Abstract
Background and objectives: In autosomal dominant polycystic kidney disease, cysts derived from tubules are detected at birth by ultrasound (threshold for detection >7.0 mm); thus, fetal cyst growth rates must exceed 2300%/yr. In adults, the combined renal cyst component enlarges at approximately 12%/yr by growth of individual cysts. To explore this discrepancy, the growth rates of individual cysts were determined in adult polycystic kidneys.
Design, setting, participants, & measurements: Diameter, volume, and growth rates of individual cysts were measured by magnetic resonance in 30 individual cysts in three adult patients over a span of 3 years. Results were confirmed in 22 cysts measured in five patients by computed tomography over a span of 11 years.
Results: Mean cyst diameters were 20.4 ± 9.9 mm (range 7.1 to 40.5 mm) at baseline and 25.8 ± 15.6 mm (range 7.8 to 49.6 mm) after 3 years. Mean cyst volumes, determined by manual segmentation and summation of magnetic resonance cross sections, were 8.7 ± 12.9 cm3 (0.3 to 43.3 cm3) and 24.2 ± 66.3 cm3 (0.3 to 364.8 cm3) after 3 years. Mean cyst growth rates ranged from 6.9 to 23.9%/yr; the maximum growth rate was 71.1%/yr, far less than required to develop a 7-mm diameter cyst in utero. Results were similar in 22 cysts examined by computed tomography.
Conclusions: It was concluded that renal cysts detected by ultrasound in newborns must have grown at exuberant rates in utero; thereafter, expansion appears to proceed at much slower rates.
Cysts develop most commonly in collecting ducts in patients with autosomal dominant polycystic kidney disease (ADPKD). Each cyst is initiated when a collecting duct epithelial cell, or small cluster of cells, begins repetitive divisions secondary to the aberrant actions of mutated polycystins 1 (PC-1) or 2 (PC-2), thereby forcing radial expansion of the tubule wall (1). In the early stages of cyst formation, a potential cavity is formed by this neoplastic growth and is filled with fluid derived primarily from the afferent flow of unreabsorbed glomerular filtrate. As cell proliferation continues, the nascent cyst expands, eventually reaching a point where the anatomic twisting caused by the expansion tears the cyst away from the parent tubule, leaving an isolated tumor (sac of fluid) (2). That point of separation is thought to occur when the cysts reach diameters of approximately 2 to 3 mm, roughly 50 times larger than the original tubule (3).
In a recent study, we used a straight-forward anatomic model and enabling equations to approximate the effect of different rates and patterns of individual cyst growth in patients with ADPKD (4). Application to a longitudinal magnetic resonance (MR) study of 241 ADPKD patients enrolled in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) revealed that in subjects aged 15 to 45 years, the kidneys enlarged at relatively constant rates within individuals, averaging 5.3%/yr among all of the subjects (5). The cystic components of the kidneys enlarged at a mean rate of 12.0%/yr (6). Cysts are independent structures, and we surmised that differences among the diameters of individual cysts within a kidney at any point in time would be a function of when each cyst was formed and their rate of growth. Formation in response to the genetic trigger in individual tubule cells would determine the total number of cysts; however, the rate of growth would be the most critical determinant of how large the individual cysts would become and the ultimate size of the cysted kidney. An earlier computed tomography (CT) study indicated that cysts within a kidney would likely grow at different rates (7), and model calculations showed that if individual cysts grew at different, although constant rates, total kidney and total cyst growth rates would remain relatively constant (4).
To gain a more quantitative understanding of cyst and kidney growth, in the study presented here we determined the rates of individual cyst growth within ADPKD kidneys and the extent to which growth rates of individual cysts within intact kidneys are sufficient to account for the clinical discovery of cysts ≥7.0 mm in diameter from birth to adulthood. The results confirm that within a polycystic kidney individual cysts grow at widely different rates; however, we are led to the unexpected conclusion that to be seen clinically with conventional imaging methods in children and young adults, cysts must experience a period of exceptionally rapid growth heretofore unobserved in human patients.
