Abstract
Background and objectives: In the United States, relatively little is known about clinical outcomes of chronic kidney disease (CKD) in vulnerable populations utilizing public health systems. The primary study objectives were to describe patient characteristics, incident ESRD, and mortality in adults with nondialysis-dependent CKD receiving care in the health care safety net.
Design, setting, participants, & measurements: Time to ESRD and time to death were examined among a cohort of 15,353 ambulatory adults with nondialysis-dependent CKD from the Community Health Network of San Francisco.
Results: The mean age of the CKD cohort was 59.0 ± 13.8 years; 50% of the cohort was younger than 60 years and 26% was younger than 50 years. Most (72%) were members of nonwhite racial-ethnic groups, 73% were indigent (annual income <$15,000) and 18% were uninsured. In adjusted analyses, blacks [hazard ratio (95% confidence interval), 4.00 (2.99 to 5.35)], Hispanics [2.20 (1.46 to 3.30)], and Asians/Pacific Islanders [3.84 (2.73 to 5.40)] had higher risks of progression to ESRD than non-Hispanic whites. The higher risk of progression to ESRD among nonwhite compared with white persons with CKD was not explained by lower relative mortality.
Conclusions: Adults with CKD stages 3 to 5 cared for within an urban public health system were relatively young and predominantly nonwhite—both factors associated with a higher risk of progression to ESRD. These findings call for targeted efforts to assess the burden and progression of CKD within other public and safety-net health systems in this country.
In the United States, ESRD affects over 500,000 Americans, disproportionately afflicts the poor and racial-ethnic minority groups, and costs society in excess of $30 billion annually (1–5). Preventing complications of chronic kidney disease (CKD) is now a focus of public health efforts to reduce racial-ethnic and socioeconomic health disparities across the nation (6).
Urban public hospitals and “safety-net” health care systems provide medical care to millions of uninsured and underserved individuals across the United States, many of whom are relatively young and of severely limited socioeconomic means. These facilities provide care to a substantially higher percentage of persons from diverse racial-ethnic, cultural, and linguistic backgrounds when compared with hospitals nationally (7,8). Although prior studies suggest that progression of CKD to ESRD may be accelerated among persons of lower socioeconomic status, relatively little is known about the demographic characteristics and outcomes of vulnerable populations with CKD in the safety-net health care setting (9).
To help understand the burden of CKD in the urban public health care setting, we describe the sociodemographics, clinical characteristics, mortality, and progression to ESRD of 15,353 adults with nondialysis-dependent CKD (stages 3 to 5) receiving care in the Community Health Network of the City and County of San Francisco. We hypothesized that nonwhite race-ethnicity would be associated with higher risks of progression to ESRD and death in this vulnerable population.
Materials and Methods
Data Sources
We obtained patient-level data from the San Francisco Department of Public Health's Community Health Network, a collection of public hospitals and clinics serving an urban poor population in San Francisco, California. Approximately half of San Francisco's 130,000 uninsured residents and one-quarter of its Medicaid population make at least one visit annually to the Community Health Network (10). Demographic information, health care utilization, diagnostic and procedural codes, and laboratory data are obtained during the course of routine clinical care at various Community Health Network sites and stored in a clinical data repository known as the Lifetime Clinical Record. Patient-level data were extracted from the Lifetime Clinical Record and linked to (1) the U.S. Renal Data System (USRDS) to exclude persons who were already receiving renal replacement therapy and to identify new cases of treated ESRD that occurred during follow-up, and (2) the California Department of Health Services Death Registry to ascertain vital status.
Study Sample
We included all adults with nondialysis-dependent CKD stages 3 to 5 receiving regular ambulatory care in the Community Health Network between January 1, 1996 and December 31, 2005. All participants were (1) age 20 years or older; (2) diagnosed with CKD, defined by at least two outpatient estimated GFR (eGFR) measurements <60 ml/min/1.73 m2 separated by at least 1 year (11); and (3) had at least one Community Health Network outpatient encounter subsequent to the initial serum creatinine date. We imposed these restrictions to ensure that the study cohort comprised persons who met the National Kidney Foundation definition for CKD stages 3 to 5 and who had regular access to outpatient care, rather than individuals with misclassified acute kidney injury or those who transiently visited the Community Health Network. We derived comparative estimates of persons with nondialysis-dependent CKD stages 3 to 5 from the general U.S. population using publicly available data from the National Health and Nutrition Examination Survey 1999 to 2006 (Supplementary Appendix A) (12).
Outcome Measures
The primary outcome measures were time to ESRD, defined as receipt of maintenance dialysis or kidney transplantation, and time to death. We defined survival time as the time of the first outpatient serum creatinine measurement <60 ml/min/1.73 m2 until ESRD, death, or the end of follow-up through December 31, 2005. Individuals were observed for a minimum of 12 months and up to 9.4 years.
