Abstract
Background and objectives: Chronic kidney disease (CKD) is a challenge for pregnancy. Its recent classification underlines the importance of its early phases. This study's aim was to evaluate outcomes of pregnancy according to CKD stage versus low-risk pregnancies followed in the same center.
Design, setting, participants, & measurements: The prospective analysis was conducted from January 2000 to May 2009 with the start of observation at referral and end of observation 1 month after delivery. Ninety-one singleton deliveries were studied; 267 “low-risk” singleton pregnancies served as controls. Because of the lack of hard end points (death, start of dialysis), surrogate end points were analyzed (cesarean section, prematurity, neonatal intensive care).
Results: CKD outcome was worse than physiologic pregnancies: preterm delivery (44% versus 5%); cesarean section (44% versus 25%); and need for neonatal intensive care (26% versus 1%). The differences were highly significant in stage 1 CKD (61 cases) versus controls (CKD stage 1: cesarean sections = 57%, preterm delivery = 33%, intensive care = 18%). In CKD, proteinuria and hypertension were correlated with outcomes [proteinuria dichotomized at 1 g/24 h at referral: need for intensive care, relative risk (RR) = 4.16 (1.05 to 16.46); hypertension: preterm delivery, RR = 7.24 (2.30 to 22.79); cesarean section, RR = 5.70 (1.69 to 19.24)]. Statistical significance across stages was reached for preterm delivery [RR = 3.32 (1.09 to 10.13)].
Conclusions: CKD is a challenge for pregnancy from early stages. Strict follow-up is needed for CKD patients, even when there is normal renal function.
Chronic kidney disease (CKD) is a growing health care problem only recently acknowledged in its full dimension (1). The recent redefinition of CKD led to the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines on diagnosis and staging of CKD; these focus attention on the earlier stages of the disease, when persistent signs of renal damage are present but renal function may still be in the normal ranges (2). Because of this broader definition, it has been calculated that 3% of women of childbearing age are affected by CKD— an impressive number when compared with the 0.1% to 1% prevalence calculated according to previous criteria (3,4).
No classification is perfect, and the CKD classification has been extensively criticized. However, the CKD classification has the advantage of simplicity and of encompassing, at least partly, for other biases, such as the lack of sensitivity of serum creatinine in the early stages of kidney disease (5).
The central role of pregnancy in the development of acute renal damage and hypertension (better known as preeclampsia) has been known for over a century, whereas the relationship between preeclampsia (PE) and the subsequent risk of CKD/ESRD was only recently elucidated (6). From the opposite point of view, it has been known for decades what a great risk renal function impairment is for the outcome of pregnancy. Small case series during the 1960s showed that fetal mortality in the presence of maternal kidney disease approached 100%, and the first case reported on dialysis was considered almost a “miracle of medicine” (7). Although this grim outcome has improved over the last decades, recent studies still show significantly poor fetal and maternal outcomes; the overall risk of negative maternal-fetal outcomes is inversely related to renal function and increases with proteinuria (8–33).
The review of 25 studies dealing with at least 25 pregnancies published in English in the last 10 years (8–32) revealed heterogeneous definitions and classifications of CKD: the categories are mostly based on serum creatinine, but the cut points are different (20,29,31); a few studies use GFR, but cut points are seldom the same, thus adding to the dispersion of the results (10). Within these limits and taking into account the different diseases considered, prematurity ranges from 5% to 100%, low birth weight rates range from 2% to 60%, and fetal death rates range from none to approximately one-third of cases (8–32). The early phases of CKD are rarely considered, which is not surprising given that they are often asymptomatic and thus can be diagnosed only if specifically searched for.
With this background, we conducted the prospective study presented here on pregnancy outcomes in CKD, defined according to the K/DOQI guidelines, over a period (2000 to 2009) in which all women were followed by the same multidisciplinary group. Although we acknowledge its limits, the K/DOQI classification was chosen as representative of the presently accepted international standards in nonpregnant women, which allows for easier comparisons across different settings (2,5).
A control group of physiologic “low-risk” pregnancies that were followed in the same setting allowed comparison of the results. The study had two main goals: (1) to analyze the overall outcome of pregnancies in CKD in our setting, stratified according to the K/DOQI definition; and (2) in particular, to compare the outcomes of the earliest stage of CKD (in which renal function is normal) with physiologic pregnancies.
Materials and Methods
Study Setting and Inclusion Criteria
The study was conducted in the Materno-Fetal Medicine Unit of Sant'Anna University Hospital in Turin, Italy. The Materno-Fetal Medicine Unit had 15 beds up until 2008 and 25 since 2009 within a university hospital with 150 beds for obstetric patients. In 2008, the Materno-Fetal Medicine Unit followed over 400 outpatients in services dedicated to maternal problems (cardiac diseases, diabetes, hypertension, epilepsy, psychiatric diseases, multiple pregnancies, kidney diseases, and other internal medicine problems). In the study period, approximately 700 deliveries per year were followed in the unit: over 70% with maternal-fetal pathology.
Starting in 2000, all patients with kidney disease were followed by the same obstetrical and nephrological team, and since 2002 this has been in a dedicated outpatient unit. Data were gathered prospectively from the start of the activity. Patients were referred from different nephrology units, regional prenatal care centers, and since 2007 from general physicians. The study included all patients referred to the outpatient unit or admitted to the obstetrics ward who had a diagnosis of CKD before or during pregnancy.
Patients and Control Population
Overall, 120 pregnancies were observed in 110 women (January 1, 2000 to May 31, 2009). Eight women had two pregnancies (two ended in spontaneous abortions); one woman had three pregnancies (two abortions).
For the study, each pregnancy was considered a separate “case.” Dropout from the study was minimal (one case). Twin pregnancies, abortions, dropouts, and ongoing pregnancies were excluded, leaving 91 singleton deliveries for the statistical analysis. The control group was a cohort of 297 singleton low-risk pregnancies cared for in the same maternal-fetal unit from 1999 to 2007 (Figure 1).
Figure 1.
Flow chart of patients and controls. Note: the patients: Spontaneous abortions: median age 33.5 years (6 primiparous); four stage 1, two stage 2 and one stage 3; two patients with previous acute pyelonephritis, two with chronic interstitial disease, one with systemic lupus erithematosus, one with kidney transplantation and one with diabetic nephropathy. Termination of pregnancy: median age 27.25 years; one primiparous; two stage 1, one stage 2 and one stage 4; one acute pyelonephritis, one membranous glomerulonephritis and 2 terminations in the same patient, with single kidney and focal glomerulosclerosis.
This control population includes the activity on physiologic low-risk pregnancies performed in the Maternal Fetal Unit the period 1999 to 2007 during which a small outpatient service was dedicated to physiologic pregnancies. New pregnant patients without known risk factors were assigned to the different outpatient units according to the initial waiting time; thus, the control population is representative of the overall population referred to the entire hospital. The service was closed in 2007 because the Materno-Fetal Unit is presently dedicated only to pathologic pregnancies. In the control population, all cases with at least two control visits during pregnancy and with complete birth data were considered. The control population was defined as low risk because all patients with diseases, risk conditions, or with multiple pregnancies were assigned to the dedicated services.