Materials and Methods
A portion of this study is based on the analysis of a mathematical model using data from the CRISP study of human subjects with ADPKD and normal GFR at enrollment into the study (4,5). Of a total of 241 patients, in 232 patients without azotemia who were 15 to 46 years old at baseline, annual changes in total cyst volume (TCV) and total kidney volume (TKV) were determined for three years. The methods used in CRISP to obtain MR images and to determine TCV and TKV (left and right volumes were combined) from those images have been reported in detail (5).
The study presented here examines the growth patterns of individual cysts that in sum account for the changes in TCV and TKV from year to year. We used gadolinium-enhanced, weighted MR T1 image data from three CRISP patients at the University of Kansas Medical Center obtained at baseline and 3 years later using the same scanner after written, informed consent in conformity to the declaration of Helsinki. In each of the patients we selected left and right renal cysts from year 3, representing a relatively wide spectrum of diameters (8 to 81.1 mm). We chose cysts that appeared circular in cross section as we examined the series of MR slices. The MR sections were generally 3 mm thick, so we were limited to selecting cysts >10 mm diameter at year 3. We then located the same cysts in the baseline scans.
The volume of an individual cyst was determined using the volumetric routine formatted in Analyze software (Mayo Foundation, Biomedical Imaging Resource, Rochester, MN); the volume of a cyst corresponds to the sum of the cross-sectional cyst areas over contiguous slices containing the cyst times the slice thickness. The mean diameter was determined independently by measuring and averaging the lengths of three transecting lines dropped at 60° angles each to the other in the slice of maximal cyst diameter. Individual cyst volume was also determined from the mean maximal radius by V = 4/3 × πr3 and the rate of change in volume from Vk = ln(Vt/Vo)/t, where k is the fractional rate of volume change in time (t) and ln is the natural logarithm; Vk × 100 = percent change.
The findings in MR scans were augmented by measurements of individual cyst growth in contrast-enhanced axial CT scans. Detailed methods of measuring total kidney and total kidney cyst volumes have been published previously (8,9). Five patients in whom three or more cysts had been measured are included. Individual cyst volumes (n = 22) were determined with the mean maximal diameter method described above at baseline and ≥3 years later.
Only descriptive statistics were used in this study.
Results
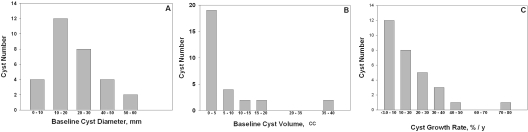
In the study presented here, we used a segmentation method to measure the volumes of ten individual cysts in each pair of kidneys from three patients at baseline and after 3 years. After written informed consent, patients were selected to represent a relatively broad spectrum of cyst diameters, cyst volumes, TCVs, and TKVs found in the CRISP cohort (Table 1). Total volumes of the combined kidneys ranged from 691 to 2684 cm3 at baseline, and the total volumes occupied by cysts ranged from 233 to 1781 cm3. Measurements of mean maximal diameter and volume of the 30 cysts were made on the baseline and year 3 images. The baseline mean maximal diameter ranged from 7.1 to 40.5 mm (mean diameter 20.4 ± 9.8 mm) and the median baseline diameter was relatively close to the mean (median 19.5 mm), indicating the chosen cysts were reasonably well distributed about the mean (Figure 1A). Mean baseline cyst volume determined by the segmentation method was 8.7 ± 12.9 cm3 and exhibited a distribution skewed toward smaller cysts, reflecting the powerful effect of the diameter cubed to exaggerate the volumes of the larger cysts (Figure 1B).
Table 1.
Individual cyst volume from stereology in MR images
| Subject | Gender | Age (years) | GFR (ml/min) | Cyst Number | Baseline Single Cyst Volume (cm3) | Single Cyst Growth Rates (%/yr) | CRISP Total Cyst Growth Rate (%/yr) | CRISP Baseline TCV (cm3) | |
|---|---|---|---|---|---|---|---|---|---|
| Y | Male | 45 | 75 | 10 | Mean | 17.6 | 21.0 | 24.1 | 1781 |
| Range | 0.30 to 40.2 | 1.6 to 71.1 | |||||||
| H | Male | 19 | 92 | 10 | Mean | 6.36 | 23.9 | 20.4 | 997 |
| Range | 11.6 to 35.8 | −2.2 to 45.2 | |||||||
| B | Female | 34 | 94 | 10 | Mean | 2.25 | 6.9 | 10.4 | 233 |
| Range | 0.26 to 6.72 | −2.3 to 28.1 |
Figure 1.
