cardiac structure constituted the primary study outcome, and no difference was observed between the two groups (4). Consequently, for the purpose of this article, all patients were included as one cohort. The study was centrally coordinated from St. John's, Canada, for Canadian patients and Manchester, England, for European patients.
Study Population
Inclusion criteria were as follows: Age ≥18 years; inception of maintenance HD within the previous 3 to 18 months; predialysis Hb level between 8 and 12 g/dl; LV volume index (LVVI) <100 ml/m2, and predialysis diastolic BP (DBP) <100 mmHg. Exclusion criteria were as follows: Clinical evidence or history of symptomatic cardiac failure or ischemic heart disease; daily prednisone dosages ≥10 mg; medical conditions that are likely to reduce epoetin responsiveness, including uncorrected iron deficiency; concurrent malignancy; blood transfusion in the preceding month; therapy with cytotoxic agents; seizure in the preceding year; hypersensitivity to intravenous iron; and current pregnancy or breastfeeding.
Description of Study Procedures
Laboratory tests were measured centrally by Quest Diagnostics (Van Nuys, CA, and Heston, UK). Hb was measured weekly for 24 weeks and biweekly thereafter. With the high target, the treatment goal was increments of 0.5 to 1.0 g/dl every 2 weeks, until achieving stability between 13.5 and 14.5 g/dl. Other treatment goals included predialysis DBP between 70 and 90 mmHg, urea reduction ratio >67%, and transferrin saturation ≥20%. On a weekly basis, midweek predialysis BP levels were communicated weekly to and treatment recommendations sent from the coordinating center. These were determined by the protocols in use at the individual treating centers, and a single, formal, standardized method was not imposed. For this study, weekly mean BPs were averaged over time. The last patient completed the study in May 2003.
Echocardiograms
Echocardiograms were performed at baseline and at 24, 48, and 96 weeks after study start. Standard echocardiographic recordings were performed within 1 kg of dry weight, usually within 24 hours after the midweek dialysis. Dry weight was the optimal postdialysis weight for BP control without symptoms of blood volume contraction. American Society of Echocardiography criteria (14) were used, and echocardiograms were read by an independent central reader, Cardialysis. LV volume, in milliliters, was calculated as 0.001047 (LV end-diastolic diameter mm)3 (15). LVM, in grams, was calculated as 0.00083 × [(end-diastolic diameter in mm + interventricular septum thickness in mm + posterior wall thickness in mm)3 − (end-diastolic diameter)3 + 0.6] (16). LVVI and LVMI were calculated as LV volume/M2 body surface area and LVM/M2 body surface area, respectively (17).
Composite Cardiovascular/Death Outcome
From the serious adverse events database, the following outcomes were identified: Myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, pulmonary edema, cerebellar infarction, cerebral hemorrhage, and cerebral vascular disorder. Time to first cardiovascular event or death was calculated, as was time to first cardiovascular event and time to death.
Risk Factors
In addition to demographic factors (age, gender, and race), baseline clinical characteristics (diabetes, primary renal disease, dialysis vintage, BP, BMI, type of vascular access) were recorded, as were conventional laboratory tests results (Hb, percentage of iron saturation, urea reduction ratio, and serum albumin) and other serologic tests at baseline. Serum concentrations of N terminal pro–B type natriuretic peptide (BNP) and cardiac troponin T were measured using diagnostic kits and performed on an Elecsys 2010 immunochemistry analyzer (Roche Diagnostics, Montreal, Québec, Canada). Serum high-sensitivity C-reactive protein was measured using CRPH reagent hit (Beckman Coulter, Fullerton, CA) and performed on an IMMAGE Immunochemistry system (Beckman Coulter). Serum IL-6 was measured using IL-6 kits and performed on a Vincel DxI 800 Access Immunoassay system (Beckman Coulter). Midweek interdialytic weight gain was measured 2 weeks after enrollment in the study. Midweek predialysis BP levels were communicated weekly to and treatment recommendations were sent from the coordinating center. These were determined by the protocols in use at the individual treating centers, and a single, formal, standardized method was not imposed. For this study, weekly mean BP were averaged over time.
Sample Size Estimate
The sample size needed to detect a 15% difference between treatment groups in the primary outcome (LVVI) was calculated as 166 per treatment group, given a two-tailed significance of 0.05, a power of 0.90, and an SD of the percentage change in LVVI of 42%. With an expected dropout rate of 40%, primarily as a result of transplantation, 277 patients were required for each treatment group. Fifty-seven percent (n = 339) of 596 patients who enrolled in the trial had an echocardiogram after 72 weeks of follow-up.