The following data were considered for cases and controls: age, parity, race, week of the first visit, educational level, body mass index, number of admissions, gestational age at delivery, type of delivery, clinical complications in the mother, fetal weight, Apgar index, sex, admission in intensive care unit, and outcome.
In 72 CKD patients referred within the 20th week, it was possible to analyze the variations in blood pressure (BP) and proteinuria throughout pregnancy.
Definitions Used
GFR Measurement.
In the study population, CKD was classified according to K/DOQI guidelines as “every anomaly of blood and urine composition, or imaging or pathologic data, lasting for at least 3 months or with a GFR below 60 ml/min for the same time period” (2,3). Because the two most widely used formulas [Cockcroft–Gault and Modifications in Diet and Renal Disease (MDRD)] have important limits in pregnancy, GFR calculation was based whenever possible on preconception data within 3 months before conception (34,35). The choice of Cockcroft–Gault was motivated by the consideration of maternal weight in the formula, thus partially adjusting for very low or very high weight. This was considered of importance in a setting such as Italy where low weight is common in women, but severe obesity is increasing. The MDRD formula was also applied, and the cases shifting from one stage to another were analyzed. When preconception data were not available, serum creatinine measured at first control in pregnancy was used. This decision may involve a bias because of the physiologic decrease of serum creatinine during the first phases of pregnancy leading to an increase in GFR over the baseline data (2,3,34,35). However, because the bias was unavoidable because of the frequent lack of recent preconception data, the patients were also stratified according to the availability of preconception data.
Other Definitions.
CKD was classified into broad categories: GN (primary and secondary); interstitial nephropathy, chronic pyelonephritis (including malformations, reflux nephropathy, and residual scars of previous pyelonephritis), diabetic nephropathy; polycystic kidney disease, isolated persistent urinary anomalies, recurrent stone disease (with multiple stones and/or kidney scars), acute pyelonephritis occurring during pregnancy, kidney transplantation [considered by definition an alteration of kidney morphology; however, the three cases in our series also displayed persistent urinary anomalies (two patients) and renal function impairment (one case)], and other-unknown. Because evidence of kidney scars is difficult to obtain during pregnancy because of the limitation on radiologic tests, the eight patients with acute pyelonephritis occurring in pregnancy were also analyzed separately; all were classified in CKD stage 1.
Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg or antihypertensive therapy; patients on antihypertensive therapy before conception were classified as affected by chronic hypertension even when antihypertensive therapy was discontinued in pregnancy.
PE was defined as the appearance of hypertension with systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg accompanied by proteinuria ≥300 mg/24 h after 20 weeks of gestational age in a previously normotensive woman (36). Because the definition of PE may partly overlap with CKD, the CKD patients were also stratified according to the presence and degree of proteinuria and hypertension. The definition of PE was applied only to CKD patients with no hypertension and proteinuria <0.3 g before the 20th week of gestational age.
A newborn was defined as small for gestational age (SGA) when the birth weight was below the 10th centile according to Italian birth weight references; cut points below the 5th and the 10th centile were tested (37).
Preterm delivery was defined as delivery before 37 completed weeks of gestational age (38); a further analysis was performed for “more severe” preterm deliveries, defined as deliveries before 34 completed weeks (39).
Prenatal and Intrapartum Care
Prenatal and intrapartum care for low-risk pregnancies was based on current guidelines (40,41). The frequency of nephrological and obstetric control visits for CKD patients was individualized (weekly/monthly). The nephrologist controlled hospitalized CKD patients at least once a week.
At each clinical consultation, BP was measured at least once, weight was recorded, fetal well being was assessed, and fetal growth was controlled by serial measurements of symphysis fundus height. Ultrasound biometry and Doppler velocimetry of uterine and umbilical arteries were individualized (every 2 to 4 weeks in cases at risk of fetal growth restriction). In patients at risk of proteinuria and urinary infections, urinalysis and urine cultures were controlled frequently (usually weekly in the first and last trimesters); the patients and their general physicians were instructed to call in case of abnormal results. In acute pyelonephritis, stone disease, reflux nephropathy, and other malformations, ultrasounds of the mother's kidneys were scheduled every 3 months. All patients were instructed to measure BP at home; hypertensive cases were asked to keep a diary with two to three measurements per day. The patients were asked to call the unit if BP rose above the target levels. Twenty-four-hour BP measurement was used in the case of discrepancies between consultations and diary, to assess nocturnal dipping, and during hospitalization in hypertensive patients.
In addition to the routine laboratory controls of pregnancy, CKD patients underwent a monthly measurement of renal function and of proteinuria, uric acid, urinalysis and urinary culture, serum electrolytes, coagulation parameters, and blood cell counts; other laboratory data were required on demand.
Hospitalization was required for uncontrolled or new onset of hypertension, worsening of renal function, new onset or worsening of proteinuria, upper urinary tract infection, and for any problem of mother and/or fetus (usually abnormal fetal growth with severely abnormal umbilical Doppler). Patients with severe CKD referred from distant settings were routinely hospitalized in the last phases of pregnancy.
The BP therapeutic goal was ≤130/80 mmHg. Drugs of first choice were nifedipine or α-methyldopa, with the latter preferred in the case of intense proteinuria or peripheral edema. Beta-blockers or doxazosine were used in the case of insufficient response or severe side effects with the above drugs.
In every case, the aim was to delay delivery as much as possible until 36 weeks according to the recent approaches to “late preterm” deliveries (42).
Indications for early delivery were severe worsening of maternal and/or fetal conditions until 32 weeks of gestational age or less severe worsening after 32 weeks. In addition to the classic hallmarks of PE or HELLP syndrome, worsening of maternal conditions included severe and noncontrolled hypertension, rapidly increasing proteinuria with nephrotic syndrome, or rapid increase in serum creatinine alone or in combination. For fetal conditions, worsening included abnormal fetal heart rate tracings at any gestational age, absent end diastolic flow velocities at Doppler interrogation of the umbilical arteries at or after 32 weeks of gestational age, and no fetal growth over 2 weeks at later gestational age. In these cases, betamethasone for induction of lung maturation was routinely administered at standard doses. Cesarean section was performed for fetal indications before or during labor or in cases of unfavorable conditions for induction or lack of response to induction. The main indications for admission to the neonatal intensive care unit (NICU) were birth weight <1500 g, gestational age <34 weeks, Apgar score below 7 at 5 minutes, and need for intubation.
Statistical Analyses
The data were gathered prospectively, periodically controlled, and entered into the electronic database. The start of observation was referral to the unit, and the end of observation was 1 month after delivery. For women with severe CKD (stages 3 to 5), efforts were made to gather renal functional data and long-term data on the babies at 6 months and 1 year after delivery.
Descriptive analysis was performed as appropriate (mean and SD for parametric and median and range for nonparametric data). The t test, χ2 test, and ANOVA were used for comparisons between cases and controls and among groups. Significance was set at <0.05.