Histograms of cyst diameter, volume, and growth rate distribution. (A) Baseline cyst diameter determined from the average maximal diameter. (B) Baseline cyst volume determined by the segmentation method. (C) Cyst growth rate determined by the segmentation measurement method.
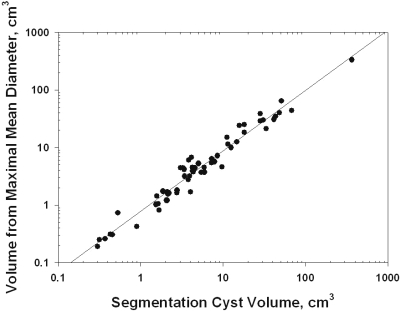
In a previous study, we had assumed that cysts are spheres so that the diameter, plane surface area, cross-section area, and volume have fixed relationships (4). In the study presented here, we selected cysts for analysis that appeared circular during the examination of sequential slices by MR. To determine how closely cysts selected on this basis approximated a spherical configuration, we compared volumes determined with the segmentation method to volumes calculated from the independent measurement of the mean maximal diameter using a standard formula. There was excellent correlation (R2 = 0.959) between cyst volumes determined by the segmentation and diameter methods at baseline and year 3 (Figure 2). The average cyst volumes determined by the segmentation and mean maximal diameter methods were not significantly different (Δ0.92 ± 3.55 cm3, P > 0.10).
Figure 2.
Validation of methods. The relation between cyst volumes determined by segmentation and diameter methods. R2 = 0.959. Thirty cysts measured at baseline and after 3 years.
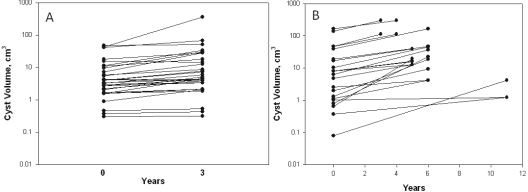
Over a 3-year interval, the volume increased in 28 of 30 cysts (Figure 3A). In paired determinations of individual cysts, the average volumes increased 15.5 ± 58.3 cm3; by contrast, the median volume change of the same cysts was 2.15 cm3. The relative change in volume from baseline (Vt/Vo) was 1.94 ± 1.43 (P < 0.001); the median relative change was 1.58. The mean rate of change in the volumes of individual cysts, determined from [ln(Vyear 3/Vbaseline)]/3 × 100, was 17.3 ± 16.4%/yr (P < 0.01) with a range of −2.2 to 45.2%/yr. Similar to the pattern for cyst volume, the rate of cyst volume change was skewed toward the lower rates of growth (Figure 1C).
Figure 3.
Volume change in 52 single cysts. (A) Volume determined from MR scans by stereology method over 3 years. Relative change was 1.94 ± 1.43; P < 0.001. (B) Volume determined from contrast-enhanced CT scans by maximal diameter method over 3 to 11 years. Relative change was 8.82 ± 12.94; P < 0.001.
We also measured changes in the mean diameters of 22 cysts over periods >3 years in CT images of kidneys from five nonazotemic patients reported previously from our laboratory (Table 2) (7). TCVs of these patients fell within the range of values observed in the three CRISP subjects. Volume increased in all cysts, ranging from 0.21 to 162 cm3 (Figure 3B). The relative change in volume from baseline (Vt/Vo) was 8.82 ± 12.94 (P < 0.001). The total change in cyst volumes determined in CT scans was greater that in MR scans because the mean interval of observation was longer. On the other hand, the range of single cyst growth rates (1.7 to 68.1%/yr) determined in CT scans was similar to the range in MR scans.
Table 2.
