Abstract
Background and objectives: Recurrence of the original kidney disease after renal transplantation is an increasingly recognized cause of allograft loss. Idiopathic membranous nephropathy (iMN) is a common cause of proteinuria that may progress to ESRD. It is known that iMN may recur after kidney transplantation, causing proteinuria, allograft dysfunction, and allograft loss. Limited data regarding the frequency and treatment of recurrent iMN are available.
Design, setting, participants, & measurements: In this single-center study, all patients who had iMN and were receiving a first kidney transplant were included. We retrospectively assessed the incidence of biopsy-confirmed recurrent iMN and compared clinical characteristics of patients with and without recurrence. In addition, the effect of treatment with rituximab on proteinuria and renal allograft function in patients with recurrent iMN was examined
Results: The incidence of recurrent iMN was 44%, and recurrences occurred at a median time of 13.6 months after transplantation. Two patterns of recurrence were identified: Early and late. No predictors of recurrence or disease progression could be identified. Treatment with rituximab was effective in four of four patients in stabilizing or reducing proteinuria and stabilizing renal function.
Conclusions: Recurrence of iMN is common even in the era of modern immunosuppression. Rituximab seems to be a valuable treatment option for these patients, although lager studies are needed to confirm our data.
Recurrent disease is considered to be the third most common cause of progressive renal failure in kidney transplant patients (1,2). Idiopathic membranous nephropathy (iMN) is a leading cause of nephrotic syndrome in adults and progresses to ESRD in 30 to 40% of patients despite intensive treatment protocols (3). Recurrence of iMN has been reported to occur in 7 to 42% of patients in various series, resulting in reduced allograft survival (1,4–13). Recurrence tends to occur early in the posttransplantation period, and male patients with hypertension and heavy proteinuria in the native kidney are considered to be at increased risk for recurrence (6,7). Limited data are available on the treatment of recurrent iMN. Dabade et al. (14) reported that conservative therapy with tight BP control and standard transplantation immunosuppression failed to prevent increasing proteinuria in the majority of their patients. We evaluated the incidence of recurrent iMN using both clinical and histopathologic data in patients who received a transplant during a 15-year period at the Columbia University Medical Center (CUMC). Furthermore, because treatment of iMN using rituximab, a chimeric anti-CD20 mAb, has shown encouraging results in the native kidney (15,16), we used rituximab to treat a limited number of patients with recurrent disease and examined its effect on proteinuria and allograft function compared with previous conventional therapy.
Materials and Methods
Patient Data
A total of 1821 renal transplantations, 1669 of which were first transplants, were performed at the CUMC between 1992 and 2008. iMN was identified as the primary cause of ESRD in 34 of these patients. The diagnosis was confirmed by review of the native kidney biopsies and/or review of renal biopsy reports when original biopsy slides were not available. Patients with known secondary causes of MN (from systemic lupus erythematosus, hepatitis B virus, hepatitis C virus, tumor, or medications) were excluded. Clinical and laboratory information was obtained from the electronic database and charts at the CUMC. This study was approved by the institutional review board of the CUMC.
The protocol for immunosuppressive therapy changed during the course of this study. Induction therapy consisted of anti-CD25 mAbs, anti-CD3 antibodies, or antithymocyte globulin, and maintenance immunosuppression consisted of various combinations of corticosteroids, mycophenolate mofetil or azathioprine, calcineurin inhibitors (CNI; cyclosporine or tacrolimus), or sirolimus. In addition, plasmapheresis and rituximab were included in the treatment protocol for ABO-incompatible transplants and highly sensitized patients with elevated donor-specific or panel-reactive antibodies.
Indications for renal allograft biopsy included a rise in serum creatinine of >0.2 mg/dl, onset of proteinuria, or protocol biopsy in patients who received an ABO-incompatible transplant. All renal biopsies were processed according to standard techniques for light microscopy and immunofluorescence (IF) microscopy. Electron microscopy (EM) was performed in selected cases when recurrent glomerular disease was suspected (heavy proteinuria or positive IF staining). For each case, 11 glass slides were prepared and stained with hematoxylin and eosin, periodic acid-Schiff, trichrome, and Jones methenamine silver. IF was performed on 3-μm cryostat sections using polyclonal FITC-conjugated antibodies to IgG, IgM, IgA, C3, C1q, κ, λ, fibrinogen, and albumin (Dako Corp., Carpinteria, CA). Ultrastructural evaluation was performed using JEOL100S/1010 electron microscopes (Tokyo, Japan).