Independent Variables
All independent variables used in the analysis were extracted or calculated from administrative and clinical data in the Lifetime Clinical Record. The primary predictor variable was self-reported patient race-ethnicity. We categorized race-ethnicity as non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, or other race-ethnicity. We attempted to measure and control for several sociodemographic and clinical factors that could confound the association between race-ethnicity and progression to ESRD and/or death on the basis of prior studies of CKD progression (9,13–15). Covariates were defined within the 2-year period preceding and closest to the initial qualifying serum creatinine measurement. Sociodemographic covariates included patient age, sex, health insurance coverage (uninsured, Medicaid, Medicare, or commercial/other), primary spoken language (English, Spanish, Cantonese, or other), housing status (domiciled or homeless), occupational status (employed, unemployed, disabled or retired), and annual income based on administrative data. We ascertained comorbid conditions on the basis of the presence of primary discharge diagnostic codes, ambulatory diagnostic codes, and procedural codes (Supplementary Appendix B) for diabetes, hypertension, congestive heart failure, cardiovascular disease (defined as coronary artery, cerebrovascular, or peripheral vascular disease), chronic obstructive lung disease, hepatitis B virus (HBV), hepatitis C virus (HCV), HIV or AIDS, depression, tobacco smoking, alcoholism, and drug abuse. We used the re-expressed Modification of Diet in Renal Disease study equation to estimate GFR on the basis of calibrated serum creatinine, age, race, and sex (16). Additional laboratory data included urinalysis dipstick proteinuria (negative, trace to 1+, 2+, or ≥3+), hemoglobin, serum albumin, and calcium concentrations (17).
Statistical Analyses
Baseline characteristics of persons with CKD are described using mean values (±SD) and proportions. We calculated crude rates of ESRD and death per 1000 person-years stratified by race-ethnicity. We examined the associations of race-ethnicity with the risks of ESRD and death controlling for potential confounders using multivariable Cox proportional hazards regression models. The final models included all baseline demographic information, socioeconomic variables, comorbid conditions, and laboratory values described above. We tested the proportional hazards assumption using plots of log [−log (survival rate)] against log (survival time) and by comparing predicted with actual survival curves.
Missing data patterns were examined for all variables. Overall, 17% of the cohort was missing data for health insurance coverage and 27% for proteinuria by urine dipstick. Less than 1% was missing data for hemoglobin, serum albumin, and serum calcium, respectively. To avoid bias caused by excluding patients with missing data, we performed multiple imputation using the Markov chain Monte Carlo method with 100 imputations for these variables (18). To address potential nonindependence of outcomes because of informative censoring, we performed additional analyses using competing-risks survival analysis for either outcome (ESRD or death) using the method of Fine and Gray (19). Two-tailed P values <0.05 were considered statistically significant. We used Stata statistical software for all analyses (Stata version 11.0, Stata Corporation, College Station, TX). The Committee on Human Research at the University of California–San Francisco approved the study protocol.
Results
Patient Characteristics
We identified 15,353 adults receiving regular ambulatory care in the San Francisco Community Health Network who met criteria for nondialysis-dependent CKD stages 3 to 5 (Table 1). In contrast with sociodemographic estimates from the general U.S. population with CKD stages 3 to 5 (Table 2), the patients with CKD stages 3 to 5 in the San Francisco public safety-net system were younger, more racially-ethnically diverse, less likely to be female, more likely to be uninsured or enrolled in Medicaid, more likely to be poor, and less likely to speak English. The study cohort had a mean age of 59.0 years (±13.8 years); 50% of the cohort was younger than 60 years and 26% was younger than 50 years. This safety-net cohort largely comprised racial-ethnic minorities (72%), most of whom (93%) had moderate (eGFR 30 to 59 ml/min/1.73 m2) rather than advanced CKD (eGFR < 30 ml/min/1.73 m2). Those with advanced disease were more likely to be black, male, and younger than those with moderate disease (P < 0.001, respectively). Approximately 64% of individuals were enrolled in a public health insurance plan (Medicare or Medicaid), whereas 18% were uninsured. Unlike the general U.S. population with CKD, most individuals (73%) from the study cohort were indigent, with an annual income <$15,000. An estimated 6% of persons were homeless and 46% were unemployed, disabled, and/or receiving public assistance. The most prevalent comorbidities, hypertension and diabetes, affected 46% and 22% of individuals, respectively; however, the prevalence of alcoholism (8%), depression (16%), drug abuse (16%), and chronic viral diseases such as HCV (4%) and HIV (3%) were also notable. The sociodemographic and clinical characteristics of this CKD cohort were representative of the adults generally served by the Community Health Network's ambulatory care clinics (10).
Table 1.