Individual cyst volume from mean diameter in CT images
| Subject | Age (years)/Gender | Baseline eGFR (ml/min) | Cyst Number | Interval (years) | Baseline Single Cyst Volume (cm3) | Final Single Cyst Volume (cm3) | Single Cyst Growth Rates (%/yr) | Total Cyst Growth Rate (%/yr) | TCV (cm3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 32/Female | 80.3 | 5 | 8.6 | Mean | 0.6 | 8.5 | 35.4 | 12.6 | 238 |
| Range | 0.08 to 1.02 | 1.23 to 19.3 | 1.7 to 68.1 | |||||||
| MI | 42/Female | 94.6 | 6 | 4.3 | Mean | 75.7 | 173.6 | 22.1 | 11.0 | 654 |
| Range | 18.8 to 164 | 47.6 to 299 | 15.0 to 30.4 | |||||||
| W | 25/Female | 92.9 | 3 | 6.0 | Mean | 1.9 | 5.9 | 18.4 | 10.3 | 105 |
| Range | 1.15 to 2.57 | 4.18 to 9.18 | 8.1 to 25.6 | |||||||
| G | 27/Female | 135 | 4 | 6.0 | Mean | 4.7 | 30.4 | 38.4 | 24.6 | 432 |
| Range | 0.79 to 10.3 | 18.8 to 44.5 | 24.4 to 55.7 | |||||||
| BO | 25/Female | 117.0 | 4 | 5.0 | Mean | 9.57 | 19.9 | 14.4 | 15.9 | 610 |
| Range | 4.8 to 17.1 | 12.7 to 38.7 | 8.9 to 19.4 |
In the eight combined CRISP and CT imaging subjects, the rate of individual cyst growth ranged from −2.2 to 71.1%/yr. Although the mean rates of single cyst growth tended to be positively associated with the rate of TCV increase in the kidneys of the combined eight patients (r2 = 0.203), the correlation did not reach statistical significance (P > 0.20). The most rapid rate of growth (71.1%/yr) found in the 52 cysts from eight patients included in the study presented here would appear to establish the upper range of cyst growth in these kidneys. To be distinctly visible by radiologic imaging techniques, cysts must exceed threshold diameters and volumes, which for ultrasound is 7.0 to 10 mm (0.18 to 0.52 cm3) and for CT/MR is 2 mm (0.0042 cm3) (10). We sought to determine if the rates of growth found in this report were sufficient to account for the observed diameters were the cysts to have originated in normal-sized renal tubules. For this analysis, we made the enabling assumptions that the tubules and cysts are regular spheres and that evolving cysts expand at a constant rate without regard to underlying mechanisms.
Consider first the growth of solitary cysts initiated in normal-sized renal tubules. Evidence indicates that most of the cysts in ADPKD develop in collecting ducts beginning in utero and may continue to develop throughout the patient's lifetime (11,12). Direct measurements of microdissected human cortical and outer medullary collecting ducts reveal outer diameters ranging from 40 to 100 μm (13,14). Fetal collecting ducts are probably smaller. How fast would cysts have to grow to reach the minimum threshold diameter of 2.0 mm for CT or MR detection were they to originate in the larger collecting ducts (100 μm)? Computation reveals that under these conditions a cyst would have to grow at a rate of 20%/yr to be detected by the age of 45, or 899%/yr to be detected in the first year of life (Table 3). The growth rate requirements are greater, 26 and 1174%/yr, respectively, for tubules initially 40 μm in diameter. Although these projected growth rates yield cyst volumes that may be feasible in older patients, the higher growth rate requirements for children and young adults are out of bounds in light of the study presented here. Because the initial tubule diameter of 100 μm and the imaging diameter detection limit of 2.0 mm define the optimum conditions for cyst enlargement and detection, the formation of cysts in smaller tubules or the use of a less sensitive imaging detection apparatus would require even greater rates of cyst growth to meet the threshold size targets.
Table 3.