All patients received a similar conservative treatment regimen that included loop diuretics if they had edema, hepatic hydroxymethyl glutaryl–CoA reductase inhibitor if they had hyperlipidemia, and an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker if tolerated. A low-sodium diet (<4 g/d NaCl) was recommended for patients with edema. Rituximab, when given, was dosed either according to the “lymphoma protocol,” which consists of four weekly doses of 375 mg/m2, or the “autoimmunity protocol,” which consists of two doses of 1 g 2 weeks apart. To minimize infusion reactions to rituximab, patients were premedicated with acetaminophen (1000 mg), diphenhydramine hydrochloride (50 mg), and methylprednisolone (100 mg). Treatment decisions were left to the treating physician's discretion.
Statistical Analysis
Data are expressed as mean ± SD (unless otherwise specified). The χ2 test was used to compare proportions: t test when normally distributed parameters were evaluated and nonparametric tests when normally distributed parameters were compared. Kaplan-Meier plots were constructed to assess recurrence over time. Multivariate analysis was performed using the Cox proportional hazards model. Statistical significance was assumed at P < 0.05.
Results
Patient Characteristics
Characteristics of the 34 transplant patients with iMN are summarized in Table 1. The diagnosis of iMN was made 8.52 ± 5.51 years before transplantation, and the mean time to ESRD was 7.63 ± 5.88 years. Nineteen of the 34 patients were treated on average for 12.6 months with hemodialysis and/or peritoneal dialysis before transplantation. A total of 20.6% of patients received a kidney from a deceased donor, 55.9% from a living-related donor, and 23.5% from a living-unrelated donor (Table 1).
Table 1.
Characteristics of patients who had iMN and received kidney transplant with or without recurrence of iMN
| Variable | All Patients | Recurrent iMN | No Recurrence | P |
|---|---|---|---|---|
| No. of patients | 34 | 15 | 19 | |
| Age at diagnosis of MN | 34.79 ± 12.55 | 36.99 ± 14.12 | 33.06 ± 11.26 | NS |
| Age at renal transplantation | 43.06 ± 13.06 | 44.53 ± 14.15 | 42.04 ± 12.43 | NS |
| Gender (female/male) | 15/19 | 7/8 | 8/11 | NS |
| Race (white/black/Hispanic) | 21/3/10 | 8/1/6 | 13/2/4 | NS |
| Immunosuppressive therapy for iMN | 23 | 9 | 14 | NS |
| Time from MN to ESRD (years) | 7.63 ± 5.88 | 7.38 ± 5.08 | 7.82 ± 6.59 | NS |
| Dialysis before transplantation | 18 | 7 | 11 | NS |
| Time on dialysis (months) | 18.25 ± 20.48 | 5.69 ± 8.61 | 24.17 ± 24.54 | NS |
| Type of organ (LRRT/LURT/CRT) | 19/18/7 | 10/4/1 | 9/4/6 | NS |
| Induction immunosuppression (%) | 71 | 60 | 83 | NS |
| Rituximab treatment | 0 | 4 | 0 | |
| Follow-up after transplantation (months) | 62.89 ± 51.74 | 70.02 ± 60.16 | 57.25 ± 44.91 | NS |
NS = P > 0.05. LRRT, living-related renal transplant; LURT, living-unrelated renal transplant; CRT, cadaveric renal transplant.
Sixty-five percent of patients received induction therapy with either antithymocyte globulin or anti-CD25 antibodies, and maintenance immunosuppression consisted of various combinations as mentioned already. After transplantation, all patients were regularly seen in our transplant center with a median follow-up of 50 months (range 4.5 to 196.6 months).
Diagnosis of Recurrent iMN
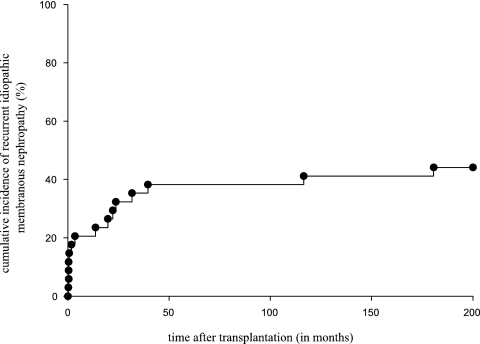
Fifteen (44%) of the 34 patients developed recurrent iMN, diagnosed at a median time of 13.6 months (range 0.1 to 180.6 months) after renal transplantation (Figure 1). Six patients' recurrent iMN was diagnosed within the first month after transplantation (“early recurrence”), with the earliest recurrence noted on day 6 after transplantation. In that patient, a postreperfusion biopsy did not show histologic evidence of MN, arguing against donor-transmitted disease.