Because no maternal or fetal deaths were observed and none of the patients who delivered needed dialysis during pregnancy or in the first months after delivery, we limited our analysis to short-term maternal-fetal outcomes (surrogate outcomes), including preterm delivery (<37 and <34 weeks), SGA, admission to the NICU, and cesarean section. Univariate odds ratios and multivariate regressions were calculated for the chosen outcomes (SPSS, Inc.) (43).
Common parameters were analyzed with the control pregnancies as reference data; analysis of the effect of hypertension, proteinuria, and diagnosis was performed only within the CKD cohort. The following comparisons were made: CKD all stages versus controls; CKD stage 1 versus controls; CKD stage 1 versus stages 2 to 5; CKD stage 1 versus stage 2; and CKD stage 1 versus stages 3 to 5. Further analyses included referral to the unit before or after 12 weeks of gestational age, new diagnosis of CKD versus known CKD, hypertension (yes versus no), proteinuria at referral present versus absent and with three strata (<300 mg/d, 300 mg to <1 g/d, and ≥1 g/d), maternal age (dichotomized at the median), parity (primiparous versus other), educational level (primary school versus others), and race (Caucasian versus others).
Results
Baseline Data
The baseline data are reported in Table 1 for all patients (120 cases), patients who delivered a single baby (91 cases), all of the control population (297 pregnancies), and controls who delivered and for whom the follow-up was available (267 pregnancies). In both cases and control population, there was no difference in any of the tested parameters between all referred pregnancies and women who delivered, indicating the absence of attrition bias after referral (Table 1). CKD patients were about 2 years older, and the prevalence of Caucasian origin and educational level were higher in this group (Table 1). The differences in age and educational level were significant if women of Caucasian origin only were considered. As for the non-Caucasian patients, they were mainly of African origin in cases and controls (patients who delivered: cases 7 of 91, 7.6%; controls 37 of 267, 13.8%), followed by Asian origin in cases (2.2%) and Latin American in controls (8.2%). The interpretation of these differences is only tentative because they might merely be due to the relatively small number of cases; however, one can speculate that better educated, Caucasian women are more frequently referred to the appropriate settings before or during pregnancy.
Table 1.
Main data at baseline in all cases, cases who delivered a singleton, all controls, and controls who delivered a singletona
| All Cases (n = 120) | Cases Who Delivered a Singleton (n = 91) | All Controls (n = 297) | Controls Who Delivered a Singleton (n = 267) | Patients versus Controls (all cases referred) | Patients versus Controls (only singleton delivery) | |
|---|---|---|---|---|---|---|
| Maternal age, years, mean ± SDb | 31.26 ± 5.36 | 31.16 ± 5.33 | 29.44 ± 5.31 | 29.41 ± 5.31 | P = 0.0016 | 0.043 |
| Nulliparous, %c | 60 | 61.53 | 60.30 | 61.4 | NS | NS |
| Week of referral, median (range)d | 12 (4 to 38) | 13 (4 to 38) | 12 (4 to 32) | 12 (4 to 32) | NS | NS |
| Caucasian race, %c | 89.16 | 89 | 77.4 | 77.2 | P = 0.005 | P = 0.022 |
| Educational level > 8th grade, %ce | 61.34 | 65.9 | 45.4 | 46 | P = 0.0053 | P = 0.001 |
| BMI, mean ± SDbf | 23.34 ± 4.64 | 23.44 ± 4.78 | 22.54 ± 3.61 | 22.51 ± 3.63 | NS | NS |
No statistical difference between patients who delivered and all referred cases or between all controls and controls who delivered for all parameters.
t test.
χ2 test.
Mann–Whitney test.
Educational level available data: 119 of 120 in cases and 295 of 297 in controls.
BMI, body mass index available data: 119 in cases and 296 in controls.
In 21 of 120 cases (17.5%), the diagnosis of CKD was made during pregnancy (16 delivered a single baby, 2 twin pregnancies, 2 ongoing, 1 decided to terminate the pregnancy). The baseline data did not differ between patients diagnosed with a renal disease in pregnancy and all other cases. In CKD patients, stratified according to the functional stage (Cockcroft–Gault), there was no significant difference among stages for age, parity, race, educational level, or body mass index (Table 2).
Table 2.
The main clinical data of CKD patients
| Data | CKD Stage 1: GFR > 90 ml/min (n = 61) | CKD Stage 2: GFR 60 to 89 ml/min (n = 15) | CKD Stage 3: GFR 30 to 59 ml/min (n = 11) | CKD Stage 4: GFR 15 to 29 ml/min and CKD Stage 5: GFR < 5 ml/min (n = 4) | Statistical Significance across Stages | Statistical Significance Stage 1 versus All Other Stages |
|---|---|---|---|---|---|---|
| Maternal age, years, mean ± SDa | 30.95 ± 5.58 | 31.26 ± 5.02 | 33.36 ± 4.12 | 28 ± 4.96 | NS | NS |
| Nulliparous, %b | 35 (57.37%) | 9 (60%) | 9 (81.8)% | 3 (75%) | NS | NS |
| Caucasian race, %b | 57 (93.44%) | 12 (80%) | 10 (90.91%) | 2 (50%) | P = 0.033 | NS |
| Educational level > 8th grade, %b | 40 (65.57%) | 6 (40%) | 11 (100%) | 3 (75%) | NS | NS |
| BMI, mean ± SDa | 24.11 ± 5.12 | 21.53 ± 4.70 | 22.1 ± 2.75 | 20.5 ± 1.47 | NS | NS |
| Week of referral, median (range)c | 15 (4 to 38) | 12 (6 to 26) | 8 (5 to 20) | 7 (6 to 28) | NS | NS |
| Hypertension, % | 16 (26.23%) | 6 (40%) | 8 (72.7%) | 1 (25%) | P = 0.032 | P = 0.044 |
| Creatinine, mg/dl, median (range)c | 0.65 (0.4 to 1.10) | 0.89 (0.64 to 1.68) | 1.45 (1.12 to 1.9) | 2.82 (2 to 3.8) | P < 0.0001 | P < 0.0001 |
| GFR, ml/min, mean ± SD * | 129.17 ± 36.38 | 75.12 ± 10.6 | 48.2 ± 7.8 | 23.07 ± 3.07 | P < 0.0001 | P < 0.0001 |
| Proteinuria, g/24 h, median (range)c | 0.10 (0 to 7.8) | 0.15 (0 to 6.8) | 0.7 (0 to 4) | 0.56 (0.3 to 0.65) | P = 0.01 | P = 0.001 |
| Proteinuria ≤ 0.3 g/24 h, %b | 44 (72.13%) | 9 (64.28%) | 4 (33.3%) | 1 (25%) | NS | P = 0.032 |
| Proteinuria > 0.3 g ≤ 1 g/24 h, %b | 11 (18.03%) | 2 (14.28%) | 2 (16.6%) | 3 (75%) | P = 0.054 | NS |
| Proteinuria > 1 g/ 24 h, %b | 6 (9.83%) | 4 (26.66%) | 5 (45.4%) | 0 | P = 0.004 | P = 0.032 |
| Renal diseases | GN 13; PN 18; APN 5; K stones 6; transplant 2; PKD 6; Diab 2; other 9 | GN 5; PN: 6; APN 1; Diab 2; other 1 | GN 2; PN 4; transplant 1; Diab 3; other 1 | PN 4 |
PN, interstitial nephritis and chronic pyelonephritis; APN, acute pyelonephritis; K stones, kidney stones (recurrent); PKD, polycystic kidney disease; Diab, diabetic nephropathy; other, persistent isolated urinary anomalies.
