Abstract
Background and objectives: Sickle cell anemia-associated nephropathy is a growing matter of concern because renal failure affects most aging sickle cell anemia patients. Glomerular damage is a common feature revealed by a microalbuminuria or a macroalbuminuria. Although glomerular hyperfiltration has been described for decades in this population, its prevalence in young adults is unknown.
Design, setting, participants, & measurements: To address this issue, as well as the clinical and biologic correlates of hyperfiltration, a single-center, cross-sectional study of 280 homozygous SS disease patients was performed.
Results: The prevalence of hyperfiltration assessed by Modification of Diet in Renal Disease estimated GFR was 51%. Among patients with hyperfiltration, 49% had hyperfiltration alone, whereas 36% and 15% had an associated microalbuminuria or macroalbuminuria, respectively. Estimated GFR sensitivity and specificity for hyperfiltration were 94% and 63%, respectively, in a selected subgroup of 48 patients (measured GFR was assessed by urinary 51Cr EDTA clearance). In patients with no albuminuria, hyperfiltration status was significantly associated with a young age (years), the absence of alpha thalassemia, a lower hemoglobin level (g/dl), and a lower fetal hemoglobin. The role of chronic hemolysis was further strengthened by multivariate analysis showing a correlation between estimated GFR and a low plasma fetal hemoglobin level, a young age, and a high reticulocyte count (r2 = 0.54).
Conclusions: Together, the data suggest that the pathophysiology of hyperfiltration would rather be attributable to the hemolysis-associated vasculopathy rather than a viscosity-vaso-occlusive process.
Sickle cell anemia-associated nephropathy (SCAN) is a growing matter of concern because renal failure affects from 12% to 21% of adult patients (1,2) and up to 80% of aging patients (3). Thus, the early recognition of SCAN at the time of chronic kidney disease (CKD) stage I (4) and the focus on the early steps of the natural history of this nephropathy, together with the recognition of the associated clinical and biologic risk factors are of major interest. Previous works have reported the occurrence of SCAN very early in childhood, as soon as 7 years of age (5), with a prevalence of microalbuminuria of 26.5% increasing up to 40% in young adults. Macroalbuminuria is reported in 26% to 40% of patients with sickle cell anemia (SCA) depending on the age and may lead to nephrotic syndrome (1), revealing a focal glomerulosclerosis.
The high prevalence of microalbuminuria in patients with SS disease and the suggested sequence of events leading from microproteinuria to macroproteinuria, and ultimately chronic renal failure (6), are very similar to type 1 diabetic nephropathy history, where a glomerular hyperfiltration is the early step. In accordance with this view, the beneficial effects of angiotensin-converting enzyme inhibitors on microalbuminuria have been reported in SCAN patients (7). Surprisingly, although hyperfiltration has been reported in several studies (6,8,9), its prevalence is at present unknown.
The focus of our study was to determine the prevalence of glomerular hyperfiltration in a cohort of young adults with SS disease and to identify the factors associated with a high risk of hyperfiltration. Our results suggest that chronic hemolysis may be a relevant pathologic feature accounting for the presence of a high GFR.
Materials and Methods
Patient Population and Methods
The study was performed according to national ethics laws. The patients attended the Sickle Cell Center of Tenon Hospital (Paris, France). A total of 280 adult patients with homozygous SS hemoglobinopathy (between January 2007 and December 2008) with no acute illness at the time of the evaluation (no history of vaso-occlusive crisis, acute chest syndrome, fever in the last month, and no ongoing pregnancy or urinary tract infection) underwent a biologic evaluation including blood and urine samples taken on the basis of a routine clinic visit. Fetal hemoglobin (HbF) was quantified by high-performance ion-exchange chromatography, and α-globin gene number was determined using PCR analysis. Patients with HIV infection, hepatitis B or C infection, systemic lupus erythematosus, rheumatoid arthritis, and diabetes mellitus were also excluded from the present report.