Statistical Analysis
Baseline characteristics were described by n (%) or by median with 25th and 75th percentiles. Linear regression (for continuous variables) and logistic regression (for binary variables) were used to evaluate associations of echocardiographic measurements. Cox regression was used to evaluate baseline associations of the composite cardiovascular/death outcome. Regarding echocardiographic and clinical outcomes, two modeling strategies were used: Model 1 adjusted for age and gender; and model 2 included all variable from model 1 with P < 0.05, as well as age and gender. In each of these models, parameter estimates for continuous variables were computed for intervals corresponding to half the interquartile range. Because some patients underwent echocardiography only at baseline, all analysis of longitudinal echocardiographic outcomes were repeated using the conservative strategy of carrying forward the first observation; outcomes were not meaningfully changed with this sensitivity analysis, and parameter estimates are not reported here. P < 0.05 was used throughout to indicate statistical significance.
Results
A total of 596 patients were enrolled in 95 treatment centers in 10 countries between February 2000 and June 2001. Table 1 shows baseline characteristics. Median age was 51.5 years, 39.6% were female, and the vast majority were white. Only 17.8% had diabetes as the cause of ESRD, and patients with symptomatic cardiac disease were excluded. Incident patients who started dialysis between 3 and 18 months before study entry were enrolled, and median time on dialysis was 9 months. The vast majority (84.2%) had a fistula as vascular access.
Table 1.
Baseline characteristics and serial BP values
| Characteristic | Value |
|---|---|
| Demographic characteristics (n = 596) | |
| age (years; median [25th to 75th percentiles]) | 51.5 (39 to 62) |
| female (%) | 39.6 |
| nonwhite race (%) | 10.6 |
| Clinical characteristics (n = 596) | |
| renal disease (%) | |
| glomerulonephritis (%) | 28.7 |
| diabetes (%) | 17.8 |
| hypertension (%) | 8.1 |
| polycystic kidney disease (%) | 9.1 |
| other/unknown (%) | 36.4 |
| dialysis duration (months; median [25th to 75th percentiles]) | 9 (6 to 14) |
| dialysis access (%) | |
| fistula | 84.2 |
| graft | 5.5 |
| catheter | 10.2 |
| assigned to high Hb target (%) | 49.7 |
| epoetin dosage (U/wk; median [25th to 75th percentiles]) | 6000 (4000 to 8000) |
| BMI (kg/m2; median [25th to 75th percentiles]) | 25.5 (22.6 to 29.3) |
| SBP (mmHg; median [25th to 75th percentiles]) | 143 (130 to 155) |
| DBP (mmHg; median [25th to 75th percentiles]) | 80 (72 to 90) |
| interdialytic weight gain (kg; median [25th to 75th percentiles])a | 2.1 (1.4 to 2.8) |
| Routine laboratory tests (median [25th to 75th percentiles]; n = 596) | |
| Hb (g/dl) | 11.1 (10.3 to 1.8) |
| serum albumin (g/L) | 4.0 (3.8 to 4.1) |
| urea reduction ratio (%)b | 67.0 (59.9 to 72.5) |
| Specialized laboratory tests (median [25th to 75th percentiles]; n = 487) | |
| BNP (mg/ml) | 288 (135 to 647) |
| Troponin T (ng/ml) | 0.02 (0.01 to 0.05) |
| C-reactive protein (mg/L) | 3.47 (1.47 to 7.75) |
| IL-6 (pg/ml) | 4.38 (2.67 to 8.68) |
| Echocardiographic parameters (median [25th to 75th percentiles]; n = 596) | |
| LVMI (g/m2) | 108 (91 to 135) |
| LVVI (ml/m2) | 70 (53 to 84) |
| fractional shortening (%) | 36 (30 to 41) |
| Serial BP, first to last echocardiogram (median [25th to 75th percentiles]; n = 524) | |
| SBP (mmHg) | 140 (132 to 150) |
| DBP (mmHg) | 80 (74 to 86) |
Interdialytic weight gain was missing for 13 patients.
Urea reduction ratio was missing for 43 patients.
Serial Echocardiograms
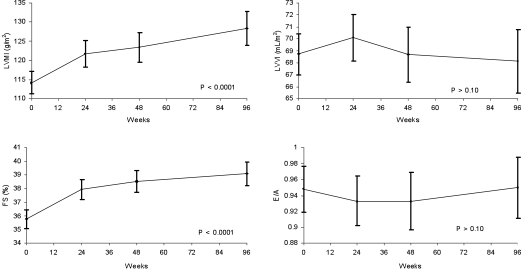
Progressive LVH was observed during 96 weeks of follow-up (Figure 1). Mean LVMI was 114 g/m2 at baseline, 121 at 24 weeks, 123 at 48 weeks, and 128 at 96 weeks (P < 0.0001 for trend). Mean LVVI was unchanged over time: 68 ml/m2 at baseline, 70 at 24 weeks, 69 at 48 weeks, and 68 at 96 weeks. Comparable values for fractional shortening demonstrated an increase during follow-up: 36, 38, 39, and 39%, respectively (P < 0.0001 for trend). E/A ratio, an index of LV diastolic function, was unchanged (0.95, 0.93, 0.93, and 0.95, respectively).