Volume growth rate required to exceed 2.0-mm diameter imaging threshhold
| Age (years) | Rate (%/yr) |
|
|---|---|---|
| Initial Diameter = 40 μm | Initial Diameter = 100 μm | |
| 45 | 26 | 20 |
| 35 | 33 | 26 |
| 25 | 47 | 36 |
| 15 | 78 | 60 |
| 5 | 235 | 180 |
| 1 | 1174 | 899 |
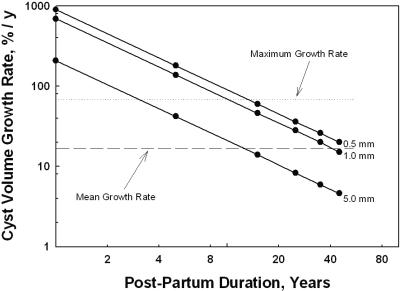
From this analysis it appeared that total and individual cyst growth rates much greater than those recorded in CRISP or in the study presented here would be required to account for detectable cysts in children and young adults with ADPKD. This raises the possibility that cysts formed in the fetal kidney might grow at rates fast enough in utero to achieve a size at birth from which the lower growth rates observed postpartum could generate cysts conventionally seen in children and adults with ADPKD. It is not uncommon to detect cysts 10 mm in diameter or larger in the kidneys of ADPKD children as young as 1 year old (15–19). The hypothetical relationships between the growth rate and postpartum duration for cysts to reach a diameter of 10 mm are illustrated in Figure 4 for cysts with diameters at birth of 0.5, 1.0, and 5.0 mm. Cysts growing at the maximal volume rate observed in the study presented here (71.1%/yr) could reach a 10-mm diameter between 3 and 4 years of age were the diameter 5.0 mm at birth. On the other hand, cysts growing at the mean rate of 17%/yr observed in the stereology measurements of the study presented here would spend approximately 10 years reaching a diameter of 10 mm. Figure 4 also illustrates that starting diameters <5 mm at birth would require even more time to reach the 10-mm target cyst diameter.
Figure 4.
Duration of postpartum growth required to generate a cyst 10 mm in diameter. Horizontal dotted line shows the maximal growth rate (71.1%/yr) observed in a single cyst in the study presented here. The dashed line shows the mean rate of cyst growth (17%/yr) in the stereology study presented here. Regression lines signifying starting cyst diameters at birth of 0.5, 1.0, and 5.0 mm are shown.
Tubule development and growth is unquestionably robust during the last 6 months of fetal development. If it is assumed that a cyst reaching a diameter of 5.0 mm at birth grew over the last 6 months of gestation from a tubule 100 μm in diameter, a growth rate exceeding 2300%/yr would be required.
Discussion
The interpretations based on the new data presented in this study depend on the assumption that renal cysts can be modeled as simple spheres. Casual inspection of CT and MR images and of gross and microscopic sections of human ADPKD confirm that most of the cysts approximate spherical geometry, although occasional specimens appear elongated, twisted, and occasionally septated. In this study, we selected for detailed analysis cysts that appeared spherical upon the examination of serial MR slices. The close relation between the cyst volume derived from measurement of the maximal mean diameter and the volume determined by segmentation strongly supports the spherical assumption (Figure 2). Because the mean values of the cysts and the mean TCV growth rates were reasonably well matched to the TKV, TCV, and growth rate values for the intact organs, it seems sensible to suppose that the spherical cysts selected in this study reflected the growth behavior of other cysts in those kidneys with perhaps more complex morphology.
The cyst volume increased in 50 of 52 cysts measured in this study over 3- to 11-year intervals (Figure 3). In the majority, cyst growth rates were less than 30%/yr, equivalent to a doubling time >2.6 years. Renal cysts have been likened to solid tumor masses in which unbridled cell proliferation drives volume expansion (20). But cysts are more complex than most solid tumors, for the mass effect is a product of cell proliferation operating in conjunction with net fluid secretion, and G-protein-mediated levels of cyclic AMP regulate both components (21). An equivalent amount of volume displacement by a cyst requires less cellular proliferation than in a solid tumor, with fluid accumulation in the cyst cavity making up the difference. Consider the mass of cells that would be required to form a 10-mm diameter (0.52 cm3) solid tumor composed of cells with uniform volume (cell length, height, width = 10 × 10 × 10 μm = 1 × 10−9 cm3). This hypothetical solid tumor would contain 5.2 × 1010 cells (32 serial cell doublings from the original). By contrast, the epithelial monolayer in the fluid-filled cyst would contain 3.14 × 106 cells, requiring only 22 serial cell doublings from the original to match the same diameter as the cellular tumor. These calculations illustrate that the rate of cell proliferation within individual renal cysts is slow in comparison to solid tumor masses in which tumor doubling times of a few months are common, and especially slow when compared with cell culture in which doubling times are measured in hours.