We collected the clinical and laboratory values of interest during the visit when the urine specimen was obtained. Albumin excretion rate (AER), expressed as milligrams per millimole creatinine, was defined as normoalbuminuria (AER ≤ 3 mg/mmol creatinine), microalbuminuria (AER from 3 to 30 mg/mmol creatinine), or macroalbuminuria (AER >30 mg/mmol creatinine). Other laboratory values were measured using standard hospital laboratory techniques. We measured serum creatinine with enzymatic technic using a Kone creatinine analyzer (Thermo Clinical Labsystems Oy, Finland). Estimated GFR (eGFR) was calculated according to the three variable Modification of the Diet in Renal Disease (MDRD) formulae: 175 × [creatinine (μmol/L)/88.9]−1154× [age (years)]−0203 × 0742 (if female), but also according to Cockroft and Gault formula (10). We defined renal insufficiency as an eGFR <60 ml/min per 1.73 m2 (11) and renal hyperfiltration as an eGFR >130 ml/min per 1.73 m2 for women and >140 ml/min per 1.73 m2 for men (12).
We assessed the measured glomerular filtration rate (mGFR) by 51Cr-EDTA renal clearance in 48 SCA patients that were referred to our department of physiology as described previously (10). Briefly, we injected 1.8 to 3.5 MBq of 51Cr-EDTA (GE Healthcare, Velizy, France) intravenously as a single bolus. We then determined average urinary 51Cr-EDTA clearance but also average plasmatic 51Cr EDTA and urinary creatinine clearance during five to six consecutive 30-minute clearance periods. The GFR measurements were standardized for body surface area (1.73 m2). Hyperfiltration was defined above a threshold value of 110 ml/min per 1.73 m2 for mGFR in accordance with published data (13) and our laboratory normal upper limits established from potential kidney donors (i.e., 110 ml/min per 1.73 m2 corresponding to the mean of mGFR + 2 SD in this population).
Statistical Analyses
Statistics were performed using Statview software (SAS Institute Inc.). Descriptive statistics were used to evaluate male and female patients' related characteristics. Quantitative data were expressed as mean ± SD or median (including maximum and minimum) and as percentage for categorical variables. Differences between groups were tested using an unpaired t test for continuous variables and χ2 test for categorical variables. The alpha level was set to 0.005 for comparing the biologic parameters according to gender, and it was set to 0.2 and 0.05 for the univariate and the properly multivariate phases, respectively, of the multivariate analysis.
We assessed the performance of the MDRD equation, Cockroft and Gault formula, urinary creatinine clearance, and plasmatic 51Cr EDTA clearance compared with the gold standard urinary 51Cr EDTA clearance mGFR. The sensitivity, specificity, and predictive positive value of each estimator to diagnose true hyperfiltration were evaluated.
Stepwise logistic regression analysis was applied to estimate odds ratios (OR) and 95% confidence intervals (95% CIs) for each factor according to hyperfiltration (assessed by eGFR). Simple and multiple linear regression analyses were used to investigate the associations between eGFR and clinical/biologic factors. Associations were first analyzed without adjustments and then with adjustments for potential confounders. Other potential confounders tested included biologic hemolysis biomarkers, BP, and usual hematologic and biochemical parameters, including albuminuria.
Results
Clinical and Biologic Parameters
Clinical and biologic parameters of the studied population (all patients with SS disease) are shown in Tables 1 and 2. The populations of 111 men and 169 women were not statistically different for clinical sickle cell complications, such as leg ulceration, retinopathy, osteonecrosis, and pulmonary hypertension. Our population was mostly composed of young adults, because 90% were younger than 40 years, with a median age of 24.1 years (16 to 55 years) and 26.3 years (17 to 61 years) for men and women. Blood hemoglobin concentration was higher in men than women, and HbF was statistically higher in women (P = 0.001). As expected, plasma creatinine and GFR assessed by 3v-MDRD formula were higher in men than women.
Table 1.