Baseline characteristics of 15,353 adults with nondialysis-dependent CKD stages 3 to 5
| Characteristic | eGFR, ml/min/1.73 m2 |
|||
|---|---|---|---|---|
| 45 to 59 (n = 12,262) | 30 to 44 (n = 2049) | 15 to 29 (n = 776) | <15 (n = 266) | |
| Race-ethnicity, N (%) | ||||
| non-Hispanic white | 3441 (28) | 584 (29) | 213 (27) | 63 (24) |
| non-Hispanic black | 2249 (18) | 585 (29) | 272 (35) | 85 (32) |
| Hispanic | 2353 (19) | 316 (15) | 122 (16) | 52 (19) |
| Asian/Pacific Islander | 2897 (32) | 506 (25) | 15 (20) | 60 (22) |
| other race-ethnicity | 322 (3) | 58 (3) | 12 (2) | 6 (2) |
| Age, mean (SD), years | 60 (13) | 58 (15) | 56 (15) | 51 (15) |
| Age category, years, % (95% CI) | ||||
| <40 | 918 (7) | 220 (11) | 117 (15) | 61 (23) |
| 40 to 49 | 1990 (16) | 401 (20) | 188 (24) | 70 (26) |
| 50 to 59 | 2933 (24) | 525 (26) | 201 (26) | 63 (24) |
| 60 to 69 | 3879 (32) | 460 (22) | 124 (16) | 45 (17) |
| ≥70 | 2542 (21) | 443 (22) | 146 (19) | 27 (10) |
| Female, N (%) | 6782 (55) | 954 (47) | 320 (41) | 95 (36) |
| Unemployed, N (%) | 5723 (47) | 930 (45) | 333 (43) | 115 (43) |
| Homeless, N (%) | 612 (5) | 148 (7) | 74 (9) | 24 (9) |
| Annual income ≥$15,000, N (%) | 9021 (75) | 1387 (68) | 507 (65) | 175 (66) |
| Primary health coverage, N (%) | ||||
| uninsured | 2299 (19) | 285 (14) | 102 (13) | 25 (9) |
| Medicaid | 2727 (22) | 531 (26) | 202 (26) | 99 (37) |
| Medicare | 5101 (42) | 699 (34) | 237 (31) | 67 (25) |
| commercial or other | 262 (2) | 43 (2) | 18 (2) | 6 (2) |
| missing | 1873 (15) | 491 (24) | 217 (28) | 69 (26) |
| Primary language, N (%) | ||||
| English | 7946 (64) | 1535 (75) | 611 (78) | 206 (77) |
| Spanish | 1491 (12) | 186 (9) | 63 (8) | 31 (12) |
| Cantonese | 1518 (12) | 170 (8) | 53 (7) | 19 (7) |
| other | 1307 (11) | 158 (8) | 49 (6) | 11 (4) |
| Comorbid conditions, N (%) | ||||
| diabetes | 2809 (23) | 414 (20) | 130 (17) | 33 (12) |
| hypertension | 6036 (49) | 756 (37) | 221 (28) | 66 (25) |
| cardiovascular disease | 2253 (18) | 303 (15) | 92 (12) | 18 (7) |
| congestive heart failure | 797 (7) | 139 (7) | 38 (5) | 12 (4) |
| chronic obstructive lung disease | 1970 (16) | 285 (14) | 97 (12) | 20 (7) |
| AIDS/HIV | 567 (5) | 99 (5) | 37 (5) | 8 (3) |
| HBV infection | 140 (1) | 17 (1) | 12 (2) | 0 (0) |
| HCV infection | 499 (4) | 103 (5) | 41 (5) | 14 (5) |
| alcoholism | 851 (7) | 207 (10) | 92 (12) | 28 (11) |
| depression | 2006 (16) | 298 (14) | 93 (12) | 23 (9) |
| drug abuse | 1762 (14) | 416 (20) | 190 (24) | 52 (20) |
| tobacco smoking | 501 (4) | 94 (5) | 35 (5) | 8 (3) |
| Laboratory measurements | ||||
| urinalysis proteinuria, N (%) | ||||
| negative (<30 mg/dl) | 8091 (66) | 966 (47) | 239 (31) | 36 (13) |
| trace to 1+ (30 to 99 mg/dl) | 2231 (18) | 509 (25) | 225 (29) | 57 (22) |
| 2+ (100 to 299 mg/dl) | 1242 (10) | 351 (17) | 182 (23) | 82 (31) |
| ≥3+ (≥300 mg/dl) | 698 (6) | 223 (11) | 129 (17) | 91 (34) |
| hemoglobin, mean (SD), g/dl | 13.1 (1.9) | 12.3 (2.3) | 11.9 (2.4) | 10.9 (2.8) |
| serum albumin, mean (SD), g/dl | 4.0 (0.6) | 3.7 (0.8) | 3.4 (0.8) | 3.4 (0.7) |
| Serum calcium, mean (SD) mg/dl | 9.1 (0.6) | 9.0 (0.8) | 8.8 (0.9) | 8.6 (1.4) |
Table 2.