This brings into question whether or not the rates of total kidney and total cyst growth measured in the kidneys of nonazotemic CRISP volunteers could account for the enormous size that kidneys attain in the usual lifetime of an ADPKD patient. Given the optimum scenario of a cyst developing in a relatively large tubule (100 μm diameter) and growing at the fastest growth rate observed in any of the kidneys (71.1%/yr), detection of that cyst by the most sophisticated imaging equipment would not be possible before the age of approximately 10 years (Table 3). At growth rates representative of most cysts in this study (5 to 40%/yr) the age of detection is >15 years of age. It is generally appreciated that individual renal cysts can be detected in children with ADPKD during the first year of life, and massive cystic change has been described in newborn children and adolescents as well. How then do we account for the fact that cysts enlarge to several centimeters in diameter in children and young adults but, when measured directly, exhibit apparent growth rates too slow to account for their origin from renal tubules?
Perhaps they grow more rapidly in a postnatal period that was not included in the study presented here. To address this concern, we reviewed key studies of children, two in Colorado (19,22) and one in Czechoslovakia (23), in which ultrasound measurements of TKV were determined sequentially over a time interval long enough to override the lack of sensitivity of the measurements. In both studies, the rates of renal enlargement in the cystic kidneys exceeded the normal renal growth rate by approximately 50%. However, the overall growth rate was only approximately 8%/yr, slightly larger than observed in the CRISP study of adults. The rate of total kidney growth reflects the geometric sum of the individual cyst growth rates with the organ. Although the rates of kidney and total cyst growth may be slightly greater than in adults, they are vastly insufficient to account for the appearance of visible cysts in the kidneys of children and adults with ADPKD that derive from normal-sized renal tubules. More likely is the possibility that renal cysts formed in utero and, subjected to the strong progrowth environment of the developing fetus, may grow at extraordinary rates that gives their size a “head start” sufficient to enable the slower compounding effect of postpartum growth to produce cysts that fall within the visible diameter thresholds of our imaging methods.
To see how this may work, we consider three cysts, each originating in 100-μm diameter tubules and enlarging at accelerated rates for 6 months in the fetal kidney. To reach terminal diameters of 0.5, 1.0, and 5.0 mm at birth, the cyst growth rates would have to be 966, 1382, and 2347%/yr, respectively. Now consider how fast these cysts would have to grow in the postpartum period to reach a terminal diameter of 10 mm (Figure 4). The largest cyst at birth (5 mm) growing at near the maximum rate observed in this study (71%/yr) could generate a 10-mm diameter specimen by the age of 3. The smaller cysts at birth (0.5 and 1.0 mm) would not reach the same threshold for another 8 to 9 years. Cysts 5 mm in diameter at birth growing at the mean growth rate observed in the stereology studies in Table 1 (17%/yr) would not reach the 10-mm threshold diameter for more than 10 years and the 0.5- and 1.0-mm cysts after more than 40 years. What these examples reveal is that biasing the assumptions to favor detection by initiating the cysts in 100-μm tubules and giving them a “head start” in utero failed to generate cyst diameters that have been observed in children <3 years old. The most economical explanation to account for these new findings is to suppose that human renal cysts experience a period of greatly accelerated cellular proliferation and enlargement in the fetal environment.
There is experimental precedent in support of the hypothesis that cysts formed in utero may grow at faster rates than in adult environments. As noted previously, homozygous pkd1 and pkd2 mice exhibit florid cyst formation in utero but die before or during birth of nonrenal causes (24,25). Several laboratories have generated mice in which pkd1 can be conditionally inactivated shortly after birth, thereby avoiding cyst formation in utero (26–28). The surprising finding was that if the provocation to form cysts in the kidneys of these mice was delivered within a 3-week “window” after birth, the kidneys erupted in diffuse cystogenesis and enlarged to such an extent that they failed to function after a few weeks. By contrast, inactivating the genes after 3 weeks led only to focal cyst formation and much slower cyst enlargement as had been observed initially in heterozygous mice. These observations were interpreted to demonstrate that cyst formation and growth were exquisitely sensitive to developmental factors that dissipate shortly after birth.