Baseline characteristics of the study population
| Whole Population (n = 280) | |
|---|---|
| Age, yrs | 26,0 (16 to 61) |
| BMI, kg/m2 | 20.75 (14.0 to 34.8) |
| MAP, mmHg | 81.2 (57 to 106) |
| Priapism, % male | 19 |
| Leg ulcer, % | 10.3 |
| Retinopathy, % | 29.6 |
| Pulmonary hypertension, % | 13 |
| Osteonecrosis, % | 25.9 |
| Transfusion, % | 11.5 |
| Hydroxyurea, % | 17.4 |
BMI, body mass index. MAP, mean arterial pressure.
Table 2.
Biological parameters according to gender
| Male (n = 111) | Female (n = 169) | P | |
|---|---|---|---|
| Hb, g/dl | 8.9 ± 1.4 | 8.4 ± 1.2 | 0.002 |
| HbF, % | 5 | 8.2 | <0.0001 |
| LDH, IU/L | 450 ± 190 | 421 ± 181 | 0.21 |
| Reticulocyte count, ×103/mm3 | 333 ± 148 | 325 ± 127 | 0.65 |
| Ferritinemia, μg/ml | 490 ± 897 | 656 ± 1393 | 0.3 |
| Alpha thalassemia, % | 42.8 | 37.8 | 0.45 |
| Plasma creatinine, μmol/L | 64 ± 27 | 56 ± 20 | 0.005 |
| MDRD, ml/min per 1.73 m2 | 148 ± 47 | 126 ± 41 | <0.0001 |
| Urinary protein/creatinine, mg/mmol | 37.6 ± 85 | 48 ± 126 | 0.47 |
| Microalbuminuria | 59 | 53 | 0.32 |
| Macroalbuminuria | 13.5 | 19 | 0.28 |
Hb, hemoglobin; LDH, lactate dehydrogenase.
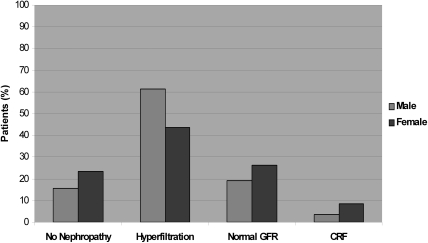
As shown Figure 1, only 15.6% of male and 23.3% of female patients were free of SCAN (NS). Indeed, hyperfiltration status alone or associated with albuminuria occurred in 51% of our population, with a significant difference between gender (60% versus 42% for men and women, respectively; P = 0.003). The prevalence of hyperfiltration was very similar (53%) according to the Cockroft and Gault formula. Chronic renal failure was encountered only in 3.6% and 8.6% of men and women, respectively (NS).
Figure 1.
Distribution of patients with SS disease according to GFR.
Comparison of eGFR to mGFR
Comparison of eGFR to mGFR (assessed by urinary 51Cr EDTA clearance), performed in a subgroup of 48 patients who were referred to further nephrological evaluation, is shown in Table 3. Among this subgroup, 66% had hyperfiltration assessed by mGFR (urinary 51Cr EDTA clearance >110 ml/min per 1.73 m2), whereas MDRD eGFR assessed hyperfiltration in 72% of the sample according to previously published criteria (threshold was defined above 130 and 140 ml/min per 1.73 m2 for women and men, respectively) (13). As shown, MDRD was a robust predictor for hyperfiltration compared with Cockroft and Gault eGFR or a six-period urinary creatinine clearance or even plasmatic 51Cr EDTA clearance, despite a lack of accuracy due to a systematic overestimation of MDRD. Thus, the value of 51% of patients with hyperfiltration (among our population of 280 homozygous SCA patients) assessed by MDRD eGFR seems a relevant finding and further allowed us to study the associated significant clinical and biologic factors.
Table 3.