Figure 1.
Echocardiographic results at baseline and at 24, 48, and 96 weeks after entry into the study. FS, fractional shortening.
Baseline Associations
After adjustment for age and gender, the following variables were associated with higher baseline LVMI: Male gender, lower BMI, higher systolic (SBP) and DBP, lower Hb, lower serum albumin, higher BNP, and higher Troponin T (Table 2). In the expanded multivariate model, only male gender and higher BNP were associated with higher LVMI (Table 2).
Table 2.
Associations between baseline risk factors and baseline LVMI, LVVI, and fractional shortening
| Parameter | Increment or Reference Category | Unadjusted (β [SE]) | P | Adjusted, Model 1 (β [SE]) | P | Model 2 (β [SE]) | P |
|---|---|---|---|---|---|---|---|
| LVMI | |||||||
| female | Male | −9.28 (2.86) | <0.01 | −9.35 (2.86) | <0.01 | −10.52 (2.95) | <0.01 |
| BMI | 1.5 kg/m2 | −1.02 (0.38) | <0.01 | −1.04 (0.39) | <0.01 | ||
| SBP | 13.5 mmHg | 3.01 (0.90) | <0.01 | 2.69 (0.90) | <0.01 | ||
| DBP | 10.0 mmHg | 2.95 (1.11) | <0.01 | 3.14 (1.13) | <0.01 | ||
| Hb | 0.4 g/dl | −1.00 (0.46) | 0.03 | ||||
| serum albumin | 0.1 g/dl | −0.98 (0.46) | 0.03 | −1.17 (0.47) | 0.01 | ||
| BNP | 76 ng/ml | −0.93 (0.12) | <0.01 | 0.93 (0.11) | <0.01 | 0.87 (0.13) | <0.01 |
| Troponin T | 0.005 ng/ml | 0.26 (0.10) | <0.01 | 0.20 (0.10) | 0.04 | ||
| LVVI | |||||||
| female | Male | −3.60 (1.76) | 0.04 | −3.54 (1.76) | 0.04 | ||
| hypertensive renal disease | GN | −7.86 (3.43) | 0.02 | −7.63 (3.53) | 0.03 | −8.01 (3.62) | 0.02 |
| dialysis duration | 1.5 months | −0.58 (0.26) | 0.03 | −0.56 (0.30) | 0.03 | −0.61 (0.28) | 0.04 |
| catheter | Fistula | −7.02 (2.84) | 0.01 | −6.22 (2.87) | 0.03 | ||
| BMI | 1.5 kg/m2 | −1.10 (0.23) | <0.01 | −1.07 (0.24) | <0.01 | −0.81 (0.29) | <0.01 |
| interdialytic weight gain | 0.7 kg | −1.22 (0.58) | 0.04 | −1.40 (0.59) | 0.02 | ||
| BNP | 76 ng/ml | 0.33 (0.08) | <0.01 | 0.33 (0.08) | <0.01 | 0.31 (0.08) | <0.01 |
| Fractional shortening | |||||||
| age | 6.3 years | 0.34 (0.14) | 0.02 | 0.32 (0.14) | 0.02 | ||
| female | Male | 2.16 (0.70) | <0.01 | 2.11 (0.70) | <0.01 | 2.02 (0.78) | <0.01 |
| diabetic renal disease | GN | 2.41 (1.04) | 0.02 | ||||
| polycystic kidney disease | GN | 2.60 (1.31) | 0.05 | ||||
| BNP | 76 ng/ml | −0.07 (0.03) | 0.04 | −0.06 (0.03) | 0.04 | −0.08 (0.03) | <.01 |
| Troponin T | 0.005 ng/ml | 0.05 (0.02) | 0.04 | 0.06 (0.02) | 0.04 | 0.07 (0.03) | <0.01 |
Parameter estimates for continuous variables were computed for intervals equal to one half the interquartile range. All of the variables shown in Table 1 were considered; only those with the P < 0.05 are shown. Model 1 adjusted for age and gender; model 2 adjusted for age, gender, and variables with P < 0.05 in model 1. GN, glomerulonephritis.
After adjustment age and gender, the following variables were associated with lower baseline LVVI: Female gender, hypertension as cause of ESRD, having a dialysis catheter, lower dialysis vintage, and lower BMI (Table 2). High BNP was associated with higher LVVI. In the expanded multivariate model, independent associations included hypertension as cause of ESRD, dialysis duration, BMI, and BNP (Table 2). Independent associations with fractional shortening were female gender, BNP, and Troponin T levels (Table 2).