In rodents, renal development and nephronogenesis continue for approximately 2 to 3 weeks after birth, which may account for the window during which inactivation of pkd1 will initiate robust cystogenesis. By contrast, in humans nephronogenesis ceases at approximately 34 weeks of gestation (29). Thus, it seems reasonable to suppose that the enhanced environment for cystogenesis that operates throughout most of pregnancy in humans may subside in the neonatal period. It is tempting to ascribe this differential growth behavior to the state of renal development and growth factors in utero, but it is undoubtedly far more complex.
Is it possible that most of the renal cysts we see by ultrasound, CT, and MR in our ADPKD patients were formed in utero? There are hints that this may be the case. Cysts usually form and grow in kidney pairs to approximately the same extent in humans with ADPKD (5,30). Although quite rare, patients have been described with unilateral expression of renal cysts. Cyst formation in these rare cases, from whatever the inciting mechanism, either had not occurred or the cysts remained below a clinically detected threshold in the null, compared with the contralateral cystic kidney. These exceptions open the possibility that the formation and growth of cysts may be regulated by stochastic as well as intrinsic and humoral factors in utero.
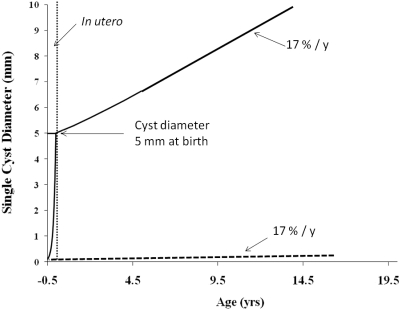
The pathogenesis of ADPKD can be viewed as occurring in two principal stages: formation and progression. Perhaps we should consider adding to the progression term of that formula a new subcomponent—in utero extraordinary cell proliferation and cyst growth. How this might appear over the long term is illustrated in Figure 5, in which exponential cyst growth at warp speed (2347%/yr) during approximately 6 months in utero raises the diameter to 5 mm at birth, to be followed by much slower growth (17%/yr) for the rest of the patient's life. This illustration demonstrates how plausible rates for in utero and postpartum cell proliferation and cyst growth might generate cysts that would reach diameters of 10 mm observed by ultrasound in a child 15 years old. By contrast, a cyst formed immediately postpartum and growing at 17%/yr would remain invisible to ultrasound detection for decades.
Figure 5.
Influence of in utero accelerated growth on the enlargement of individual cysts postpartum. Cyst originating in 100-μm collecting duct in utero grows at a rate of 2347%/yr for 6 months, then at a rate of 17%/yr thereafter. Dashed line at bottom indicates grow of cyst at 17%/yr after formation in utero. Vertical dashed line indicates birth.
The study presented here provides new information to investigators aiming to treat ADPKD with targeted agents. Although we did not address the formation of cysts explicitly, the analysis leads us to think that a great deal of cyst formation may occur in utero in view of the abundant number of relatively large cysts detected in children and young adults. Therein lies a potential problem because the administration of drugs or other nostrums to pregnant women or infants to delay cyst formation will require an extraordinary degree of safety. The extent to which new cysts form during and after birth is unknown, but such knowledge is obviously important in regard to the design of practical therapeutic agents. Studies are needed to fill in the age gaps inaccessible to us during the analysis presented here. Do myriads of microscopic cysts form in utero and remain below the resolution of ultrasound as we have hypothesized? Is there a postpartum period of exuberant cyst formation and growth that is not reflected in the published studies of total kidney enlargement measured by ultrasound? Clearly, investigation of the earliest stages of cyst formation and growth with more sensitive technologies is justified and needed.
Conclusion
We conclude that renal cysts detected by ultrasound in newborns must have grown at exuberant rates in utero; thereafter, expansion appears to proceed at much slower rates.
Disclosures
None.