Comparison between different eGFR and mGFR methods relative to urinary 51Cr EDTA mGFR to assess the prevalence of hyperfiltration status among 48 homozygous SCA patients with proteinuria
| Hyperfiltration, % | GFR, ml/min per 1.73 m2 | Se, % | Sp, % | PP, % | |
|---|---|---|---|---|---|
| MDRD | 72 | 161 (44 to 300) | 94 | 63 | 82 |
| Cockroft | 45 | 135 (62 to 320) | 58 | 69 | 79 |
| Creatinine clearance | 62 | 144 (64 to 285) | 87 | 88 | 90 |
| Plasmatic mGFR | 74 | 121 (62 to 171) | 97 | 69 | 89 |
| Urinary mGFR | 66 | 123 (54 to 188) | 100 | 100 | 100 |
Prevalence of Albuminuria According to Hyperfiltration Status
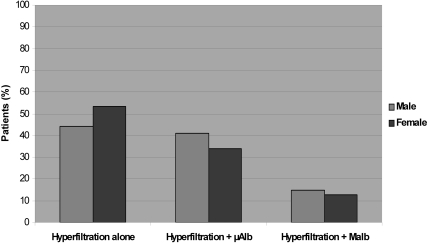
Hyperfiltration (according to eGFR) was present in 52% and 40% of microalbuminuric and macroalbuminuric patients (NS), respectively, underscoring the high prevalence of hyperfiltration in this population. However, as shown Figure 2, among the group with hyperfiltration, most of the patients had either no albuminuria (49%) or microalbuminuria (36%), and only 15% had macroalbuminuria.
Figure 2.
Distribution of patients with SS disease with hyperfiltration according to albuminuria. μAlb, microalbuminuria; Malb, macroalbuminuria.
Risk Factors Associated with Hyperfiltration Status
The prevalence of sickle cell anemia-related organ injury (retinopathy, osteonecrosis, priapism, pulmonary arterial hypertension, leg ulceration) was not significantly different between the groups with and without hyperfiltration (data not shown).
To identify the factors that could account for hyperfiltration and rule out potential bias such as albuminuria, we compared the patients with hyperfiltration alone (n = 63) to patients with no CKD (n = 56). As shown Table 4, the mean age was younger (P = 0.0003), with no difference for gender or body mass index, whereas several hemolysis-related biomarkers, such as HbF, hemoglobin concentration, plasma bilirubin, and the absence of thalassemia, were significantly different between the two groups. Using a multiple logistic regression analysis, we found that hyperfiltration status was independently associated with a young age (years) (OR: 0.79, 95% CI: 0.71 to 0.89; P = 0.0001), the absence of alpha thalassemia (OR: 5.2, 95% CI: 1.73 to 15.5; P = 0.003), a lower HbF (OR: 0.87, 95% CI: 0.78 to 0.97; P = 0.015), and a lower hemoglobin level (grams per deciliter) (OR: 0.62, 95% CI: 0.39 to 0.99; P = 0.049), suggesting that chronic hemolysis would be a related factor. In accordance with this view, multivariate analysis (Table 5) performed in the homogenous subgroup composed of patients with four-gene α-globin (i.e., no alpha thalassemia, n = 53), showed that plasma HbF level, age, and reticulocyte count were all independently related to eGFR (r2 = 0.54), with HbF explaining 29.9% of variance. Of notice, this association was not affected after further adjustment for hemoglobin level, lactate dehydrogenase, plasma bilirubin, and other potential confounders.
Table 4.
Characteristics of SCA patients with hyperfiltration alone (n = 63) and normal GFRa
| Hyperfiltration Alone (n = 63) | Normal GFR (n = 56) | P | |
|---|---|---|---|
| Age, yrs | 21.9 | 27.1 | 0.0003 |
| BMI, kg/m2 | 20.6 | 20.5 | 0.87 |
| Gender, % male | 40 | 30 | 0.23 |
| eGFR (MDRD), ml/min per 1.73 m2 | 160 | 104 | <0.0001 |
| eGFR (Cockroft), ml/min per 1.73 m2 | 162 | 115 | <0.0001 |
| Hb, g/dl | 8.5 | 9.2 | 0.002 |
| Bilirubin, IU/L | 57 | 42 | 0.004 |
| HbF, % | 5.5 | 8.7 | 0.003 |
| Ferritin, μg/L | 702 | 360 | 0.16 |
| No thalassemia, % | 62 | 40 | 0.02 |
| LDH, IU/L | 397 | 360 | 0.09 |
| Reticulocyte count 103/mm3 | 335 | 298 | 0.07 |
BMI, body mass index; Hb, hemoglobin; LDH, lactate dehydrogenase.