ANOVA.
χ2 or Fisher test.
Kruskal–Wallis test.
The MDRD formula would have reclassified 11 of 120 cases. The shift was from a lower to a higher stage in five women (three from stage 2 to stage 3; two from stage 1 to stage 2), all with pregravidic weight >100 kg (range 101 to 112 kg); and from a higher to a lower stage in six cases (five from stage 2 to stage 1; one from stage 3 to stage 2), five of six with pregravidic weight <50 kg.
The median week of referral was higher, albeit nonsignificantly, in CKD stage 1 versus all others.
Pregravidic serum creatinine (within 3 months of preconception) was available in approximately half of the cases (43 of 91 patients); whereas 21 cases were new diagnoses, in the remaining patients no recent renal functional control was available. In none of the cases with available recent pregravidic data would the CKD stage have changed if calculated upon the first control available during pregnancy.
GN, interstitial nephropathies, and pyelonephritis were the most common renal diseases in all groups. In keeping with the definitions of CKD, the mean creatinine and GFR varied significantly across the stages. The prevalence of hypertension and proteinuria increased significantly. The exception was the low prevalence of hypertension in stages 4 to 5, presumably because of the low risk of hypertension in interstitial nephropathies (Table 2).
Hypertension, Proteinuria, and Serum Creatinine over the Gestational Period
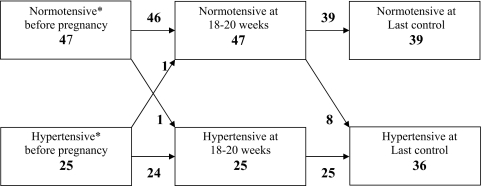
For this analysis, 72 patients who delivered a single baby and were referred within the 20th week of gestation were considered. The trends toward increased prevalence of hypertension and proteinuria are shown in Figures 2 and 3 and Table 3: 47 patients (65%) were normotensive before pregnancy; 9 developed hypertension—1 within the 20th week and 8 thereafter. Twenty-five patients were hypertensive at the start of pregnancy. Although antihypertensives were often reduced or discontinued in the early stages, only one patient remained normotensive by the 20th week (Figure 2). A similar trend was observed for proteinuria and for serum creatinine (Figure 3, Table 3).
Figure 2.
Modification of proteinuria from first to last control in 72 patients referred within the 20th week of gestation. The last control was within 2 weeks of delivery.
Figure 3.
Prevalence of hypertension before pregnancy (according to the anamnesis) through the control at 18 to 20 weeks and the last control before delivery in 72 patients referred within the 20th week of gestation.
Table 3.
Modifications over time (first versus last control) in the 72 cases referred within the 20th week to the unit who delivered a single baby
| CKD Stage (cases) | Proteinuria First Control, g/24 h, median (range) | Proteinuria Last Control, g/24 h, median (range) | First versus Last Control (Wilcoxon test) | Serum Creatinine First Control, mg/dl, median (range) | Serum Creatinine Last Control, mg/dl, median (range) | First versus Last Control (Wilcoxon test) |
|---|---|---|---|---|---|---|
| 1 (n = 45) | 0.11 (0 to 5.2) | 0.16 (0 to 9.4) | P = 0.014 | 0.67 (0.4 to 1.1) | 0.74 (0.4 to 0.9) | P = 0.005 |
| 2 (n = 13) | 0.13 (0 to 6.8) | 0.35 (0 to 10.1) | P = 0.084 | 0.89 (0.6 to 1.7) | 1 (0.7 to 1.8) | P = 0.142 |
| 3 (n = 11)a | 0.7 (0 to 4) | 2.45 (0.5 to 17.3) | P = 0.004 | 1.50 (1.1 to 1.9) | 1.78 (1.1 to 5) | P = 0.013 |
| 4 to 5 (n = 3) | 0.63 (0.3 to 0.6) | 1.63 (0.7 to 2.6) | NA | 2.74 (2 to 3.8) | 2.7 (1.73 to 3.7) | NA |
| All cases (n = 72) | 0.19 (0 to 6.8) | 0.31 (0 to 17.3) | P < 0.001 | 0.74 (0.4 to 3.8) | 0.79 (0.4 to 5) | P < 0.001 |
NA, not assessed (three cases).
One patient doubled serum creatinine level.
The incidence of PE was 1.1% in the control low-risk group. Only 1 CKD patient developed PE out of 36 who were normotensive and with proteinuria <0.3 g at the 20th week.
Maternal and Fetal Outcomes: Overall Data
No maternal or fetal death occurred within 1 month after delivery (end of follow-up) in the study group or control group. In the study group, one child was affected by dwarfism (achondroplasia), one child had a single kidney, and one baby delivered at the 30th week developed mild neurologic sequelae after cerebral hemorrhage.
The main maternal-fetal data at delivery are reported in Table 4. Differences among cases and control population were assessed as all cases versus controls, CKD stage 1 versus controls, and CKD stage 1 versus all other stages. All major outcomes were significantly worse in CKD patients compared with controls except for SGA.
Table 4.