Figure 1.
Cumulative incidence of recurrent iMN was constructed using Kaplan-Meier plots.
Table 1 summarizes the baseline characteristics of renal transplant patients with and without recurrence of iMN. Those with recurrent disease underwent dialysis for a shorter period of time, but this trend did not reach statistical significance. The mean serum creatinine was 1.93 ± 0.85 mg/dl with a mean blood urea nitrogen level of 31 ± 9 mg/dl at the time of diagnosis of recurrent disease. Twelve (80%) patients had proteinuria (defined as ≥2+ on dipstick, >150 mg/24 h, or a urine protein/urine creatinine ratio of >200 mg/g), and eight patients had nephrotic-range proteinuria (Table 2). Three patients without proteinuria had a recurrence of iMN diagnosed on kidney biopsy performed for protocol reasons and/or suspicion of allograft rejection. Among the six patients who were discovered to have recurrent iMN on biopsies performed for graft dysfunction alone, urine protein was trace (n = 2), 380 mg/g creatinine; two patients had 2+ protein on dipstick, which was quantified as 670 and 1320 mg/g creatinine, respectively; and one patient had 3+ protein on dipstick, which was not quantified. Of the seven patients whose recurrence was diagnosed within the first 6 months after transplantation, four (57%) had significant proteinuria. In all 15 patients, the mean serum total protein, serum albumin, and serum total cholesterol were 5.8 ± 1.6 g/dl, 3.5 ± 0.7 g/dl, and 250 ± 130 mg/dl, respectively. Three patients had a serum albumin level of <3 mg/dl. At the time of diagnosis, there was no significant difference in serum creatinine or GFR among the patients with and without recurrent disease. As expected, serum total protein and albumin were lower and proteinuria higher in patients with recurrent disease (Table 2). Using multiple regression analysis, no factors that could predict the development of recurrent iMN were identified.
Table 2.
Presentation of recurrent iMN compared with data for patients without recurrent disease (12-month data)
| Variable | Patients with Recurrent iMN | Patients without Recurrence | Pa |
|---|---|---|---|
| Serum creatinine (mg/dl) | 1.93 ± 0.85 | 1.71 ± 0.59 | NS |
| Serum BUN (mg/dl) | 30.9 ± 9.2 | 27.6 ± 13.0 | NS |
| Estimated GFR (ml/min per 1.73 m2)b | 44.27 ± 18.34 | 46.38 ± 15.17 | NS |
| Serum total protein (g/dl) | 5.8 ± 1.6 | 7.2 ± 0.4 | <0.001 |
| Serum albumin (g/dl) | 3.5 ± 0.7 | 4.5 ± 0.4 | <0.001 |
| Serum cholesterol (mg/dl) | 250 ± 130 | 194 ± 52 | NS |
| Proteinuria (g/24 h or g/g creatinine) | 6.18 ± 2.77 or 2.70 ± 1.98 | 0.44 ± 0.60 | <0.001 |
NS = P > 0.05. BUN, blood urea nitrogen.
Values obtained at diagnosis of recurrence were compared with values obtained 1 year after transplantation in patients without recurrence.
The GFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula.
Histology of Recurrent iMN
The biopsy findings in the 15 patients with recurrent iMN are summarized in Table 3. Among the 15 patients with recurrent iMN, indications for allograft biopsy included graft dysfunction (n = 6), graft dysfunction and nephrotic-range proteinuria (n = 3), nephrotic-range proteinuria alone (n = 5), and protocol biopsy in the patient who underwent an ABO-incompatible transplant (n = 1). Among the nine patients who had recurrent iMN and graft dysfunction, allograft biopsy also revealed acute cellular rejection (n = 4), antibody-mediated rejection (n = 1), CNI toxicity (n = 1), acute tubular necrosis (n = 1), thrombosis (n = 1), or no specific changes (n = 1). Among the other six patients with stable graft function, one protocol biopsy of an ABO-incompatible graft showed isolated C4d staining, four showed no other pathologic alteration, and one showed pathologic features of acute cellular rejection (i.e., subclinical acute cellular rejection). Subepithelial deposits were identified by light microscopy in nine of 15 cases, and well-established basement membrane spikes were identified in seven cases (Ehrenreich-Churg stage 2 MN) (17). Three of the 15 biopsies had no subepithelial deposits or spikes by light microscopy but demonstrated granular IgG (±C3) staining by IF (stage 1 MN). All six cases of recurrent iMN diagnosed within the first month after transplantation were stage 1. The typical coarse granular deposition of IgG along the glomerular basement membrane was present in 14 of the 15 cases by IF. Complement (C3) deposition was noted in 10 of the 15 cases. EM was available for nine cases and demonstrated podocyte foot process fusion and subepithelial electron-dense deposits. In total, eight cases were diagnosed as stage I MN and seven as stage II MN. Interstitial fibrosis and tubular atrophy of ≥15% was present in seven of the 15 biopsies.