Characteristics of adults with nondialysis-dependent CKD stages 3 to 5 from the general U.S. populationa
| Characteristic | eGFR, ml/min/1.73 m2 |
|||
|---|---|---|---|---|
| 45 to 59 (n = 1239) | 30 to 44 (n = 399) | 15 to 29 (n = 93) | <15 (n = 23) | |
| Race-ethnicity, % (95% CI) | ||||
| non-Hispanic white | 86 (83 to 88) | 86 (81 to 89) | 68 (54 to 79) | 72 (47 to 89) |
| non-Hispanic black | 6 (5 to 7) | 8 (5 to 11) | 13 (7 to 21) | 18 (6 to 43) |
| Hispanic | 5 (3 to 8) | 4 (2 to 9) | 5 (2 to 10) | 9 (3 to 23) |
| other race-ethnicity | 3 (2 to 4) | 3 (1 to 5) | 15 (5 to 33) | 0 (NC) |
| Age, mean (95% CI), years | 67 (66 to 68) | 74 (73 to 76) | 72 (67 to 76) | 56 (46 to 66) |
| Age category, years, % (95% CI) | ||||
| <40 | 3 (2 to 5) | 0 (0 to 2) | 5 (1 to 20) | 24 (6 to 59) |
| 40 to 49 | 8 (6 to 11) | 3 (1 to 7) | 3 (1 to 8) | 8 (1 to 40) |
| 50 to 59 | 17 (14 to 20) | 7 (4 to 11) | 3 (1 to 9) | 28 (9 to 59) |
| 60 to 69 | 23 (21 to 26) | 15 (11 to 20) | 22 (14 to 34) | 23 (7 to 54) |
| ≥70 | 49 (45 to 53) | 75 (68 to 81) | 67 (51 to 80) | 19 (6 to 44) |
| Female, % (95% CI) | 61 (59 to 64) | 66 (61 to 71) | 54 (43 to 64) | 68 (40 to 88) |
| Unemployed, % (95% CI) | NA | NA | NA | NA |
| Homeless, % (95% CI) | NA | NA | NA | NA |
| Annual income ≤$15,000, % (95% CI) | 27 (23 to 30) | 41 (34 to 48) | 28 (19 to 39) | 39 (16 to 68) |
| Primary health coverage, % (95% CI)b | ||||
| uninsured | 5 (3 to 7) | 2 (1 to 6) | 5 (1 to 20) | 17 (4 to 49) |
| Medicaid | 6 (4 to 7) | 6 (4 to 9) | 9 (4 to 18) | 12 (3 to 38) |
| Medicare | 58 (53 to 62) | 76 (70 to 81) | 76 (63 to 86) | 33 (12 to 63) |
| commercial or other | 64 (60 to 68) | 57 (51 to 63) | 59 (50 to 67) | 56 (28 to 81) |
| English spoken at home, % (95% CI) | 93 (90 to 95) | 92 (87 to 96) | 96 (92 to 98) | 91 (77 to 97) |
| Comorbid conditions, % (95% CI)c | ||||
| diabetes | 18 (15 to 23) | 27 (22 to 34) | 43 (31 to 57) | 29 (10 to 60) |
| hypertension | 68 (64 to 71) | 80 (76 to 83) | 86 (74 to 93) | 85 (52 to 97) |
| cardiovascular disease | 19 (16 to 22) | 33 (28 to 39) | 41 (30 to 53) | 22 (6 to 53) |
| congestive heart failure | 6 (5 to 7) | 13 (10 to 17) | 20 (13 to 29) | 29 (9 to 61) |
| chronic obstructive lung disease | 11 (9 to 13) | 9 (7 to 13) | 14 (6 to 28) | 5 (1 to 23) |
| AIDS/HIV | 0 (NC) | 0 (NC) | 0 (NC) | 0 (NC) |
| HBV infection | 0 (0 to 1) | 1 (0 to 3) | 0 (NC) | 0 (NC) |
| HCV infection | 1 (0 to 2) | 1 (0 to 2) | 1 (0 to 3) | 0 (NC) |
| alcoholism | 10 (8 to 13) | 8 (5 to 12) | 13 (7 to 23) | 14 (3 to 48) |
| depression | NA | NA | NA | NA |
| drug abuse | 5 (3 to 7) | 1 (0 to 5) | 2 (0 to 7) | 11 (2 to 50) |
| tobacco smoking | 12 (10 to 15) | 7 (4 to 13) | 13 (6 to 25) | 41 (16 to 71) |
| Laboratory measurements | ||||
| albuminuria, % (95% CI) | ||||
| <30 mg/dl | 96 (95 to 97) | 87 (83 to 90) | 69 (53 to 81) | 59 (31 to 83) |
| 30 to 99 mg/dl | 2 (1 to 3) | 6 (4 to 9) | 8 (4 to 17) | 4 (1 to 13) |
| 100 to 299 mg/dl | 1 (0 to 2) | 5 (3 to 8) | 12 (5 to 24) | 7 (2 to 23) |
| ≥300 mg/dl | 0 (0 to 1) | 2 (1 to 3) | 11 (5 to 22) | 30 (10 to 61) |
| hemoglobin, mean (SEM), g/dl | 14.2 (0.07) | 13.4 (0.11) | 12.4 (0.19) | 12.6 (0.64) |
| serum albumin, mean (SEM), g/dl | 4.2 (0.01) | 4.1 (0.03) | 4.0 (0.04) | 4.1 (0.16) |
| serum calcium, mean (SEM) mg/dl | 9.5 (0.02) | 9.5 (0.04) | 9.4 (0.10) | 9.2 (0.29) |
NC, not calculable because of fewer than five observations (point estimates may also be unstable); NA, not available.