The main maternal-fetal outcomes in the study groupa
| Outcomes | Controls (n = 267)b | CKD Stage 1: GFR > 90 ml/min (n = 61) | CKD Stage 2: GFR 60 to 89 ml/min (n = 15) | CKD Stage 3: GFR 30 to 59 ml/min (n = 11) | CKD Stages 4 to 5: GFR < 30 ml/min (n = 4) | CKD All Stages (n = 91) | Statistical Significance (cases versus controls) | Statistical Significance (stage 1 versus controls) | Statistical Significance (stage 1 versus stages 2 to 5) |
|---|---|---|---|---|---|---|---|---|---|
| Cesarean section, % | 66 (24.7%) | 35 (57.4%) | 6 (40%) | 9 (81.8%) | 3 (75%) | 53 (58.2%) | P < 0.0001 | P < 0.0001 | NS |
| Delivery <37 weeks, % | 13 (4.9%) | 20 (32.9%) | 6 (40%) | 10 (90.9%) | 4 (100%) | 40 (44%) | P < 0.0001 | P < 0.0001 | P = 0.004 |
| Delivery <34 weeks, % | 4 (1.5%) | 9 (14.8%) | 2 (13.3%) | 6 (54.5%) | 2 (50%) | 19 (20.9%) | P < 0.0001 | P < 0.0001 | NS |
| Gestational age, weeks, mean ± SD | 39.2 ± 1.91 | 36.9 ± 2.9 | 36.7 ± 2.9 | 33.6 ± 2.8 | 32 ± 3.2 | 36.2 ± 3.2 | P < 0.0001 | P < 0.0001 | P = 0.005 |
| Fetal weight, g, mean ± SD | 3268.3 ± 500.4 | 2795.7 ± 745 | 2761.3 ± 518 | 2050.5 ± 696 | 1246.3 ± 397.1 | 2631.7 ± 786.5 | P < 0.0001 | P < 0.0001 | P = 0.004 |
| SGA, % (<10th centile) | 28 (10.5%) | 8 (13.1%) | 1 (6.7%) | 3 (27.3%) | 3 (75%) | 15 (16.5%) | NS | NS | NS |
| Admission to NICU, % | 3 (1.1%) | 11 (18%) | 3 (20%) | 6 (54.5%) | 4 (100%) | 24 (26.7%) | P < 0.0001 | P < 0.0001 | P = 0.02 |
Limiting the analysis to stage 1, two cut points of proteinuria were tested: (≥ 0.3 g/24 h: 44 cases; >0.3 g/24 h: 17 cases; ≥ 1 g/24 h: 55 cases; >1 g/24 h: 6 cases). Statistical differences were found as for cesarean section gestational age with both cut points and with need for NICU, fetal weight, and delivery <34 weeks with the cut point at 1 g/d. Statistically significant differences between controls and stage 1 persist omitting the cases with proteinuria >1 g/d from stage 1 CKD.
Incidence of PE in controls = 1.1%. Statistical tests included χ2 and t test.
The differences were also significant when comparison was limited to CKD stage 1 patients versus controls.
Within CKD stage 1, presence/absence of proteinuria (≥0.3 g/24 h) and proteinuria dichotomized at 1 g/24 h were significant predictors of outcome: statistical differences were found for cesarean section and gestational age with both cut points and for admission to NICU, low fetal weight, and delivery <34 weeks with the cut point at 1 g/24 h. However, statistically significant differences between control low-risk population and stage 1 CKD patients persisted even when omitting the cases with proteinuria >1 g/24 h.
Most outcomes, including gestational age at delivery, fetal weight, and need for NICU admission, were better in CKD stage 1 than in all of the other stages; however, the differences were mainly because of worse outcomes in the last three stages (Table 4).
Preterm delivery was mostly iatrogenic for maternal (26 cases) or fetal indication (8 cases) or a combination of both (3 cases); only three of them were spontaneous.
Four pregnancies were terminated during the first or early second trimester: two because of fetal problems (severe fetal infection; severe multiple malformations). In two cases (severe renal failure and nephrotic syndrome), the women chose to terminate the pregnancy after thorough counseling by the caregivers and discussion of the risks and possibility of success; the decision was also affected by the prevision of long periods of hospitalization. In such a setting, the distinction between therapeutic and elective abortion may be difficult. One woman who started pregnancy in stage 4 CKD underwent preemptive transplantation 20 months after delivery. Two patients (CKD stages 2 and 4 at referral) are in a strict predialysis follow-up. One patient started dialysis 8 months after abortion of her fourth pregnancy (CKD stage 4 at the beginning of her fourth pregnancy).
Odds Ratios and Logistic Regression Analysis
Odds ratios were calculated in univariate and multivariate analyses as for cesarean section, preterm delivery, and need for NICU admission. The outcome of SGA was not analyzed because of its very low incidence in patients and controls. Odds ratios were significantly high for all of the selected outcomes when all patients versus all controls were considered (Table 5); likewise the odds ratios were significantly high when CKD stage 1 patients versus controls were considered (Table 6).
Table 5.
Summary data from the logistic regression analyses comparing all CKD cases versus controls for the chosen outcomesa
| Outcome | Cesarean Sections (n = 116) | Preterm Delivery <37 Weeks (n = 52) | Preterm Delivery <34 Weeks (n = 23) | Need for NICU (n = 27) |
|---|---|---|---|---|
| Controls (260)b | 4.52 (2.68 to 7.63) | 16.84 (7.99 to 35.50) | 15.80 (5.03 to 49.64) | 30.77 (8.65 to 109.46) |
| All CKD patients (91) | ||||
| Maternal age ≤30 years (194) | 0.92 (0.56 to 1.50) | 1.23 (0.60 to 2.50) | 2.14 (0.78 to 5.83) | 1.22 (0.47 to 3.13) |
| Maternal age >30 years (157) | ||||
| Nulliparous (221) | 1.08 (0.65 to 1.77) | 0.97 (0.47 to 2.01) | 0.47 (0.16 to 1.39) | 0.40 (0.14 to 1.19) |
| Pluriparous (130) | ||||
| Non-Caucasian (69) | 1.01 (0.55 to 1.86) | 0.63 (0.25 to 1.58) | 0.56 (0.16 to 1.97) | 0.51 (0.15 to 1.74) |
| Caucasian (282) | ||||
| Referral ≤12 weeks (193) | 0.83 (0.51 to 1.34) | 0.99 (0.49 to 1.97) | 1.04 (0.41 to 2.67) | 0.90 (0.36 to 2.23) |
| Referral >12 weeks (158) |
Data presented as odds ratio (95% confidence interval).
Complete data available for 260 of 267 cases.
Table 6.
Summary data from the logistic regression analyses comparing CKD stage 1 cases and controls for the chosen outcomesa
| Outcome | Cesarean Sections (n = 98) | Preterm Delivery <37 Weeks (n = 32) | Preterm Delivery <34 Weeks (n = 13) | Need for NICU (n = 14) |
|---|---|---|---|---|
| Controls (260)b | 4.37 (2.38 to 8.02) | 9.75 (4.25 to 22.39) | 8.99 (2.53 to 31.92) | 15.17 (3.90 to 59.04) |
| CKD stage 1 (61) | ||||
| Maternal age ≤30 years (181) | 0.97 (0.58 to 1.64) | 1.49 (0.65 to 3.41) | 3.48 (0.87 to 13.97) | 1.89 (0.55 to 6.53) |
| Maternal age >30 years (140) | ||||
| Nulliparous (198) | 1.10 (0.65 to 1.85) | 1.18 (0.52 to 2.69) | 0.62 (0.17 to 2.22) | 0.41 (0.10 to 1.60) |
| Pluriparous (123) | ||||
| Non-Caucasian (63) | 1.05 (0.55 to 2.01) | 0.97 (0.31 to 3.16) | 1.49 (0.17 to 12.81) | 1.51 (0.17 to 13.16) |
| Caucasian (258) | ||||
| Referral ≤12 weeks (176) | 0.76 (0.46 to 1.27) | 0.93 (0.41 to 2.10) | 0.73 (0.21 to 2.49) | 1.05 (0.32 to 3.46) |
| Referral >12 weeks (145) |
Data presented as odds ratio (95% confidence interval).
Complete data available for 260 of 267 cases.