LV Growth
A total of 524 patients had sequential determinations of LVMI; comparing first and last studies, LVM increased in 326 (62.6%) patients. With binary logistic regression (Table 3), unadjusted associations of an increase in LVMI included female gender, “other” renal disease, longer dialysis vintage, time-integrated SBP, and lower BNP levels. Age- and gender-adjusted significant predictors of increased LVMI on last echocardiogram compared with baseline included male gender, longer dialysis vintage, high time-integrated SBP, and lower BNP (Table 3). The expanded multivariate model revealed persistent associations with higher LVMI for the last three variables.
Table 3.
Associations among baseline risk factors; serial BP values; and changes in LVMI, LVVI, and fractional shortening between first and last echocardiograms
| Parameter | Increment or Reference Category | Unadjusted (OR [95% CI]) | P | Adjusted, Model 1 (OR [95% CI]) | P | Adjusted, Model 2 (OR [95% CI]) | P |
|---|---|---|---|---|---|---|---|
| ΔLVMI >0 g/m2 (326/524) | |||||||
| female gender | Male | 0.69 (0.48 to 0.99) | 0.05 | ||||
| “other” renal disease | GN | 1.58 (1.01 to 2.47) | 0.04 | 1.69 (1.06 to 2.68) | 0.03 | 1.72 (1.02 to 2.90) | 0.04 |
| months on dialysis | 1.5 months | 1.06 (1.01 to 1.12) | 0.03 | 1.06 (1.00 to 1.12) | 0.05 | ||
| serial SBP | 4.1 mmHg | 1.07 (1.02 to 1.13) | <0.01 | 1.06 (1.01 to 1.12) | 0.02 | 1.09 (1.03 to 1.15) | <0.01 |
| BNP | 76 ng/ml | 0.98 (0.96 to 1.00) | 0.01 | 0.98 (0.96 to 1.00) | 0.01 | 0.98 (0.96 to 1.00) | 0.01 |
| ΔLVMI >26.8 g/m2 (174/524)a | |||||||
| age | 6.3 years | 1.09 (1.01 to 1.17) | 0.03 | 1.09 (1.01 to 1.18) | 0.02 | 1.09 (1.01 to 1.18) | 0.02 |
| serial SBP | 4.1 mmHg | 1.08 (1.02 to 1.13) | <0.01 | 1.06 (1.01 to 1.12) | 0.02 | 1.06 (1.01 to 1.12) | 0.02 |
| New LVH (122/300) | |||||||
| female gender | Male | 1.84 (1.11 to 3.03) | 0.02 | 1.84 (1.11 to 3.05) | 0.02 | 2.23 (1.30 to 3.84) | <0.01 |
| serial SBP | 4.1 mmHg | 1.08 (1.01 to 1.16) | 0.03 | 1.08 (1.01 to 1.16) | 0.03 | ||
| ΔLVVI >0 ml/m2 (258/524) | |||||||
| female gender | Male | 0.65 (0.45 to 0.94) | 0.02 | ||||
| diabetes | GN | 0.56 (0.33 to 0.95) | 0.03 | 0.52 (0.30 to 0.91) | 0.02 | 0.51 (0.29 to 0.90) | 0.02 |
| months on dialysis | 1.5 months | 1.07 (1.01 to 1.12) | 0.01 | 1.07 (1.01 to 1.12) | 0.02 | 1.06 (1.01 to 1.12) | 0.02 |
| high target Hb | Low | 0.70 (0.50 to 0.99) | 0.05 | 0.68 (0.48 to 0.98) | 0.04 | ||
| ΔLVVI >9.6 ml/m2 (177/524)a | |||||||
| urea reduction ratio | 6.3% | 0.90 (0.81 to 1.00) | 0.05 | 0.90 (0.81 to 1.00) | 0.05 | ||
| ΔFS <0 (203/522) | |||||||
| age | 6.3 years | 1.14 (1.06 to 1.23) | <0.01 | 1.14 (1.06 to 1.22) | <0.01 | 1.12 (1.04 to 1.21) | <0.01 |
| catheter | Fistula | 1.82 (1.03 to 3.21) | 0.04 | ||||
| BMI | 1.5 kg/m2 | 1.08 (1.03 to 1.13) | <0.01 | 1.06 (1.01 to 1.11) | 0.02 | 1.06 (1.01 to 1.11) | 0.02 |
| urea reduction ratio | 6.3% | 0.88 (0.79 to 0.98) | 0.02 | ||||
| ΔFS <−1.8% (174/522)b | |||||||
| age | 6.3 years | 1.12 (1.05 to 1.20) | <0.01 | 1.12 (1.04 to 1.20) | <0.01 | 1.11 (1.04 to 1.20) | <0.01 |
| BMI | 1.5 kg/m2 | 1.05 (1.00 to 1.10) | 0.04 | ||||
| albumin | 0.1 | 0.94 (0.89 to 0.99) | 0.03 | ||||
| urea reduction ratio | 6.3% | 0.88 (0.79 to 0.98) | 0.02 | 0.88 (0.79 to 0.98) | 0.02 | 0.88 (0.79 to 0.98) | 0.02 |
Parameter estimates for continuous variables were computed for intervals equal to one half the interquartile range. All of the variables shown in Table 1 were considered; only those with the P < 0.05 are shown. Model 1 adjusted for age and gender; model 2 adjusted for age, gender, and variables with P < 0.05 in model 1. Δ, last value minus first value; GN, glomerulonephritis; FS, fractional shortening; OR, odds ratio.