Data estimated from the National Health and Nutrition Examination Survey (1999 to 2006).
Percentages may not total 100 due to multiple sources of health coverage.
Participants were considered to have diabetes if a physician had informed them that they had “sugar diabetes,” if they were receiving insulin or an oral hypoglycemic agent, or if they recorded a fasting plasma glucose concentration ≥126 mg/dl or a nonfasting plasma glucose concentration ≥200 mg/dl (2); hypertension if they reported taking a prescribed antihypertensive agent or if their systolic blood pressure was ≥140 mmHg or if their diastolic blood pressure was ≥90 mmHg at the time of the examination (3,4); cardiovascular disease if they reported a history of angina, coronary artery disease, stroke, or lower extremity amputation; congestive heart failure if they reported a history of “heart failure”; chronic obstructive lung disease if they reported a history of emphysema or chronic bronchitis; HBV, HCV, and HIV on the basis of the presence of a positive antibody test for HBV surface antigen, HCV, and HIV, respectively; and alcoholism if they reported drinking five or more drinks of any alcoholic beverage almost every day. We considered participants to suffer from drug abuse if they reported use of cocaine, heroin, methamphetamine, or other street drug, and we considered participants as tobacco smoking if they reported current cigarette use “every day” or “some days.”
ESRD and Death
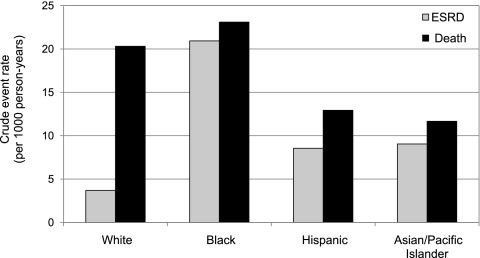
Overall, we observed 559 cases of ESRD during 55,538 person-years of follow-up and 984 deaths over 57,698 person-years of follow-up, respectively (the difference in survival time was accounted for by those who eventually died). Crude rates of progression to ESRD were highest among blacks—over 2-fold higher than among any other racial-ethnic group. Hispanics and Asians/Pacific Islanders progressed to ESRD at approximately twice the rate as that observed in non-Hispanic whites (Figure 1). Although crude mortality rates were highest among blacks, differences compared with other racial-ethnic groups were smaller in magnitude than racial-ethnic differences in rates of progression to ESRD. Notably, among all racial-ethnic groups, the rates of progression to ESRD peaked in the youngest age group of 20 to 39 years {20.5 [95% confidence interval (CI), 16.9 to 24.9] per 1000 person-years compared with 4.1 (95% CI, 3.1 to 5.4) per 1000 person-years in those ≥70 years}.
Figure 1.
Crude rates of ESRD and death by race-ethnicity.
In analyses adjusted for sociodemographic factors, comorbid conditions, and laboratory abnormalities, nonwhite race-ethnicity was independently associated with the risk of progression to ESRD (Table 3A). Other significant covariates associated with a higher risk of progression to ESRD included diabetes [hazard ratio (95% CI): 2.92 (2.39 to 3.55)] and the presence and degree of albuminuria [hazard ratio (95% CI): 4.21 (2.49 to 7.14) for trace to 1+; 15.10 (9.33 to 24.49) for 2+; and 26.72 (16.48 to 43.32) for ≥3+]. In Asians/Pacific Islanders, the risk estimate for ESRD increased after covariate adjustment (negative confounding) primarily because of the older age distribution of this group relative to non-Hispanic whites (Table 3A). In contrast, the relative risk of death among racial-ethnic groups did not differ in fully adjusted models, with the exception of Asians/Pacific Islanders, in whom the risk of death was 25% lower than in non-Hispanic whites (Table 3B).
Table 3A.
Relative rates of progression to ESRD by race-ethnicity
| Group | Patients (%) | Person-Years | ESRD | Unadjusted Hazard Ratio of ESRD (95% CI) | Adjusted Hazard Ratio of ESRD (95% CI)ab |
|---|---|---|---|---|---|
| Non-Hispanic white | 4301 (28) | 15,672 | 58 | 1.00 (Referent) | 1.00 (Referent) |
| Non-Hispanic black | 3191 (21) | 12,564 | 263 | 5.67 (4.27 to 7.54) | 4.00 (2.99 to 5.35) |
| Hispanic | 2843 (19) | 9823 | 84 | 2.30 (1.65 to 3.22) | 2.20 (1.46 to 3.30) |
| Asian/Pacific Islander | 4620 (30) | 16,025 | 145 | 2.43 (1.79 to 3.30) | 3.84 (2.73 to 5.40) |
| Other | 398 (2) | 1454 | 9 | 1.66 (0.82 to 3.36) | 1.74 (0.86 to 3.52) |
Model includes age, gender, race-ethnicity, health insurance coverage, housing status, annual income, eGFR, diabetes, hypertension, cardiovascular disease, congestive heart failure, chronic obstructive lung disease, HIV/AIDS, HBV, HCV, smoking, drug abuse, alcoholism, depression, hemoglobin, serum calcium, serum albumin, and urinalysis proteinuria.