The data were more sparse for the comparison among functional stages. Proteinuria, hypertension, and functional stage were correlated with the different outcomes, but in our series none was a strong single determinant of all of them (Table 7).
Table 7.
Summary data from the logistic regression analyses comparing CKD stage 1 cases versus all other stages for the chosen outcomesa
| Outcome | Cesarean Sections (n = 53) | Preterm Delivery <37 Weeks (n = 40) | Preterm delivery <34 Weeks (n = 19) | Need for NICU (n = 24) |
|---|---|---|---|---|
| CKD stage 1 (61) | 0.69 (0.24 to 2.01) | 3.32 (1.09 to 10.13) | 1.73 (0.52 to 5.76) | 2.32 (0.77 to 6.99) |
| CKD stages 2 to 5 (30) | ||||
| Maternal age ≤30 years (39) | 0.55 (0.20 to 1.52) | 0.55 (0.18 to 1.67) | 1.20 (0.35 to 4.10) | 0.67 (0.22 to 2.04) |
| Maternal age >30 years (52) | ||||
| Nulliparous (63) | 1.78 (0.623 to 5.11) | 1.03 (0.34 to 3.12) | 0.30 (0.07 to 1.27) | 0.49 (0.14 to 1.72) |
| Pluriparous (28) | ||||
| Non-Caucasian (10) | 1.41 (0.28 to 7.13) | 0.99 (0.17 to 5.70) | 0.99 (0.17 to 5.75) | 0.97 (0.19 to 4.87) |
| Caucasian (81) | ||||
| Referral ≤12 weeks (45) | 0.80 (0.30 to 2.13) | 1.32 (0.46 to 3.80) | 1.48 (0.44 to 4.92) | 1.19 (0.40 to 3.59) |
| Referral >12 weeks (46) | ||||
| Proteinuria ≤ 1 g/24 h (76)b | 3.14 (0.55 to 17.89) | 2.50 (0.50 to 12.36) | 2.56 (0.62 to 10.63) | 4.16 (1.05 to 16.46) |
| Proteinuria >1 g/24 h (15)b | ||||
| Normotension (31)b | 5.70 (1.69 to 19.24) | 7.24 (2.30 to 22.79) | 3.35 (0.94 to 11.91) | 1.78 (0.54 to 5.83) |
| Hypertension (60)b |
Data presented as odds ratio (95% confidence interval).
Proteinuria and hypertension defined at the first clinical control.
Discussion
The recent broader definition of CKD according to the K/DOQI guidelines (2) has yet to be extensively applied in studies on the outcomes of pregnancy in renal diseases. Despite its limits, and enhanced by the difficulties in assessing renal function in pregnancy, a homogeneous classification may be of help in the setting of a rather confusing and heterogeneous definition of renal diseases and maternal-fetal outcomes (5,8–32). The uncertainty is greater for the early stages of CKD, which are more easily missed unless specifically searched for and because of the physiologic reduction of serum creatinine in the early stages of pregnancy (34,35,44).
One of the characteristics of our cohort is the higher prevalence of stage 1 CKD versus later stages, reflecting the expected distribution in the overall population in this age group.
Our study takes into account 91 singleton pregnancies and compares them with a control population of low-risk single pregnancies followed in the same period in the same setting. This is one of the largest single-center series published in the last decade (8–32). Overall, our results are favorable in cases with absent-mild and severe renal function impairment (Tables 3 and 4). Despite a trend toward a moderate increase of serum creatinine (median + 0.25 mg/dl), only one patient (CKD stage 3 at the start of pregnancy) doubled serum creatinine and none started dialysis within 6 months after delivery (Table 3). This compares favorably with literature data reporting a severe decline in kidney function in approximately 25% to 50% of cases. The comparison with the literature is often difficult because of the different categorizations of results, although a doubling of serum creatinine or an increase of at least 1 mg/dl of serum creatinine is most frequently used (8,10,18–20). In one of the largest series on pregnancy in severe CKD and using GFR-based definitions, Imbasciati (10) reports an increased risk for progression to dialysis in patients with GFR <40 ml/min and proteinuria over 1 g/d. However, in this large multicenter series the time span of enrollment was wider (1977 to 2004), and recent changes in the global care of the CKD patients as well as changing policies toward delivery may have played an important role. For example, their series reports a higher prevalence of SGA babies and a lower prevalence of prematurity as compared with ours, probably reflecting a different policy toward delivery in case of flattening of the growth curve (10).
The prevalence of stillbirth, neonatal, and perinatal deaths reported in papers dealing with at least 25 pregnancies reaches 8% to 15% in different subgroups (8,9,14,22,23,28) and is in the range of 1% to 7% in most series (10,13,19,20,26,27,32); a few maternal deaths are reported (all in lupus patients) related to disease flare-ups (14,23,27).
In two recent large surveys (1990 to 2002 and 2002 to 2005) based on International Classification of Diseases version 9 data that retrieved 83 and 101 cases with CKD, respectively, the prevalence of live birth was significantly associated with a cutoff of maternal serum creatinine at 1.1 mg/dl. Interestingly, as in our series, the differences with the overall population were also observed for mild degrees of renal function impairment, with minor differences in the risk of adverse perinatal outcome in women with severe CKD compared with milder disease. However, because in our population no neonatal or perinatal death occurred, the mathematical model proposed by the authors was not applicable in our context (45,46)
In our study, because of the absence of such hard end points, surrogate end points were studied, including cesarean section, preterm delivery, SGA, and need for NICU admission. All of the surrogate outcomes except SGA are closely correlated with the presence of CKD; this holds true for all cases and for the stage 1 CKD patients versus controls (Tables 4 to 6). The relative risk of cesarean section is approximately 4 comparing all CKD patients with low-risk controls and limiting the comparison to stage 1 CKD patients. A similar pattern is observed for prematurity (strictly defined as delivery before 37 completed weeks of gestation or as before 34 weeks) and for the need for intensive care for the newborn. The wide confidence intervals reflect the relatively low number of cases and underline the need to increase the number of patients (Tables 4 to 6). The close cooperation with a skilled neonatal unit certainly contributed to the generally favorable outcomes (only one baby with neurologic sequelae) and was in turn a “historical” determinant of the policy of the maternal-fetal unit (Tables 5 to 7).
It is somehow surprising the lack of difference in SGA between the control population and the study group; this is presumably related to our policy of scheduling delivery in all cases in which the fetal growth curve tends to level off, thus avoiding SGA in the face of a higher incidence of preterm babies (10). The higher incidence of SGA with more severe renal function impairment (CKD stages 3 to 5) suggests a threshold effect above CKD stage 2.
The analysis within the CKD group was aimed at determining the effect of three major renal parameters (renal function, hypertension, and proteinuria) on the outcome. We observed a trend toward increased risk of adverse outcomes along with renal function impairment, in keeping with the classical tenet that the risk of poor outcomes is inversely related to renal function (4,5,44). However, statistical significance was only reached in the univariate analysis and was not uniformly confirmed in the across-stages multivariate analysis (Tables 4 and 7). This may be a reflection of the relatively low number of cases with renal function impairment (15 in CKD stages 3 to 5), thus reducing the statistical power of the across-stages comparison. It is also conceivable that our policy of stricter follow-up according to the severity of kidney disease may have attenuated the effect of these determinants on the overall prognosis.