Highest tertile of change between first and last echocardiograms.
Lowest tertile of change between first and last echocardiograms.
Because it was possible that the association between higher BNP and higher LVMI at baseline may have biased the association between BNP and LVMI during follow-up, we performed a post hoc analysis restricted to patients without LVH at baseline (n = 345; 87% with repeat echocardiograms during the trial). With the expanded multivariate modeling approach, high time-integrated SBP and other disorder as cause of ESRD were associated with an increase in LVMI. The adjusted odds ratio for SBP was 1.22 per 9-mm interval (half the interquartile range; 95% confidence interval [CI] 1.05 to 1.43; P = 0.008) and for other disorder 2.11 (95% CI 1.07 to 4.17; P = 0.03) compared with glomerulonephritis. Independent associations for the highest tertile of LV growth were older age and higher time-integrated SBP (Table 3).
LV Volume Increase
A total of 524 patients had sequential determinations of LVVI; comparing first and last studies, LV volume increased in 258 (49.2%) patients. With unadjusted binary logistic regression (Table 3), longer dialysis vintage was associated with an increase in LVVI, and diabetes as renal disease was associated with a decrease in LVVI. Baseline associations of increase in LVVI on last echocardiogram included longer dialysis vintage and being allocated to the low Hb group. In addition, diabetes as cause of ESRD had an odds ratio of 0.68 (Table 3). None of the biomarkers was associated with increase in LVVI.
The only association identified for the highest tertile of change in LVVI between first and last echocardiogram was low urea reduction ratio (Table 3). Independent associations for the lowest tertile of change between first and last echocardiogram were older age and lower urea reduction ratio (Table 3).
Clinical Outcomes
The composite cardiovascular event/death outcome occurred in 99 patients during the trial. Age was a significant predictor of these events. With age and gender adjustment, diabetes as cause of ESRD, low baseline Hb, high baseline epoetin dosage, low serum albumin, high SBP, high LVMI, and high BNP all were associated with the composite outcome (Table 4). With expanded multivariate modeling, LVMI was not among the associations identified, which were as follows: Age, diabetes, high SBP, and high BNP (Table 4).
Table 4.
Associations between baseline risk factors and cardiovascular events and death
| Parameter | Increment or Reference Category | Unadjusted (HR [95% CI]) | P | Adjusted, Model 1 (HR [95% CI]) | P | Adjusted, Model 2 (HR [95% CI]) | P |
|---|---|---|---|---|---|---|---|
| Cardiovascular event or death (99/596) | |||||||
| LVMI | 21.8 g/m2 | 1.13 (1.00 to 1.27) | 0.04 | 1.14 (1.01 to 1.29) | 0.03 | ||
| age | 6.3 years | 1.24 (1.13 to 1.35) | <0.01 | 1.23 (1.13 to 1.35) | <0.01 | 1.24 (1.11 to 1.39) | <0.01 |
| diabetic renal disease | GN | 2.85 (1.54 to 5.26) | <0.01 | 1.90 (1.00 to 3.61) | 0.05 | 2.31 (1.04 to 5.14) | 0.04 |
| “other” renal disease | GN | 1.91 (1.07 to 3.42) | 0.03 | ||||
| catheter | Fistula | 1.73 (1.01 to 2.97) | 0.05 | ||||
| epoetin dosage | 1000 U | 1.05 (1.01 to 1.09) | <0.01 | 1.06 (1.02 to 1.10) | <0.01 | ||
| SBP | 13.5 mmHg | 1.16 (1.02 to 1.32) | 0.02 | 1.16 (1.02 to 1.32) | 0.02 | 1.19 (1.02 to 1.39) | 0.03 |
| Hb | 0.4 g/dl | 0.92 (0.87 to 0.98) | 0.01 | 0.92 (0.86 to 0.98) | 0.01 | ||
| serum albumin | 0.1 g/dl | 0.90 (0.85 to 0.95) | <0.01 | 0.91 (0.86 to 0.97) | <0.01 | ||
| BNP | 76 ng/ml | 1.03 (1.01 to 1.04) | <0.01 | 1.03 (1.02 to 1.05) | <0.01 | 1.03 (1.01 to 1.05) | <0.01 |
| Troponin T | 0.005 ng/ml | 1.01 (1.00 to 1.02) | 0.02 | ||||
| Cardiovascular event (78/596) | |||||||
| LVMI | 21.8 g/m2 | 1.15 (1.00 to 1.31) | 0.05 | ||||
| fractional shortening | 5.5% | 0.87 (0.76 to 1.00) | 0.05 | ||||
| age | 6.3 years | 1.29 (1.17 to 1.43) | <0.01 | 1.29 (1.16 to 1.42) | <0.01 | 1.33 (1.17 to 1.52) | |