Multiple imputation performed for missing values of health insurance coverage, hemoglobin, serum calcium, serum albumin, and urinalysis proteinuria.
Table 3B.
Relative rates of death by race-ethnicity
| Group | Patients (%) | Person-Years | Deaths | Unadjusted HR of Death (95% CI) | Adjusted HR of Death (95% CI)ab |
|---|---|---|---|---|---|
| Non-Hispanic white | 4301 (28) | 15,857 | 322 | 1.00 (Referent) | 1.00 (Referent) |
| Non-Hispanic black | 3191 (21) | 13,600 | 314 | 1.13 (0.97 to 1.32) | 0.99 (0.84 to 1.16) |
| Hispanic | 2843 (19) | 10,132 | 131 | 0.64 (0.52 to 0.78) | 0.94 (0.74 to 1.20) |
| Asian/Pacific Islander | 4620 (30) | 16,628 | 194 | 0.58 (0.48 to 0.69) | 0.76 (0.61 to 0.95) |
| Other | 398 (2) | 1482 | 23 | 0.77 (0.51 to 1.18) | 0.83 (0.55 to 1.28) |
Model includes age, gender, race-ethnicity, health insurance coverage, housing status, annual income, eGFR, diabetes, hypertension, cardiovascular disease, congestive heart failure, chronic obstructive lung disease, HIV/AIDS, HBV, HCV, smoking, drug abuse, alcoholism, depression, hemoglobin, serum calcium, serum albumin, and urinalysis proteinuria.
Multiple imputation performed for missing values of health insurance coverage, hemoglobin, serum calcium, serum albumin, and urinalysis proteinuria.
In adjusted analyses, diabetes [hazard ratio (95% CI): 1.29 (1.10 to 1.52)], congestive heart failure [1.39 (1.13 to 1.72)], cardiovascular disease [1.25 (1.05 to 1.48)], chronic obstructive lung disease [1.21 (1.02 to 1.43)], HBV [1.68 (1.01 to 2.81)], HCV [1.62 (1.22 to 2.16)], HIV/AIDS [1.87 (1.47 to 2.38)], alcoholism [1.52 (1.23 to 1.88)], and hypoalbuminemia [1.43 (1.30 to 1.59)] were each associated with a higher risk of death. These results did not change materially in analyses stratified by age, sex, and diabetes status or when competing-risks survival analysis was performed for progression to ESRD or death (Supplementary Appendix C).
Discussion
In contrast to general population estimates and prior studies of CKD in the United States, we found that in this public safety-net health care setting, CKD afflicted a large fraction of younger adults, most of whom are from racial-ethnic minority groups (13,14,20). Most individuals in our study cohort were indigent, approximately 40% were either uninsured or enrolled in Medicaid, and one-third spoke a primary language other than English—vulnerable populations that have been underrepresented in prior studies of CKD (13–15). In this resource-poor environment, we found that blacks, Hispanics, and Asians/Pacific Islanders with CKD were at higher risk of developing ESRD compared with whites. The elevated risk of progression to ESRD among nonwhite compared with white patients was not explained by a lower relative risk of death. These observations illustrate a compelling need for further studies to characterize the burden of CKD within public hospital and safety-net health systems in this country.
Our findings extend the observations of prior studies of CKD to a racially-ethnically diverse, low-income population in a public health care setting (13–15). Peralta et al. described a higher risk of progression to ESRD among Hispanics compared with non-Hispanic whites with CKD stages 3 to 5 from Kaiser Permanente, an integrated health care system in northern California (13). O'Hare et al. demonstrated a lower risk of progression to ESRD with older age in a national cohort of veterans with CKD stages 3 to 5 (14). The population cared for within the Community Health Network of San Francisco extends our understanding of the epidemiology of CKD by including a large proportion of persons who are homeless, uninsured, linguistically isolated, and suffering from abject poverty, populations rarely examined in epidemiologic studies of chronic disease. In contrast to these previously published studies and estimates from a nationally representative sample of Americans from the National Health and Nutrition Examination Survey, which found CKD stages 3 to 5 to be relatively uncommon in younger persons (13,14,20), one-half of persons with CKD stages 3 to 5 receiving ambulatory care in our study were younger than 60, and over one-fourth were younger than 50. The high prevalence of CKD stages 3 to 5 and the large proportion of relatively young, nonwhite adults in our study setting are striking and provide rationale for studies of targeted CKD screening and risk factor management programs within public health systems in this country (21).