Because we used broad definitions of CKD and included patients with minor signs of renal impairment, we wondered if the outcome of the first functional stage was driven by a few intensely proteinuric patients. The persistence of statistical significance when we omitted the cases with proteinuria over 1 g/d at referral confirms that the effect in CKD stage 1 is not merely explainable by a small subset with a bad prognosis (Table 4).
Therefore, our data can be summarized by underlining that the major difference compared with low-risk controls is due to the presence or absence of CKD, and that proteinuria, degree of renal function impairment, and hypertension are modulators of the overall prognosis (Tables 4 to 7). These findings strongly support a policy of early referral of CKD patients, preconception counseling, and strict follow-up in women with kidney disease even in the absence of impaired renal function. Of note, in 21 patients (approximately 20% of the referred cases), CKD was diagnosed during pregnancy, confirming the importance of pregnancy as an occasion for early diagnosis of diseases that are often asymptomatic, at least in the early phases.
Our study has some peculiarities, strengths, and weak points that may at least partly explain the differences from previous literature reports. The main strengths are the homogeneity of follow-up, the relatively large number of cases, and the use of a validated means to assess and stratify CKD (K/DOQI staging) never before used (2,3). The lack of hard end points and the need for surrogate end points can be considered a weakness and strength. The weakness is statistical, because only surrogate end points could be analyzed. The strength is the generally favorable results: although approximately one-third of cases had moderate-severe renal function impairment, nephrotic proteinuria, and hypertension, the overall outcomes were good and compare favorably with recent literature reports, underlining the importance of being followed in a tertiary center (8–32).
A weak point, common to all large clinical studies on CKD, is the heterogeneity of referral and the lack of preconception data, because the decrease in serum creatinine is often preserved in CKD and the first data in pregnancy may underscore the severity of the disease (4,5,34,35,44).
The low prevalence of women of African origin in our series and the higher educational level as compared with low-risk pregnancies is difficult to interpret. Because in Italy the basic care for pregnancy is free and comprehensive care is free for high-risk pregnancies, a selection bias based on census is unlikely. Thus, our data may suggest that better educated and Caucasian women have easier access to settings with higher attention to kidney diseases that timely refer them to tertiary care centers. However, no difference of educational level or ethnicity was observed stratifying the women as for week of referral, thus pointing to the need to further analyze this issue to improve timely referral of high-risk pregnancies.
Another limit is the assessment of renal function in pregnancy because all of the well established formulas display important limits in pregnancy (34,35); therefore, our working choice to use the Cockcroft–Gault formula whenever possible on preconception data took into account the preanalytical biases and practical limits of 24-hour urine collection and the importance of patients' weight in our population (2,3,34,35,44).
Another major strength of our study is the availability of a large control population uniformly followed in the same maternal-fetal unit in the same period. Because the unit provides specific follow-up according to maternal diseases, the control group consisted of women known to be nonhypertensive, nondiabetic, and not affected by other diagnosed diseases, thus making the comparison clear with respect to other studies comparing nephropathic versus non-nephropathic women. The incidence of PE in our low-risk control group (1.1%) is in keeping with other low-risk multicenter Italian series in which the incidence of PE was 1.5% in the low-risk control group (47) and is lower than the 3% to 5% incidence recorded in Europe in the overall population, which reflects the careful evaluation of the women at baseline and possibly the availability of a series of outpatient services for diabetic or hypertensive patients.
A further strong point is the very low incidence of loss to follow-up in the study group, presumably linked to the flexibility and personalization of the follow-up and the close cooperation with the caregivers. The incidence of loss to follow-up was also low in the control group (Figure 1).
Further analyses and long-term studies in carefully studied controls matched with larger patient populations are needed to verify the risk patterns in other settings as a basis for defining optimal follow-up strategies for the increasing number of pregnant CKD patients.
In conclusion, CKD poses a challenge for pregnancy from the early stages of the disease. Although the risks connected with severe CKD are well known, this study underlines the importance of early diagnosis and strict follow-up from the early stages of CKD. It also underlines the importance of pregnancy as an occasion for the early diagnosis of CKD. Three major points may be identified: (1) there are less favorable outcomes in patients with stage 1 kidney disease as compared with low-risk populations, thus requiring differentiation of preconception counseling and prenatal care (low- versus high-risk pregnancies); (2) attention should be paid to nephropathies first diagnosed in pregnancy and to pregnancy as an occasion for timely CKD diagnosis; and (3) further studies using strict and shared definitions and classifications of nephropathies in pregnancy are needed to verify management protocols.
Disclosures
None.
Acknowledgments
The authors thank Dr. P. Christie for careful language editing. Preliminary data were presented in abstract form at the American Society for Nephrology Congress in San Diego, California, October 27 through November 1, 2009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Graves JW: Diagnosis and management of chronic kidney disease. Mayo Clin Proc 83: 1064– 1069, 2008 [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification: Am J Kidney Dis 39 [ Suppl 1]: S1– S266, 2002 [PubMed] [Google Scholar]
- 3.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211– 215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou S: Historical perspective of pregnancy in chronic kidney disease. Adv Chronic Kidney Dis 14: 116– 118, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ikizler TA: CKD classification: Time to move beyond KDOQI. J Am Soc Nephrol 20: 929– 930, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800– 809, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Confortini P, Galanti G, Ancona G, Giongio A, Bruschi E, Lorenzini E: Full-term pregnancy and successful delivery in a patient on chronic hemodialysis. Proc Eur Dial Transplant Assoc 8: 74– 80, 1971 [Google Scholar]