Group of homozygous SCA patients defined on the following criteria: no albuminuria and an eGFR >60 ml/min per 1.73 m2.
Table 5.
MDRD eGFR according to HbF among a selected subgroup of patientsa
| R2, % | B | P | |
|---|---|---|---|
| Univariate analysis | 29.9 | −0.56 | <0.0001 |
| Multivariate analysis | |||
| M = Age + reticulocyte count | 54.4 | −0.393 | 0.0004 |
| M + BMI | 54.4 | −0.41 | 0.0005 |
| M + LDH | 56.2 | −0.43 | 0.0003 |
| M + Hb | 53.9 | −0.348 | 0.0061 |
| M + bilirubin | 53.5 | −0.382 | 0.0011 |
Data are standardized regression coefficient (B) for the association of HbF and eGFR. BMI, body mass index; LDH, lactate dehydrogenase; Hb, hemoglobin.
Group of non-alpha thalassemic SCA patients (n = 53) defined on the following criteria: no albuminuria and an eGFR >60 ml/min per 1.73 m2
Discussion
Our data show that among 280 patients with SS disease ages 18 to 61 years, the prevalence of albuminuria was around 60% (40% microalbuminuria and 19% macroalbuminuria), in accordance with previous reports (6). In contrast, our lower prevalence of chronic renal failure (around 7%) is probably explained by the young age of our adult population (median age 25 years; range: 17 to 61 years).
Our finding of a hyperfiltration assessed by eGFR formula in up to 51% of patients of 280 homozygous SCA patients (and 66% in a selected subgroup of 48 patients assessed by mGFR) is a surprising feature. To our knowledge, a prevalence of hyperfiltration was previously addressed in only one report showing a lower prevalence of 30.5% with the same MDRD criteria: eGFRs above 130 and 140 ml/min per 1.73 m2, respectively, in women and men (12). Our higher figure is probably explained by the different sampling: our population is younger and is exclusively composed of homozygous SS patients, whereas the Marouf et al. study (12) was performed in a population composed of 48% of SS genotype and 52% of S/thalassemia genotype. Moreover, Marouf et al. reported a discordant prevalence of hyperfiltration in 44% and 10.2% of patients using, respectively, Cockcroft-Gault or cystatin C clearance, raising the issue of a potential bias in calculated eGFR compared with mGFR. The prevalence of true glomerular hyperfiltration in patients with SS disease assessed by mGFR, using urinary 51Cr EDTA renal clearance, has not been investigated to our knowledge, although a previous study performed in young men and women with SS disease (including some patients with CKD) has reported high mean mGFR values of 136 and 117 ml/min per 1.73 m2, respectively (13). Other studies support this finding using other mGFR methods, such as inulin (9) or iothalamate clearance (14). Our mGFR data from the subgroup of 48 patients validate the MDRD eGFR approach for the unbiased whole population (sensitivity 94%, specificity 63%, and predictive positive value 82%) because it gives a better estimate than Cockroft-Gault formula to assess hyperfiltration status, despite a systematic overestimation of GFR (see Table 3). The most likely explanations for such an overestimation are probably a lower mean body weight, a higher GFR in patients with SS disease compared with the MDRD population study (15), and an increased creatinine tubular secretion, which is positively correlated to mGFR (data not shown). Although the threshold value for hyperfiltration may be a matter of debate (16), depending on the method of reference for mGFR, our threshold of 110 ml/min per 1.73 m2 corresponded to the mean plus 2 SD of the value found in a control population selected among potential kidney graft donors previously investigated in our center.
Thus, our data show an 84% prevalence of SCAN in our population when we consider hyperfiltration together with albuminuria or renal failure as relevant criteria. It means that SCAN, although often unnoticed in the initial phase, is the most frequent feature among the many other chronic complications of SS disease. Indeed, our reported prevalence of 28% for priapism, 11% for leg ulcers, 27% for retinopathy, and 30% for osteonecrosis are in accordance with other reports (2,17).