The higher risk of progression to ESRD among members of racial-ethnic minority groups in our study is consistent with ESRD risk estimates from cohorts in other U.S. health care settings (2,5,13). However, the persistence of racial-ethnic differences in ESRD risk in this low-income population suggests that factors other than socioeconomic status may play a more important role in the progression of established CKD to ESRD than previously suggested (2,5,13,22). The higher risk of progression to ESRD among nonwhite individuals in our study may reflect racial-ethnic differences in factors such as impediments to healthy behaviors (e.g., crime, drug abuse, overcrowding, and limited access to healthy foods or outdoor parks) (23,24), treatment adherence, patient-provider miscommunication, or distrust of the health care system (25). Notably, one-third of individuals with CKD stages 3 to 5 in our study cohort spoke a primary language other than English. Thus, it is possible that racial-ethnic minority groups may face additional challenges to navigating the public health care system because of factors such as cultural or linguistic isolation, limited English proficiency, and/or inadequate health literacy (26–28). Differing racial-ethnic patterns of obesity, insulin resistance, and CKD etiology could also partially explain this phenomenon. For example, rates of GFR decline may be faster in nonwhite versus white persons because of a higher prevalence and severity of glomerular diseases such as diabetes and IgA nephropathy in the former (1,15,29). The influence of chronic viral diseases such as HBV, HCV, and HIV and the contribution of psychosocial factors such as alcoholism, depression, drug abuse, and suicide to disparate rates of progression to ESRD and death in vulnerable populations with CKD warrant further investigation.
The largely nonwhite racial composition of our cohort is consistent with 2007 data from the National Association of Public Hospitals in which over 52% of patients discharged from member hospitals were of racial-ethnic minority backgrounds (7). Relative to public hospital estimates nationally, our cohort's disproportionately large Asian/Pacific Islander representation reflects the high concentration of these racial-ethnic groups in San Francisco (30). Conversely, blacks accounted for over 20% of individuals with CKD stages 3 to 5 receiving public health care during the study period but only 7% of adults in San Francisco in 2003 (30). Recent studies suggest that the care of nonwhite Americans may be concentrated in the hands of relatively few providers (31,32) This concentration of care for nonwhite patients raises the question of whether interventions targeted at the relatively small group of medical providers and facilities that serve racial-ethnic minority communities may help to reduce disparities in CKD outcomes (33).
The health insurance status of our CKD cohort is consistent with national data of persons generally served by urban public hospitals (34). Over 40% of individuals with CKD in our study were uninsured or enrolled in Medicaid. Uninsured persons and Medicaid enrollees with CKD can be difficult to identify from other data sources because CKD has historically been poorly coded in administrative data and because there is no system for tracking the care of uninsured patients within the United States (35,36). In a sense, these individuals with CKD represent a “blind-spot” in our current surveillance system. Supportive of these observations, data from the USRDS indicated that nearly one-third of persons initiating treatment for ESRD in 2006 were uninsured or covered by Medicaid (1).
Limitations
Our study provides a unique insight into the care of low income adults with nondialysis-dependent CKD. Nonetheless, there are several limitations in interpreting the results derived from observational data. First, although diverse populations were well represented in our study, our cohort may not be representative of persons receiving care from other public hospitals or safety-net health systems in the United States (i.e., the racial-ethnic composition, prevalence of comorbidities, and existence of social or linguistic isolation may differ in other urban safety-net health care settings). Second, we were unable to fully account for changes in some sociodemographic measures such as health insurance coverage or income over the period of follow-up. Third, our assessment of comorbid conditions was based on diagnostic codes and thus likely underestimates the prevalence of comorbidities such as cardiovascular disease, diabetes, and hypertension in this population; moreover, we could not determine the severity of most of the comorbid conditions (37,38). Fourth, although we undertook efforts to restrict the cohort with “true” CKD by requiring multiple outpatient serum creatinine determinations or evidence of established care within the Community Health Network, these criteria may have introduced some selection bias for cohort entry. It is also possible that we have misclassified some persons with acute kidney injury or with near-normal kidney function as having CKD. Differential misclassification of CKD and its severity using population-based GFR estimating equations may also be operative because the Modification of Diet in Renal Disease study equation was derived in a population of largely white and black patients with moderate to advanced CKD, very few of whom had diabetes. Lastly, our study is limited by the inability to comprehensively ascertain death in persons who died outside of California and ESRD in individuals who left the United States to receive renal replacement therapy.
In conclusion, adults with CKD stages 3 to 5 in this impoverished, urban, public health care setting were relatively young and predominantly nonwhite—both potent risk factors associated with progression of CKD to ESRD. These findings suggest that more concerted efforts to assess the extent and burden of CKD within other public hospital and safety-net health systems in this country is warranted, particularly as the nation contemplates health care reform aimed toward enhancing access to effective care for uninsured and underinsured individuals.
Disclosures
None.