Table 3.
Histologic features in biopsies from patients with recurrent iMN
| Patient | Days after Tx | Indication for Biopsy | UP | GD | Light Microscopy |
IF |
Electron Microscopy |
Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glomeruli | Spikes | Deposits | F/TA (%) | IgG | C3 | IgA | IgM | C1q | SEDD | FPF/FPE (%) | TRI | ||||||
| 1 | 6 | Protocola | Neg | No | 17 | No | Yes | 0 | 2+ | 1+ | 0 | 0 | 0 | 1+ | 40 | 0 | 1 |
| 2 | 13 | GD | <0.5 | Yes | 3 | No | No | 0 | 1 to 2+ | 0 | 0 | 0 | 0 | 1+ | 95 | 0 | 1 |
| 3 | 11 | GD | 2+ | Yes | 18 | No | No | 0 | 2 to 3+ | 0 to 1+ | 0 | 0 | 0 | ND | ND | ND | 1 |
| 4 | 19 | GD | 3+ | Yes | 13 | No | Yes | 15 | 1+ | 1+ | 0 | 1+ | 0 | ND | ND | ND | 1 |
| 5 | 19 | GD | 380 mg | Yes | 17 | No | No | <5 | 2+ | 0 | 0 | 0 | 0 | ND | ND | ND | 1 |
| 6 | 53 | GD | 2+ | Yes | 24 | No | No | <5 | 3+ | 0+ | 0 | 0 | 0 | ND | ND | ND | 1 |
| 7 | 104 | GD | Trace | Yes | 5 | Yes | No | 50 | 2 to 3+ | 0 to 1+ | 0 | 0 | 0 | 3+ | 90 | 0 | 1 |
| 8 | 414 | GD/NRP | NRP | Yes | 12 | Yes | Yes | 15 | 3+ | 1+ | 0 | 0 | 0 | ND | ND | ND | 2 |
| 9 | 599 | GD/NRP | NRP | Yes | 12 | No | Yes | <5 | 3+ | 1+ | 0 | 0 | 0 | 1+ | 100 | 0 | 1 |
| 10 | 674 | NRP | NRP | No | 5 | Yes | Yes | ND | 3+ | 1+ | 0 | 0 | 0 | 2+ | 100 | 0 | 2 |
| 11 | 721 | GD/NRP | NRP | Yes | 9 | Yes | Yes | 20 | 3+ | 1 to 2+ | 0 | 0 | 0 | ND | ND | ND | 2 |
| 12 | 963 | NRP | NRP | No | 35 | Yes | No | 15 | 2 to 3+ | 1+ | 0 | 0 | 0 | 1 to 2+ | 95 | 0 | 2 |
| 13b | 1203 | NRP | NRP | No | 14 | Yes | Yes | 15 | 0 | 0 | 0 | 0 to 1+ | 0 | 1 | 30 | 0 | 2 |
| 14 | 3545 | NRP | NRP | No | 14 | No | Yes | 15 | 3+ | 1+ | 0 | 0 | 0 | 1+ | 90 | 0 | 2 |
| 15 | 5497 | NRP | NRP | No | 12 | Yes | Yes | 10 | 2 to 3+ | 1+ | 0 | 0 | 0 | 1 | Mild | 0 | 2 |
FPF/FPE, foot process fusion/foot process effacement; F/TA, fibrosis and tubular atrophy; GD, graft dysfunction; ND, not done; NRP, nephrotic-range proteinuria; SEDD, subepithelial dense deposits; TRI, tubuloreticular inclusions; Tx, transplantation; UP, urinary protein.
Patient underwent ABO-incompatible renal transplantation.
IF negative probably because of the segmental nature of the MN in this patient, which was also noted in his native biopsy.