| diabetic renal disease | GN | 2.99 (1.48 to 6.04) | <0.01 | ||||
| “other” renal disease | GN | 2.03 (1.04 to 3.95) | 0.04 | ||||
| epoetin dosage | 1000 U | 1.05 (1.00 to 1.10) | 0.03 | 1.06 (1.01 to 1.10) | 0.02 | ||
| SBP | 13.5 mmHg | 1.25 (1.08 to 1.44) | <0.01 | 1.25 (1.08 to 1.44) | <0.01 | 1.30 (1.09 to 1.55) | <0.01 |
| serum albumin | 0.1 g/dl | 0.91 (0.85 to 0.97) | <0.01 | 0.93 (0.87 to 1.00) | 0.05 | ||
| BNP | 76 ng/ml | 1.02 (1.00 to 1.04) | 0.03 | ||||
| Death (33/596) | |||||||
| age | 6.3 years | 1.19 (1.03 to 1.38) | 0.02 | 1.19 (1.02 to 1.37) | 0.02 | ||
| diabetic renal disease | GN | 3.03 (1.03 to 8.86) | 0.04 | ||||
| catheter | Fistula | 3.84 (1.81 to 8.16) | <0.01 | 2.58 (1.03 to 6.44) | 0.04 | ||
| epoetin dosage | 1000 U | 1.08 (1.02 to 1.15) | 0.01 | 1.09 (1.02 to 1.15) | <0.01 | ||
| Hb | 0.4 g/dl | 0.83 (0.75 to 0.93) | <0.01 | ||||
| serum albumin | 0.1 g/dl | 0.83 (0.77 to 0.90) | <0.01 | 0.84 (0.77 to 0.91) | <0.01 | 0.89 (0.79 to 1.00) | 0.05 |
| BNP | 76 ng/ml | 1.04 (1.03 to 1.06) | <0.01 | 1.05 (1.03 to 1.07) | <0.01 | 1.03 (1.01 to 1.05) | 0.01 |
| Troponin T | 0.005 ng/ml | 1.01 (1.00 to 1.02) | <0.01 | 1.01 (1.00 to 1.02) | 0.02 | ||
| C-reactive protein | 1 mg/L | 1.02 (1.00 to 1.04) | 0.01 | 1.02 (1.01 to 1.04) | <0.01 |
Parameter estimates for continuous variables were computed for intervals equal to one half the interquartile range. All of the variables shown in Table 1 were considered, except serial BP levels; only those with P < 0.05 are shown. Model 1 adjusted for age and gender; model 2 adjusted for age, gender, and variables with P < 0.05 in model 1. GN, glomerulonephritis; FS, fractional shortening; HR, hazard ratio.
Analysis of associations for cardiovascular events and death separately revealed that high SBP was strongly predictive of cardiovascular events (hazard ratio 1.30) and was not predictive of death (Table 4). Independent predictors of death were catheter as vascular access, hypoalbuminemia, and high BNP. Time-dependent analysis, with death as the outcome and the conditional event being occurrence of a cardiovascular event after the baseline examination, revealed that the mortality hazard ratio for the cardiovascular event was 3.8 (95% CI 1.9 to 7.6)
Discussion
The key findings in this prospective study of patients without symptomatic cardiac disease after initiation of HD were that (1) progressive concentric LVH occurred; (2) higher SBP during the study was associated with LV growth, whereas anemia and several other potential risk factors were not; and (3) higher SBP was associated with subsequent cardiovascular events.
Several variables were associated with higher baseline LVMI, but only male gender and BNP (a marker of volume overload/LV distension) retained their association in expanded multivariate models. Subsequent LV growth was associated with months on dialysis before study entry, higher time-integrated SBP, and lower BNP. It is likely that the association between lower BNP and increase in LVMI was biased by the fact that at baseline, those with higher LVMI had higher BNP levels. In the analysis of those without LVH at baseline, BNP was not associated with increase in LVMI, whereas high SBP during follow-up was associated. Furthermore, LV growth was not explained by vascular access, anemia, markers of volume overload or of inflammation, or interdialytic weight gain.
Failure to associate LV growth during the study with low Hb levels is consistent with aggregated results from multiple studies that suggested that erythropoietin treatment of severe anemia to a Hb target of approximately 11 g/d is associated with a reduction in LVMI, but in patients with moderate anemia (Hb >10 and <12 g/dl), normalization of Hb does not have a beneficial impact on LVMI (4,11). Of interest in this report is that randomization to the low target Hb group was an independent significant predictor of increase in LV volume.