Supplementary Material
Acknowledgments
This project received support from the National Institutes of Health (NIH)/National Center for Research Resources University of California–San Francisco/Clinical and Translational Science grants UL1-RR024131 and KL2-RR024130. Y.N.H. received support from Satellite Healthcare's Norman S. Coplon Extramural Grant Program. A.I.C. received support from K23-DK-080645. G.M.C. received support from N01-DK-012450 and U01-DK-066481. The funding organizations had no role in the design and conduct of the study; collection, analysis, or preparation of the data; or preparation, review, or approval of the manuscript. The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Government, NIH, or the San Francisco Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
References
- 1.U.S. Renal Data System: 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008. Available online at http://www.usrds.org/2008 Accessed September 22, 2009 [Google Scholar]
- 2.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293– 1298, 1997 [PubMed] [Google Scholar]
- 3.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV: Ethnic disparities in diabetic complications in an insured population. JAMA 287: 2519– 2527, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902– 2907, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Hall YN, Hsu CY, Iribarren C, Darbinian J, McCulloch CE, Go AS: The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int 68: 2310– 2316, 2005 [DOI] [PubMed] [Google Scholar]
- 6.National Healthcare Disparities Report 2004. Available online at http://www.qualitytools.ahrq.gov/disparitiesreport Accessed September 10, 2009
- 7.Regenstein M, Sickler D. Race, Ethnicity, and Language of Patients Washington, DC, National Public Health and Hospital Institute, 2006: 15– 16 Available online at http://www.naph.org Accessed September 24, 2009 [Google Scholar]
- 8.Gaskin DJ, Hadley J: Population characteristics of markets of safety-net and non-safety-net hospitals. J Urban Health 76: 351– 370, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merkin SS, Coresh J, Roux AV, Taylor HA, Powe NR: Area socioeconomic status and progressive CKD: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 46: 203– 213, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB: Effects of primary care coordination on public hospital patients. J Gen Intern Med 15: 329– 336, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137– 147, 2003 [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics National Health and Nutrition Examination Survey (NHANES) Analytic Guidelines. Available online at http://www.cdc.gov/nchs/nhanes/nhanes2003–2004/analytical_guidelines.htm Accessed September 10, 2009
- 13.Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892– 2899, 2006 [DOI] [PubMed] [Google Scholar]
- 14.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758– 2765, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, Collins AJ, Kusek JW, Levey AS, Greene T: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310– 1315, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461– 470, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468– 1474, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Little RJA, Rubin DB. Statistical Analysis with Missing Data New York, Wiley & Sons, 1987 [Google Scholar]
- 19.Fine JP: Regression modeling of competing crude failure probabilities. Biostatistics 2: 85– 97, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038– 2047, 2007 [DOI] [PubMed] [Google Scholar]
- 21.White SL, McGeechan K, Jones M, Cass A, Chadban SJ, Polkinghorne KR, Perkovic V, Roderick PJ: Socioeconomic disadvantage and kidney disease in the United States, Australia, and Thailand. Am J Public Health 98: 1306– 1313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS: MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185– 1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DR, Collins C: Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep 116: 404– 416, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL: Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345: 99– 106, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Murray-Garcia JL, Selby JV, Schmittdiel J, Grumbach K, Quesenberry CP, Jr: Racial and ethnic differences in a patient survey: Patients' values, ratings, and reports regarding physician primary care performance in a large health maintenance organization. Med Care 38: 300– 310, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Snyder RE, Cunningham W, Nakazono TT, Hays RD: Access to medical care reported by Asians and Pacific Islanders in a West Coast physician group association. Med Care Res Rev 57: 196– 215, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Gazmararian JA, Baker DW, Williams MV, Parker RM, Scott TL, Green DC, Fehrenbach SN, Ren J, Koplan JP: Health literacy among Medicare enrollees in a managed care organization. JAMA 281: 545– 551, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, Nurss JR: Inadequate functional health literacy among patients at two public hospitals. JAMA 274: 1677– 1682, 1995 [PubMed] [Google Scholar]
- 29.Ward MM: Socioeconomic status and the incidence of ESRD. Am J Kidney Dis 51: 563– 572, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Current Population Survey, 2000–2007 Annual Social and Economic Supplements. Historical Health Insurance Tables. U.S. Census Bureau. Available online at http://www.census.gov/hhes/www/hlthins/hlthins.html Accessed August 27, 2009
- 31.Konety SH, Vaughan Sarrazin MS, Rosenthal GE: Patient and hospital differences underlying racial variation in outcomes after coronary artery bypass graft surgery. Circulation 111: 1210– 1216, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL: Primary care physicians who treat blacks and whites. N Engl J Med 351: 575– 584, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O'Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493– 501, 2007 [DOI] [PubMed] [Google Scholar]
- 34.National Association of Public Hospitals (NAPH): America's Public Hospitals and Health Systems, 2007. Results of the Annual NAPH Hospital Characteristics Survey, pp 2– 13 Available online at http://www.naph.org/Publications/Characteristics-2007.aspx Accessed September 23, 2009
- 35.Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, Aron D, Pogach L: Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res 41: 564– 580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powe NR, Plantinga L, Saran R: Public health surveillance of CKD: Principles, steps, and challenges. Am J Kidney Dis 53[ Suppl 3]: S37– S45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iezzoni LI: Assessing quality using administrative data. Ann Intern Med 127: 666– 674, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB: Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med 119: 844– 850, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.