The mechanism leading to an increased GFR was previously shown to be associated with enhanced renal blood flow (9,13,18,19), a pathologic feature also detected in early diabetic nephropathy at the stage of hyperfiltration (20). Interestingly, mean mGFR values obtained from microalbuminuric non-insulin-dependent diabetic patients are in about the same order of magnitude (9,21). However, apart from the normoglucose regulation and normal BP in the population with SS disease, another striking difference is a low filtration fraction in hyperfiltration SCAN (9,13), conversely to diabetic nephropathy and obesity-induced hyperfiltration (20,22), where the filtration fraction is higher than in healthy control subjects (9,20).
The pathophysiology is at present unraveled and possibly relates to an increased cardiac output, as suggested by the positive correlation of hyperfiltration with low hemoglobin levels. However, the presence of an abnormal vascular tone, such as a dysregulation of the myogenic response of afferent arterioles, leading to increased intrarenal blood flow, may also be at play (9,23) through a decreased nitric oxide (NO) availability (24) and endothelin activation, which have been reported in patients with SS disease (25,26). Indeed, intravascular hemolysis is known to impair NO bioavailability, mostly through plasma-free hemoglobin and arginase (23), thus raising the issue of whether hemolysis rather than viscosity would be responsible for hyperfiltration. In accordance with this view, among nonalbuminuric SCA patients the risk factors for hyperfiltration status were a young age together with well-known chronic hemolysis biomarkers, such as a low hemoglobin level, a low HbF, and the absence of alpha thalassemia. Indeed, a lower hemoglobin level and HbF level and a high reticulocyte count are considered hallmarks of high-rate chronic intravascular hemolysis (26–28). To identify the biomarkers associated to a high GFR, we selected a homogenous group of 53 SCA patients among this population with no alpha thalassemia, because alpha thalassemia is known to decrease hemolysis and to increase viscosity vaso-occlusive events (26,27). As expected, we found a strong negative correlation between eGFR and age (R2 = 19.9%) but an even stronger negative correlation with HbF level (R2 = 29.9%), as well as a weak positive correlation with reticulocyte count. Together, these data favor the view that hyperfiltration in patients with SS disease could be related predominantly to a predisease state related to chronic hemolysis (which has been associated previously with pulmonary hypertension, priapism, leg ulceration, and strokes) than viscosity vaso-occlusive complications (which comprise vaso-occlusive pain crisis, acute chest syndromes, and osteonecrosis) (28,29). This predisease state hypothesis could explain the lack of association between eGFR and clinical features related to hemolysis vasculopathy in our cross-sectional study.
Another issue is the natural history of SCAN, especially at the stage of hyperfiltration, to prevent subsequent histologic damages. Few therapeutical trials have addressed this issue of nephroprotection at the time of albuminuria using renin angiotensin system inhibitors (1,7,30). Although these trials have shown a beneficial effect on albuminuria excretion, GFR values were not investigated except in a 2-week trial that failed to show any variation of eGFR (1). These important issues require a long-term longitudinal follow-up to assess whether a nephroprotective treatment for isolated hyperfiltration could influence the natural history of SCAN.