Treatment of Recurrent iMN
After the diagnosis of recurrent iMN, 11 patients were treated with tight BP control (target systolic BP <130 mmHg achieved in all patients) and blockade of the renin-angiotensin-aldosterone system in addition to the routine posttransplantation maintenance immunosuppression. Three patients received high-dosage corticosteroid therapy, one of whom responded with complete remission of the nephrotic syndrome and one of whom had a partial remission. The one patient who did not respond to corticosteroid therapy had focal segmental sclerosing lesions on biopsy. One patient received oral cyclophosphamide for 3 months and had a partial remission of proteinuria. Two patients reached ESRD and subsequently underwent second renal transplants without development of proteinuria; they have not had repeat biopsies to determine whether they could still have biopsy proof of recurrence despite no clinical evidence.
Effect of Treatment with Rituximab on Recurrent iMN
Because of recent reports demonstrating beneficial effects of rituximab in iMN, four patients received treatment with this agent without any definitive drug-related adverse effects (patients 8 through 11). The characteristics of these patients are summarized in Table 4. The dosage of rituximab administered was determined by the treating physician. Patient 8 received 4 weekly doses of 375 mg/m2. Patients 10 and 11 received two doses of 1 g with a 2-week interval. Patient 9 received only one dose of rituximab (1 g) because of malaise and gastroenteritis that developed 1 week after the dose, although these symptoms were unlikely to be drug related. Depletion of B cells was documented in all four patients who were treated with rituximab. Using FACS analysis, no B cells were detected for up to 6 months after rituximab administration except in the patient who received only one dose of rituximab, in whom B cells were undetectable at 3 months but reappeared at 6 months after the infusion.
Table 4.
Patients who had recurrent iMN and received rituximab treatment: Patient characteristics at the time of rituximab administration
| Variable | Patient 8 | Patient 9 | Patient 10 | Patient 11 |
|---|---|---|---|---|
| Time since renal transplantation | 20 months | 20 months | 36 weeks | 15.2 years |
| Time since diagnosis of recurrent MN | 6 months | 5 days | 34 weeks | 3 weeks |
| BP (mmHg) | 130/88 | 150/90 | 130/80 | 130/90 |
| Edema | 2 to 3+ | Trace | Trace | 0 |
| Immunosuppressive regimen | Tacrolimus, MMF | Tacrolimus, azathioprine | Tacrolimus MMF | Cyclosporine, azathioprine, prednisone |
| ARB | Losartan | Losartan | Candesartan | |
| Diuretics | Furosemide | |||
| Statins | Simvastatin | Atorvastatin | ||
| Others | Amlodipine, allopurinol, testosterone, propranolol, gabapentin, epoietin alfa | ASA, calcium carbonate, labetalol | Amlodipine, ASA, nadolol |
ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; MMF, mycophenolate mofetil.
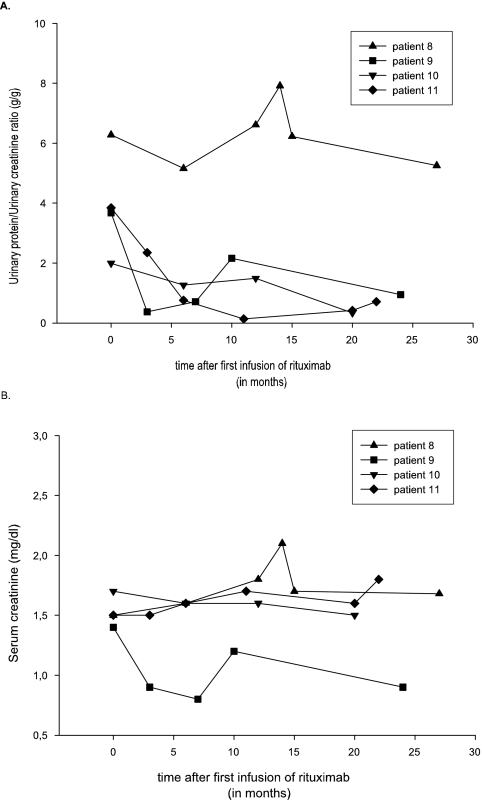
Figure 2 displays the effect on proteinuria (Figure 2A) and serum creatinine (Figure 2B) in the four patients after rituximab therapy (patients 8 through 11). After the first course of rituximab, proteinuria worsened in patient 8, stabilized in patient 10, and improved in patients 9 and 11. There was no significant change in kidney function after infusion of rituximab. A second course of rituximab was given to patients 8 and 10 because of worsening or only stabilization of proteinuria at 11 and 13 months after the initial therapy. Patient 10 received two additional infusions of 1 g, and patient 8 received four additional infusions of 375 mg/m2. Patient 8 had a 30% improvement in proteinuria with stable renal function 16 months after the last administration of rituximab, and patient 10 had stable kidney function and proteinuria was reduced to near-normal levels (0.3 g/g creatinine) 7 months after the last administration of rituximab.