Acknowledgments
The CRISP data used in this report are available to investigators from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) data repository. The authors thank our colleagues in CRISP for developing this invaluable data source. This study was supported in part by grant 5U01DK056943-09 from NIDDK (Dr. Grantham) and by the general clinical research center at the University of Kansas Medical Center. This work was presented in abstract form at the annual meeting of the American Society of Nephrology; San Diego, CA; October 27 through November 1, 2009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477– 1485, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Geiser JL, Evan AP: Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int 31: 1145– 1152, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Evan AP, McAteer JA: Cyst cell and cyst walls. In: The Cystic Kidney, edited by Gardner KDJ, Bernstein J.Boston, Kluwer, 1990, pp 21– 42 [Google Scholar]
- 4.Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, Guay-Woodford LM, Bae KT: Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 73: 108– 116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122– 2130, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013– 3019, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Sise C, Kusaka M, Wetzel LH, Winklhofer F, Cowley BD, Cook LT, Gordon M, Grantham JJ: Volumetric determination of progression in autosomal dominant polycystic kidney disease by computed tomography. Kidney Int 58: 2492– 2501, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bae KT, Commean PK, Lee J: Volumetric measurement of renal cysts and parenchyma using MRI: Phantoms and patients with polycystic kidney disease. J Comput Assisted Tomogr 24: 614– 619, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O'Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP: Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035– 1045, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bae KT, Grantham JJ: Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96– 106, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Verani R, Walker P, Silva FG: Renal cystic disease of infancy: Results of histochemical studies. A report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol 3: 37– 42, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Verani RR, Silva FG: Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: A histochemical study. Mod Pathol 1: 457– 463, 1988 [PubMed] [Google Scholar]
- 13.Jacobson HR, Gross JB, Kawamura S, Waters JD, Kokko JP: Electrophysiological study of isolated perfused human collecting ducts: Ion dependency of the transepithelial potential difference. J Clin Invest 58: 1233– 1239, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace DP, Christensen M, Reif G, Belibi F, Thrasher B, Herrell D, Grantham JJ: Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol 283: F1337– F1350, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Sedman A, Bell P, Manco-Johnson M, Schrier R, Warady BA, Heard EO, Butler-Simon N, Gabow P: Autosomal dominant polycystic kidney disease in childhood: A longitudinal study. Kidney Int 31: 1000– 1005, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Pretorius DH, Lee ME, Manco-Johnson ML, Weingast GR, Sedman AB, Gabow PA: Diagnosis of autosomal dominant polycystic kidney disease in utero and in the young infant. J Ultrasound Med 6: 249– 255, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Fick GM, Johnson AM, Strain JD, Kimberling WJ, Kumar S, Manco-Johnson ML, Duley IT, Gabow PA: Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1863– 1870, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Boyer O, Gagnadoux MF, Guest G, Biebuyck N, Charbit M, Salomon R, Niaudet P: Prognosis of autosomal dominant polycystic kidney disease diagnosed in utero or at birth. Pediatr Nephrol 22: 380– 388, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fick-Brosnahan G, Johnson AM, Strain JD, Gabow PA: Renal asymmetry in children with autosomal dominant polycystic kidney disease. Am J Kidney Dis 34: 639– 645, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Grantham JJ: Polycystic kidney disease: Neoplasia in disguise. Am J Kidney Dis 15: 110– 116, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149– 168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4: 820– 829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeman T, Dusek J, Vondrichova H, Kyncl M, John U, Misselwitz J, Janda J: Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press Monit 8: 107– 110, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J: Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 17: 179– 181, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S: Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75– 78, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Piontek KB, Huso DL, Grinberg A, Liu L, Bedja D, Zhao H, Gabrielson K, Qian F, Mei C, Westphal H, Germino GG: A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J Am Soc Nephrol 15: 3035– 3043, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ: Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16: 3188– 3196, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Starremans PG, Li X, Finnerty PE, Guo L, Takakura A, Neilson EG, Zhou J: A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5′ end of Pkd1. Kidney Int 73: 1394– 1405, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Haycock GB: Development of glomerular filtration and tubular sodium reabsorption in the human fetus and newborn. Br J Urol 81[ Suppl 2]: 33– 38, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Kistler AD, Poster D, Krauer F, Weishaupt D, Raina S, Senn O, Binet I, Spanaus K, Wuthrich RP, Serra AL: Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int 75: 235– 241, 2009 [DOI] [PubMed] [Google Scholar]