To conclude, our data show a high prevalence of glomerular hyperfiltration among patients with SS disease, with a strong correlation with chronic hemolysis, suggesting a mechanism involving both an increased cardiac output and a hemolysis-mediated abnormal vascular tone, which deserve further studies (30). Thus, hyperfiltration recognition seems a relevant feature to adjust kidney-metabolizing drugs (to adapt doses of antibiotics, morphine…) but also to address the issue of the potential benefit of nephroprotective treatments at the early stage of SCAN before the occurrence of renal histologic lesions.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Glomerular Hyperfiltration in Sickle Cell Disease,” on pages 748–749.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC: Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 326: 910– 952, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Powars DR, Chan LS, Hiti A, Ramicone H, Johnson C: Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine 84: 363– 376, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Serjeant GR, Higgs DR, Hambleton IR: Elderly survivors with homozygous sickle cell disease. N Engl J Med 356: 642– 643, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int 67: 2089– 2100, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Dharnidharka VA, Dabbagh S, Atiyeh B, Simpson P, Sarnaik S: Prevalence of microalbuminuria in children with sickle cell disease. Pediatr Nephrol 12: 475– 478, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Guasch A, Navarrete J, Nass K, Zayas CF: Glomerular involvement in adults with sickle cell hemoglobinopathies. Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 17: 2228– 2235, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Foucan L, Bourhis V, Bangou J, Mérault L, Etienne-Julan M, Salmi RL: A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med 104: 339– 342, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Barros FB, Lima CS, Santos AO, Mazo-Ruiz MF, Lima MC, Etchebehere EC, Costa FF, Saad ST, Camargo EE, Ramos C: 51Cr-EDTA measurements of the glomerular filtration rate in patients with sickle cell anaemia and minor renal damage. Nucl Med Commun 27: 959– 962, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Schmitt F, Martinez F, Brillet G, Giatras I, Choukroun G, Girot R, Bachir D, Galacteros F, Lacour B, Grünfeld JP: Early glomerular dysfunction in patients with sickle cell anemia. Am J Kidney Dis 32: 208– 214, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763– 773, 2005 [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S1– S266, 2002 [PubMed] [Google Scholar]
- 12.Marouf R, Mojiminiyi O, Abdella N, Kortom M, Al Wazzan H: Comparison of renal function markers in Kuwaiti patients with sickle cell disease. J Clin Pathol 59: 345– 351, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson J, Reid M, Hambleton I, Serjeant GR: Albuminuria and renal function in homozygous sickle cell disease. Arch Intern Med 167: 701– 708, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Herrera J, Avila E, Marín C, Rodríguez-Iturbe B: Impaired creatinine secretion after an intravenous creatinine load is an early characteristic of the nephropathy of sickle cell anaemia. Nephrol Dial Transplant 17: 602– 607, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new predictive equation. Modification of diet in Renal Disease study group. Ann Intern Med 130: 461– 470, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Berg UB: Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant 21: 2577– 2582, 2006 [DOI] [PubMed] [Google Scholar]
- 17.van Beers EJ, van Tuijn CFJ, Mac Gillavry MR, van der Giessen A, Schnog JJB, Biemond BJ; on behalf of the CURAMA study group: Sickle cell disease-related organ damage occurs irrespective of pain rate: Implications for clinical practice. Haematologica 93: 757– 760, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ettledorf JW, Smith JD, Tuttle AH, Diggs LW: Renal haemodynamic studies in adults with sickle cell anaemia. Am J Med 18: 243– 248, 1955 [DOI] [PubMed] [Google Scholar]
- 19.Guasch A, Cua M, You W, Mitch W: Sickle cell anemia causes a distinct pattern of glomerular dysfunction. Kidney Int 51: 826– 833, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA: Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17: 1703– 1709, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Vedel P, Obel J, Nielsen FS, Bang LE, Svendsen TL, Pedersen OB, Parving HH: Glomerular hyperfiltration in microalbuminuric NIDDM patients. Diabetologia 39: 1584– 1589, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, Gafter U: Obesity-induced glomerular hyperfiltration: Its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 23: 3946– 3952, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbahi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL: Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle cell disease. J Clin Invest 118: 1924– 1933, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Just A, Arendshorst WJ: Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol 569: 959– 974, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter CD, Wang XD, Tanus-Santos JE, Hogg N, Cannon R, Schechter AN, Gladwin MT: Cell-free hemoglobin limits nitric oxide bioavailability in sickle cell disease. Nat Med 8: 1383– 1389, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Steinberg MH: Predicting clinical severity in sickle anemia. Br J Haematol 129: 465– 481, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kato GJ, Gladwin MT, Steinberg MH: Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37– 47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, 6th, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr., Gladwin MT: Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood 107: 2279– 2285, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki RY, Saad STO: Enalapril reduces the albuminuria of patients with sickle cell disease. Am J Med 98: 432– 435, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT: Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84: 618– 625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]