Figure 2.
Effect of rituximab treatment on the urine protein/urine creatinine ratio (A) and serum creatinine (B).
Outcome of Patients with Recurrent iMN
Table 5 summarizes the characteristics at the end of the follow-up period for patients with and without recurrent disease (and patients who were treated with rituximab). Five patients with recurrent iMN after renal transplantation developed ESRD: One patient developed thrombotic microangiopathy after sepsis, and one patient experienced hypovolemic shock after transplant biopsy, requiring a transplant nephrectomy. Two patients progressed to ESRD as a result of iMN alone. The former two patients received a second renal transplant without clinical signs of recurrence at last follow-up (45.5 ± 7.4 months after transplantation). One patient developed severe acute cellular and antibody-mediated rejection as a result of medication noncompliance. The repeat renal allograft biopsies that were performed on this patient showed no progression of recurrent iMN. Of the patients with recurrent disease, one patient died during follow-up 26 months after transplantation (the cause of death was unknown).
Table 5.
Outcome after renal transplantation in patients with and without recurrent iMN at the end of follow-up
| Variable | Patients with Recurrent iMN (n = 15) |
Patients without Recurrence (n = 19) | Pa | |
|---|---|---|---|---|
| All Patients with Recurrent iMN (n = 15) | Rituximab Treatment (n = 4) | |||
| Patients died during follow-up | 1 | 0 | 2 | NS |
| Patients progressed to ESRD | 5 | 0 | 2 | NS |
| Serum creatinine (mg/dl)b | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.8 ± 1.1 | NS |
| Serum BUN (mg/dl)b | 30.23 ± 10.30 | 38.00 ± 6.38 | 29.44 ± 11.51 | NS |
| Estimated GFR (ml/min per 1.73 m2)b | 51.8 ± 12.2 | 45.8 ± 2.4 | 47.7 ± 20.0 | NS |
| Serum total protein (g/dl) | 6.6 ± 0.6 | 6.4 ± 0.5 | 7.0 ± 0.5 | <0.05 |
| Serum albumin (g/dl) | 3.9 ± 0.5 | 4.1 ± 0.4 | 4.3 ± 0.4 | 0.01 |
| Serum cholesterol (mg/dl) | 182 ± 43 | 196 ± 51 | 196 ± 53 | NS |
| Proteinuria (g/24 h or g/g creatinine) | 1.17 ± 1.83 | 1.80 ± 2.31 | 0.27 ± 0.25 | NS |
| Length of follow-up (months; mean ± SE) | 73.08 ± 18.87 | 80.50 ± 41.32 | 56.67 ± 9.32 | NS |
NS = P > 0.05.
Statistical tests were performed on total patients with recurrence compared with patients without recurrence if iMN after renal transplantation.
Includes only the patient who did not develop ESRD.
Among the patients without recurrence, two died during follow-up (one of posttransplantation lymphoma and one from unknown causes). Two patients progressed to ESRD, one of whom underwent a second renal transplantation. One patient developed nephrotic-range proteinuria as a result of FSGS (which improved with therapy over time). At the end of follow-up, proteinuria was higher and serum total protein and albumin were lower in the patients with recurrent disease, but there was no difference in serum creatinine or GFR between the two groups.
On both univariate and multivariate analysis, only black race was associated with allograft failure (Cox regression P = 0.003) and death (Cox regression P = 0.021) after transplantation. Recurrent iMN was not a predictor of allograft failure or patient survival.
Discussion
The reported recurrence rate of iMN ranges between 7 and 57% (1,4–13). In our study, the recurrence rate was 44%. In our study, as opposed to the study by Dabade et al. (14), surveillance allograft biopsies were not performed; therefore, early recurrence (without proteinuria) may have been missed, so the true incidence of recurrence of MN may be even higher. However, in patients with early recurrence (and absence of proteinuria), iMN was diagnosed on biopsies that were performed per protocol or to differentiate rejection from CNI toxicity.