- 8.Chopra S, Suri V, Aggarwal N, Rohilla M, Keepanasseril A, Kohli HS: Pregnancy in chronic renal insufficiency: Single centre experience from North India. Arch Gynecol Obstet 279: 691– 695, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Cavallasca JA, Laborde HA, Ruda-Vega H, Nasswetter GG: Maternal and fetal outcomes of 72 pregnancies in Argentine patients with systemic lupus erythematosus (SLE). Clin Rheumatol 27: 41– 46, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Imbasciati E, Gregorini G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, Ravani P: Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis 49: 753– 762, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Thapa L: Acute pyelonephritis in pregnancy: A retrospective study. Aust NZ J Obstet Gynaecol 47: 313– 315, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Carr DB, Koontz GL, Gardella C, Holing EV, Brateng DA, Brown ZA, Easterling TR: Diabetic nephropathy in pregnancy: Suboptimal hypertensive control associated with preterm delivery. Am J Hypertens 19: 513– 519, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Carmona F, Font J, Moga I, Làzaro I, Cervera R, Pac V, Balasch J: Class III-IV proliferative lupus nephritis and pregnancy: A study of 42 cases. Am J Reprod Immunol 53: 182– 188, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Rahman FZ, Rahman J, Al-Suleiman SA, Rahman MS: Pregnancy outcome in lupus nephropathy. Arch Gynecol Obstet 271: 222– 226, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Brown MA, Holt JL, Mangos GJ, Murray N, Curtis J, Homer C: Microscopic hematuria in pregnancy: Relevance to pregnancy outcome. Am J Kidney Dis 45: 667– 673, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hill JB, Sheffield JS, McIntire DD, Wendel GD, Jr: Acute pyelonephritis in pregnancy. Obstet Gynecol 105: 18– 23, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Trevisan G, Ramos JG, Martins-Costa S, Barros EJ: Pregnancy in patients with chronic renal insufficiency at Hospital de Clínicas of Porto Alegre, Brazil Ren Fail 26: 29– 34, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR: Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis 43: 415– 423, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Tandon A, Ibañez D, Gladman DD, Urowitz MB: The effect of pregnancy on lupus nephritis. Arthritis Rheum 50: 3941– 3946, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Misra R, Bhowmik D, Mittal S, Kriplani A, Kumar S, Bhatla N, Dadhwal V, Pandey RM: Pregnancy with chronic kidney disease: Outcome in Indian women. J Women Health 12: 1019– 1025, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Clark CA, Spitzer KA, Nadler JN, Laskin CA: Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol 30: 2127– 2132, 2003 [PubMed] [Google Scholar]
- 22.Khoury JC, Miodovnik M, LeMasters G, Sibai B: Pregnancy outcome and progression of diabetic nephropathy. What's next? J Matern Fetal Neonatal Med 11: 238– 244, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Moroni G, Quaglini S, Banfi G, Caloni M, Finazzi S, Ambroso G, Como G, Ponticelli C: Pregnancy in lupus nephritis Am J Kidney Dis 40: 713– 720, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Stehman-Breen CO, Levine RJ, Qian C, Morris CD, Catalano PM, Curet LB, Sibai BM: Increased risk of preeclampsia among nulliparous pregnant women with idiopathic hematuria. Am J Obstet Gynecol 18: 703– 708, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Mølvig J, Mathiesen ER: Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diab Care 24: 1739– 1744, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Malik GH, Al-Mohaya S, Shaikh JF, Al-Wakeel J, Al-Hozaim W, Kechrid M, Al-Duhami H, Shetia MS, El Gamal H, Hammed D: Repeated pregnancies in patients with non-immunoglobulin a mesangioproliferative glomerulonephritis. Am J Nephrol 21: 378– 382, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Huong DL, Wechsler B, Vauthier-Brouzes D, Beaufils H, Lefebvre G, Piette JC: Pregnancy in past or present lupus nephritis: A study of 32 pregnancies from a single centre. Ann Rheum Dis 60: 599– 604, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossing K, Jacobsen P, Hommel E, Mathiesen E, Svenningsen A, Rossing P, Parving HH: Pregnancy and progression of diabetic nephropathy. Diabetologia 45: 36– 41, 2002 [DOI] [PubMed] [Google Scholar]
- 29.North RA, Taylor RS, Gunn TR: Pregnancy outcome in women with reflux nephropathy and the inheritance of vesico-ureteric reflux. Aust NZ J Obstet Gynaecol 40: 280– 285, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Murakami S, Saitoh M, Kubo T, Koyama T, Kobayashi M: Renal disease in women with severe preeclampsia or gestational proteinuria. Obstet Gynecol 96: 945– 949, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Bar J, Orvieto R, Shalev Y, Peled Y, Pardo Y, Gafter U, Ben-Rafael Z, Chen R, Hod M: Pregnancy outcome in women with primary renal disease. Isr Med Assoc J 2: 178– 181, 2000 [PubMed] [Google Scholar]
- 32.Bar J, Ben-Rafael Z, Padoa A, Orvieto R, Boner G, Hod M: Prediction of pregnancy outcome in subgroups of women with renal disease. Clin Nephrol 53: 437– 444, 2000 [PubMed] [Google Scholar]
- 33.Germain S, Nelson-Piercy C: Lupus nephritis and renal disease in pregnancy. Lupus 15: 148– 155, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Alper AB, Yi Y, Webber LS, Pridjian G, Mumuney AA, Saade G, Morgan J, Nuwayhid B, Belfort M, Puschett J: Estimation of glomerular filtration rate in preeclamptic patients. Am J Perinatol 24: 569– 574, 2007 [DOI] [PubMed] [Google Scholar]
- 35.MC Smith Moran P, Ward MK, Davison JM: Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG 115: 109– 112, 2008 [DOI] [PubMed] [Google Scholar]
- 36.ACOG Committee on Obstetric Practice ACOG practice lletin: Diagnosis and management of preeclampsia and eclampsia. Int J Gynaecol Obstet 77: 67– 75, 2002 [PubMed] [Google Scholar]
- 37.Parazzini F, Cortinovis I, Bortolus R, Fedele L: Standards of birth weight in Italy. Ann Ostet Ginecol Med Perinat 112: 203– 246, 1991 [PubMed] [Google Scholar]
- 38.International Statistical Classification of Diseases and Related Health Problems, Rev. 10, Vol. 2, ICD-10, Geneva, World Health Organization, 2003 [Google Scholar]
- 39.Lachelin GCL, McGarrigle HG, Seed PT, Briley A, Shennan AH, Poston L: Low saliva progesterone concentrations are associated with spontaneous early preterm labour (before 34 weeks of gestation) in women at increased risk of preterm delivery. BJOG 116: 1515– 1519, 2009 [DOI] [PubMed] [Google Scholar]
- 40.National Collaborating Centre for Women's and Children's Health: Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline, London, Royal College of Obstetricians and Gynaecologists Press, 2008 [Google Scholar]
- 41.Intrapartum Care: Care of Healthy Women and Their Babies during Childbirth, NICE clinical guideline 55, London, National Institute for Health and Clinical Excellence, 2007 [PubMed] [Google Scholar]
- 42.Adamkin DH: Late preterm infants: Severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol 29[ Suppl 1]: S12– S17, 2009 [DOI] [PubMed] [Google Scholar]
- 43.SPSS Statistics, version 17.0, Chicago, SPSS, 2009 [Google Scholar]
- 44.Maynard SE, Thadhani R: Pregnancy and the kidney. J Am Soc Nephrol 20: 14– 22, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Modena A, Hoffman M, Tolosa JE: Chronic renal disease in pregnancy: A modern approach to predicting outcome [Abstract]. Am J Obstet Gynecol 193: S86, 2006 [Google Scholar]
- 46.Modena A, Hoffman M, Tolosa JE: Is there a difference in obstetrical outcome between mild and severe renal disease? [Abstract]. Am J Obstet Gynecol 191: S88, 2004 [Google Scholar]
- 47.Todros T, Verdiglione P, Oggé G, Paladini D, Vergani P, Cardaropoli S: Low incidence of hypertensive disorders of pregnancy in women treated with spiramycin for toxoplasma infection. Br J Clin Pharmacol 61: 336– 340, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]