It is possible that LV growth may result from stimuli that were not assessed in this study, including neurohumeral activation, LV pressure overload from noncompliant vessels as a result of arteriosclerosis, and stimuli independent of LV overload. Certainly, sympathetic overactivity occurs in dialysis patients (18), LVH has been linked to large vessel geometry and function (19), and activation of signaling pathways may contribute to LVH (20). Several signaling pathways could have a role in this process, such as the mammalian target of rapamycin (mTOR) pathway. Siedlecki et al. (21) created normotensive chronic kidney disease using a mouse model in which kidney parenchyma was resected so as to avoid excessive renin-angiotensin system activation. Progressive cardiac hypertrophy and fibrosis developed, associated with de novo protein synthesis and activation of the mTOR pathway. Administrating of rapamycin, which inhibits the mTOR pathway, prevented cardiac hypertrophy (19).
Baseline associations of the composite cardiovascular/death outcome included older age, diabetes, higher SBP, and higher BNP. The relationship between hypertension and subsequent cardiovascular events is consistent with a study that identified hypertension as a risk factor for mortality after 2 years in incident HD patients (22). The BNP association with death is consistent with the report that BNP is an antecedent association of mortality in chronic kidney disease (23). Of interest, in the current study higher LVMI in isolation predated cardiovascular events, but in the expanded multivariate model, its predictive power was negated by older age and higher SBP. This suggests that the adverse risk indicated by these variables may partly occur through the adverse impact of LVH.
One of the limitations of this study is that, by design, it excluded patients with preexisting cardiac disease. Nonetheless, it demonstrated that even in this group of relatively healthy patients, progressive LV growth occurred. Another limitation is study power. Although we enrolled 596 incident HD patients, the number of cardiovascular events (n = 78) and of deaths (n = 33) was not high and the proportion with some risk factors was low (e.g., those without fistula). A third limitation is the use of predialysis BP as a measure of hypertension, particularly because interdialytic BP may be a better measure of pressure overload.
Conclusions
Progressive LVH occurs in HD patients, is partly explained by hypertension, but is not related to moderate anemia or to a wide array of baseline risk factors.
Disclosures
P.S.P. has received research support and is an academic advisor to Ortho Biotech, Amgen, and Roche, companies that make erythropoietin products. He had full access to all of the data in the study and had final responsibility for the decision to submit for publication; R.N.F. has received research support and honoraria from Affymax, Amgen, Ortho Biotech, and Roche; and B.C. has received support from Ortho Biotech.
Acknowledgments
P.S.P. and R.N.F., the co-principal investigators, designed the trial, applied for funding to Johnson and Johnson, coordinated the study, and wrote the report.
Members of the Canadian European Study Group are as follows. EP)-INT-68 Independent Data Monitoring committee members: L.J. Wei, Boston, MA; M.-M. Samama, Paris, France; P. Ivanovich, Chicago, IL; M.A. Pfeffer, Boston, MA. Principal investigator/site: W. Hoerl, Wien, Austria; H.-K. Stummvoll, Linz, Austria; G. Mayer, Innsbruck, Austria; H. Graf, Wien, Austria; H. Holzer, Graz, Austria; Y. Vanrenterghem, Leuven, Belgium; W. Lornoy, Aalst, Belgium; C. Tielemans, Bruxelles, Belgium; M. Jadoul, Bruxelles, Belgium; P. Parfrey, St. John's, Canada; P. Barre, Montréal, Canada; A. Levin, Vancouver, Canada; P. Cartier, Montréal, Canada; N. Muirhead, London, Canada; A. Fine, Winnipeg, Canada; B. Murphy, Calgary, Canada; S. Handa, St. John's, Canada; P. Campbell, Edmonton, Canada; V. Pichette, Montréal, Canada; S. Tobe, Toronto, Canada; C. Lok, Toronto, Canada; D. Kates, Kelowna, Canada; D. Holland, Kingston, Canada; G. Karr, Penticton, Canada; G. Pylpchuk, Saskatoon, Canada; G. Wu, Mississauga, Canada; M. Vasilevsky, Montreal, Canada; E. Carlisle, Hamilton, Canada; E.R. Gagne, Fleurimont, Canada; W. Callaghan, Windsor, Canada; G. Soltys, Greenfield Park, Canada; P. Tam, Scarborough, Canada; R. Turcot, Trios-Rivieres, Canada; M. Berrall, Toronto, Canada; J. Zacharias, Winnipeg, Canada; S. Donnelly, Toronto, Canada; G. London, Fleury-Merogis, France; A. London, Aulnay sous Bois, France; F.P. Wambergue, Lille, France; H. Geiger, Frankfurt, Germany; V. Kliem, Hann Muenden, Germany; R. Winkler, Rostock, Germany; B. Kraemer, Regensburg, Germany; H. Schiffl, Munich, Germany; R. Brunkhorst, Hannover, Germany; D. Seybold, Bayreuth, Germany; M. Hilfenhaus, Langenhagen, Germany; D. Schaumann, Hameln, Germany; R. Goetz, Bad Windsheim, Germany; P. Roch, Regensburg, Germany; R. Goetz, Bad Windsheim, Germany; P. Roch, Regensburg, Germany; H.