Our data suggest two patterns of recurrence: An “early recurrence” (within the first 6 months after transplantation) with limited proteinuria and a “late recurrence” with overt nephrotic syndrome. The patients with early recurrence, in accordance with the findings in the study of Dabade et al. (14), had minor or absent manifestations of glomerular disease despite characteristic pathologic findings of MN. In contrast, nephrotic-range proteinuria was usually encountered in the patients who experienced later recurrences. One patient's recurrent iMN was diagnosed >15 years after renal transplantation. With the recent discovery of the phospholipase-A2 receptor as a potential inciting antigen in iMN (18), studies to evaluate its value to predict and detect recurrence after renal transplantation may clarify whether recurrences are truly “idiopathic” and/or related to similar antigens.
Two patients who had recurrent iMN and experienced graft failure received second renal transplants without clinical signs of recurrence, so they did not undergo allograft biopsies. This may suggest other causative factors that do not represent genetic or inherent predispositions for recurrent disease. In the patients who did not experience recurrent MN, two died with stable graft function and two developed graft loss as a result of chronic allograft nephropathy.
The impact of iMN on renal survival remains unclear. Most studies have not demonstrated an adverse effect of iMN on renal survival (1,8,19); however, Cosyns et al. (4) showed a higher incidence of allograft loss after the onset of the nephrotic syndrome; the outcome of recurrence was poor because the actual rate of graft loss among patients with recurrent disease was 38 and 52% at 5 and 10 years, respectively. In our study, the outcome in patients with recurrent iMN was not significantly inferior to those without recurrence. Although five patients with recurrence versus two patients without recurrence developed graft loss, this difference was NS. Black race was associated with both allograft failure and death after transplantation. Recurrent iMN was not a predictor of outcome in our study, but because of the limited sample size and the retrospective nature of the study, no definitive conclusions can be made. Because this study spanned a period of 16 years, we cannot define the roles of specific immunosuppressive regimens; however, there seemed to be no difference in regimens between those with and without recurrence: There was no difference in recurrence rate between patients who were treated with older, cyclosporine-based regimens and newer, tacrolimus/mycophenolate-based regimens. Previous studies that reported inferior survival made no distinction between de novo and recurrent MN or were conducted of patients with de novo MN (20). Because de novo MN is often associated with episodes of acute rejection, this could represent a selection bias.
Our study was not designed prospectively to identify risk factors for recurrence. Various reports have suggested that the risk for recurrence is greater in patients with heavy proteinuria, male gender (6,7), hypertension, better HLA matching, living-related renal transplant (8,9), and use of cyclosporine therapy after transplantation (10). In our study, there seemed to be no clear gender, age, or racial predisposition to recurrent disease. Neither the nature of the allograft, living donor versus cadaveric, nor the use of CNIs seemed to be associated with recurrent disease. With a recurrence rate up to 44% in our study as well as in others, a high grade of suspicion for recurrence is justified in patients who receive a kidney transplant for iMN.
From case reports demonstrating efficacy of rituximab in the treatment of both native and transplant iMN, we instituted a similar course of therapy in four transplant patients with recurrent disease (21–23). This is the first, although small, study of patients who had recurrent iMN and were treated with rituximab, and the results of therapy were encouraging. One patient showed stabilization of proteinuria, and the other three showed significant diminishment of proteinuria. Renal function was preserved in all patients. Comparison of rituximab treatment and other treatment protocols (e.g., pulse steroid, cyclophosphamide) was not possible because of limit sample size.
Conclusions
iMN recurs frequently in the allograft even in the era of modern transplant immunosuppression. There seem to be two patterns of recurrence: An early pattern, which may be detectable only on biopsy, and a late-onset recurrence that is usually associated with the nephrotic syndrome. In our study, we were unable to find risk factors for recurrence. Biopsy evidence of recurrence within the first week after transplantation suggests the presence of a “preexistent factor,” similar to that suspected in patients with early recurrent FSGS. Data regarding the treatment of recurrent iMN with rituximab are very limited, and our data represent the largest case series to date. Our data show favorable results for rituximab in the treatment of recurrent iMN. Prospective, randomized clinical trials are needed to compare the efficacy of conventional therapy of recurrent MN with high-dosage corticosteroids or cyclophosphamide versus newer agents such as rituximab.
Disclosures
None.
Acknowledgments
B.S. is a fellow of the Belgian American Education Foundation and received support from the Rotary Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ: Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347: 103– 109, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, McCredie M, Boyle P: Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: Results from an international comparative study. Am J Kidney Dis 35: 157– 165, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Cattran D: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188– 1194, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cosyns JP, Couchoud C, Pouteil-Noble C, Squifflet JP, Pirson Y: Recurrence of membranous nephropathy after renal transplantation: Probability, outcome and risk factors. Clin Nephrol 50: 144– 153, 1998 [PubMed] [Google Scholar]
- 5.Poduval RD, Josephson MA, Javaid B: Treatment of de novo and recurrent membranous nephropathy in renal transplant patients. Semin Nephrol 23: 392– 399, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Monga G, Mazzucco G, Basolo B, Quaranta S, Motta M, Segoloni G, Amoroso A: Membranous glomerulonephritis (MGN) in transplanted kidneys: Morphologic investigation on 256 renal allografts. Mod Pathol 6: 249– 258, 1993 [PubMed] [Google Scholar]
- 7.Truong L, Gelfand J, D'Agati V, Tomaszewski J, Appel G, Hardy M, Pirani CL: De novo membranous glomerulonephropathy in renal allografts: A report of ten cases and review of the literature. Am J Kidney Dis 14: 131– 144, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Berger BE, Vincenti F, Biava C, Amend WJ, Jr, Feduska N, Salvatierra O, Jr: De novo and recurrent membranous glomerulopathy following kidney transplantation. Transplantation 35: 315– 319, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Morzycka M, Croker BP, Jr, Siegler HF, Tisher CC: Evaluation of recurrent glomerulonephritis in kidney allografts. Am J Med 72: 588– 598, 1982 [DOI] [PubMed] [Google Scholar]
- 10.Montagnino G, Colturi C, Banfi G, Aroldi A, Tarantino A, Ponticelli C: Membranous nephropathy in cyclosporine-treated renal transplant recipients. Transplantation 47: 725– 727, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Odorico JS, Knechtle SJ, Rayhill SC, Pirsch JD, D'Alessandro AM, Belzer FO, Sollinger HW: The influence of native nephrectomy on the incidence of recurrent disease following renal transplantation for primary glomerulonephritis. Transplantation 61: 228– 234, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Obermiller LE, Hoy WE, Eversole M, Sterling WA: Recurrent membranous glomerulonephritis in two renal transplants. Transplantation 40: 100– 102, 1985 [PubMed] [Google Scholar]
- 13.Josephson MA, Spargo B, Hollandsworth D, Thistlethwaite JR: The recurrence of recurrent membranous glomerulopathy in a renal transplant recipient: Case report and literature review. Am J Kidney Dis 24: 873– 878, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Dabade TS, Grande JP, Norby SM, Fervenza FC, Cosio FG: Recurrent idiopathic membranous nephropathy after kidney transplantation: A surveillance biopsy study. Am J Transplant 8: 1318– 1322, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G: Rituximab in idiopathic membranous nephropathy: A one-year prospective study. J Am Soc Nephrol 14: 1851– 1857, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Cravedi P, Sghirlanzoni MC, Gagliardini E, Conti S, Gaspari F, Marchetti G, Abbate M, Remuzzi G: Effects of rituximab on morphofunctional abnormalities of membranous glomerulopathy. Clin J Am Soc Nephrol 3: 1652– 1659, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churg J, Ehrenreich T: Membranous nephropathy. Perspect Nephrol Hypertens 1: 443– 448, 1973 [PubMed] [Google Scholar]
- 18.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11– 21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM, Vincenti F, George V: Recurrent and de novo glomerular disease after renal transplantation: A report from Renal Allograft Disease Registry (RADR). Transplantation 68: 635– 641, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Cosyns JP, Pirson Y, Squifflet JP, Alexandre GP, van Ypersele de Strihou C, Pinn VW, Sweet SJ, Shapiro KS, Cho S, Harrington JT: De novo membranous nephropathy in human renal allografts: Report of nine patients. Kidney Int 22: 177– 183, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Gallon L, Chhabra D: Anti-CD20 monoclonal antibody (rituximab) for the treatment of recurrent idiopathic membranous nephropathy in a renal transplant patient. Am J Transplant 6: 3017– 3021, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Weclawiak H, Ribes D, Guilbeau-Frugier C, Touchard G, Kamar N, Mehrenberger M, Modesto A, Rostaing L: Relapse of membranous glomerulopathy after kidney transplantation: Sustained remittance induced by rituximab. Clin Nephrol 69: 373– 376, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Sirimongkolrat T, Premasathian N, Vongwiwatana A, Limsrichamrern S, Cheunsuchon B, Vasuvattakul S: Anti-CD20 monoclonal antibody (rituximab) for the treatment of membranous nephropathy after living-unrelated kidney transplantation: A case report. Transplant Proc 40: 2440– 2441, 2008 [DOI] [PubMed] [Google Scholar]