-P. Brasche, Ludwigshafen, Germany; V. Wizeemann, Giessen, Germany; K. Bittner, Ansback, Germany; K. Appen, Hamburg, Germany; B. Schroeder, Bad Toelz, Germany; W. Schropp, Munich, Germany; D. O'Donoghue, Salford, England; I. MacDougall, London, England; G. Warwick, Leicester, England; M. Raftery, London, England; K. Farrington, Stevenage, England; J. Kwan, Carshalton, England; P. Conlon, Dublin, Ireland; G. Mellotte, Dublin, Ireland; K. Siamopoulos, Ioannina, Greece; N. Tsaparas, Crete, Greece; D. Tsakiris, Veria, Greece; V. Vargemezis, Alexandroupolis, Greece; S. Ferenczi, Gyor, Hungary; S. Gorogh, Kisvarda, Hungary; I. Kulcsar, Szombathely, Hungary; L. Locsey, Debrecen, Hungary; K. Akocsi, Veszprem, Hungary; I. Solt, Szekesfehervar, Hungary; O. Arkossy, Budapest, Hungary; E. Kiss, Szeged, Hungary; J. Manitius, Bydgoszcz, Poland; B. Rutkowski, Gdansk, Poland; A. Wiecek, Katowice, Poland; W. Sulowicz, Krakow, Poland; A. Ksiazek, Lublin, Poland; S. Czekalski, Poznan, Poland; M. Klinger, Wroclaw, Poland; M. Mysliwiec, Bialystok, Poland; H. Augustyniak-Bartosik, Milicz, Poland; J. Imeila, Warszaqa-Miedzylesie, Poland; A. Sydor, Tarnow, Poland; R. Rudka, Bytom, Poland; M. Kiersztejn, Chrzanow, Poland; R. Wnuk, Oswiecim, Poland; A. Milkowsk, Krakow, Poland; F. Valderrabano, Madrid, Spain; P. Aljama, Córdoba, Spain; H. Alcocer, Valencia, Spain; A. Purroy, Pamplona, Spain.
We are grateful to Janet Morgan in Canada and to Aileen Foley in England for coordinating patient enrollment and treatment.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186– 192, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277– 1285, 1996 [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int 54: 1720– 1725, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D: Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16: 2180– 2189, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 11: 912– 916, 2000 [DOI] [PubMed] [Google Scholar]
- 6.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metvier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol 12: 2759– 2767, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Stewart GA, Gansevoort, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG: Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int 67: 217– 226, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS: Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol 4: 1486– 1490, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379– 1385, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 28: 53– 61, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P: Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: A meta-analysis. Clin J Am Soc Nephrol 4: 755– 762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon LP, Parfrey PS: Cardiovascular aspects of chronic kidney disease. In: Brenner and Rector's The Kidney, 8th Ed., edited by Brenner BM.Philadelphia, Saunders Elsevier, 2008, pp 1697– 1727 [Google Scholar]
- 13.Parekh RS, Platinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klap MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335– 1342, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Sahn DJ, DeMaria A, Kisslo J, Weyman A: Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 58: 1072– 1083, 1978 [DOI] [PubMed] [Google Scholar]
- 15.Pombo JF, Troy BL, Russell RO, Jr: Left ventricular volumes and ejection fraction by echocardiography. Circulation 43: 480– 490, 1971 [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB: Detection of left ventricular hypertrophy by M-mode echocardiography: Anatomic validation, standardization, and comparison to other methods. Hypertension 9: II9– II26, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Lin CL, Hsu PY, Yang CT, Yang HY, Yang TY, Huang WH, Huang CC: LV mass index significantly impacts on patient and renal outcomes in patients with coronary artery bypass grafting and poor left-ventricular function. Ren Fail 25: 287– 295, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Rump LC, Amann K, Orth S, Ritz E: Sympathetic overeactivity in renal disease: A window to understand progression and cardiovascular complications of uraemia? Nephrol Dial Transplant 15: 1735– 1738, 2000 [DOI] [PubMed] [Google Scholar]
- 19.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, Metivier F: Cardiac and arterial interactions in end-stage renal disease. Kidney Int 50: 600– 608, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ritz E: Left ventricular hypertrophy in renal disease: Beyond preload and afterload. Kidney Int 75: 771– 773, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Siedlecki AM, Jin X, Muslin AJ: Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int 75: 800– 808, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, Bedrick EJ, Meyer KB, Johnson HK, Zager PGMedical Directors of Dialysis Clinic Inc.: Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17: 513– 520, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LSCreed Investigators: Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol 12: 1508– 1515, 2001 [DOI] [PubMed] [Google Scholar]