Abstract
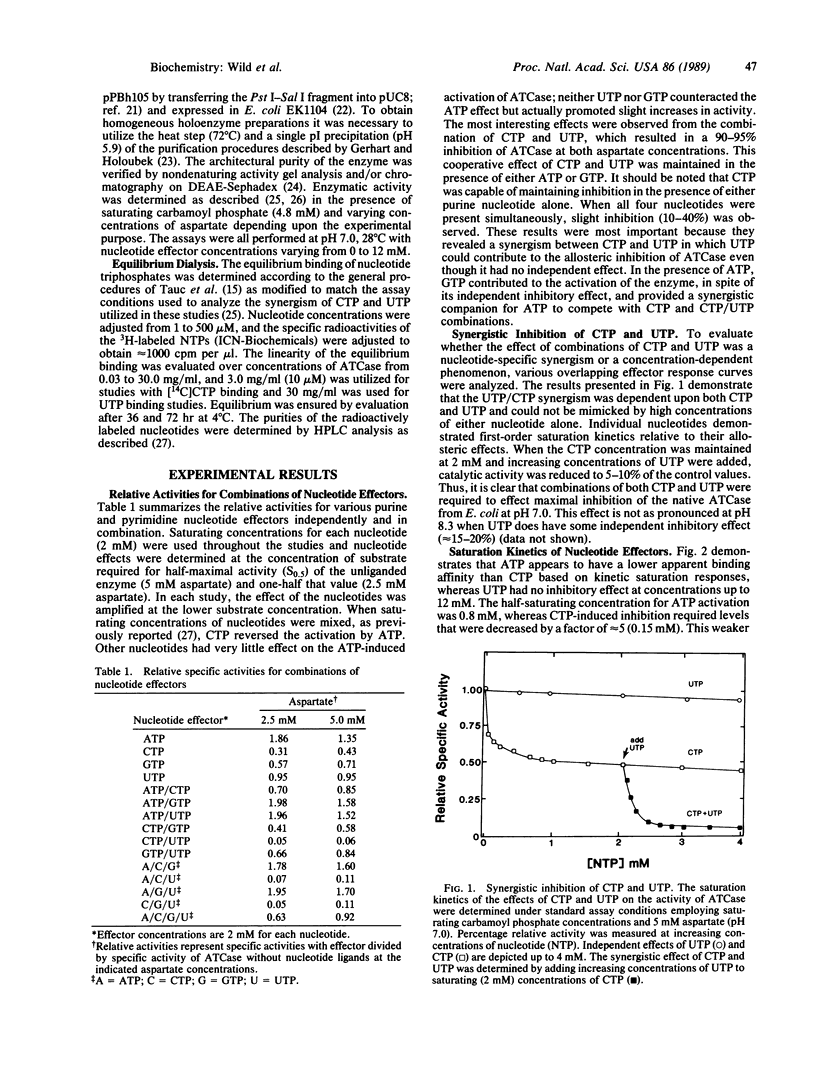
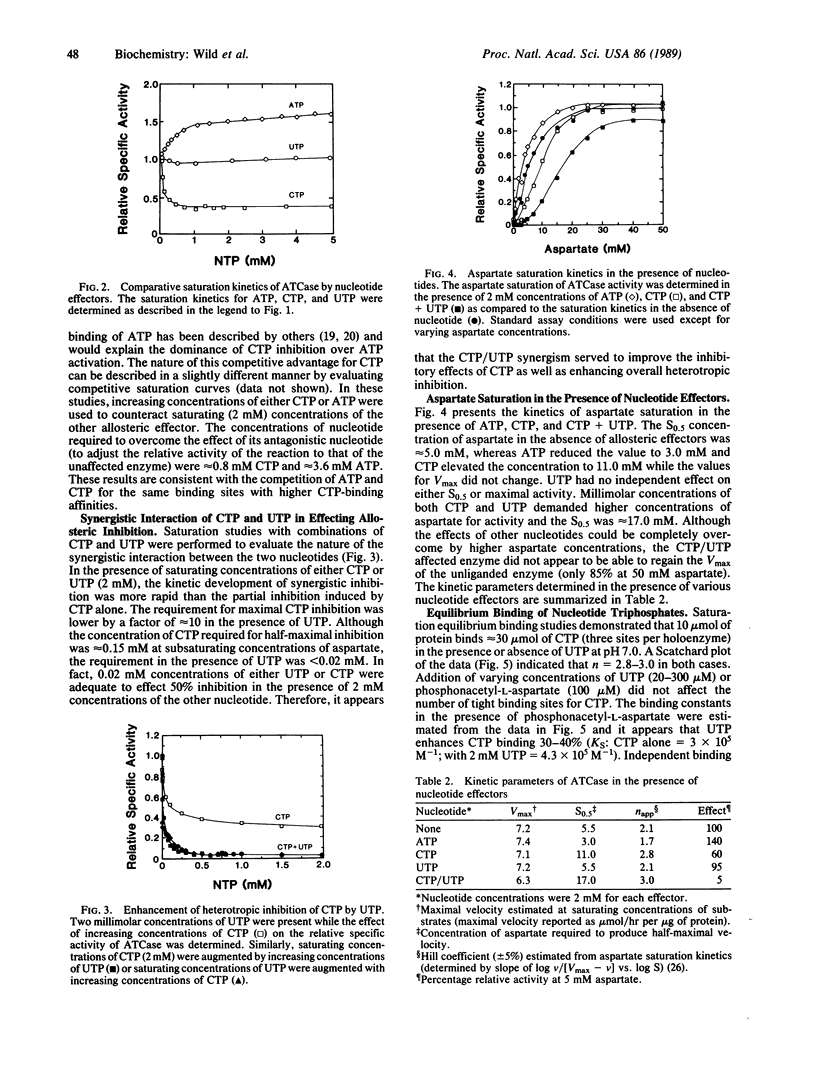
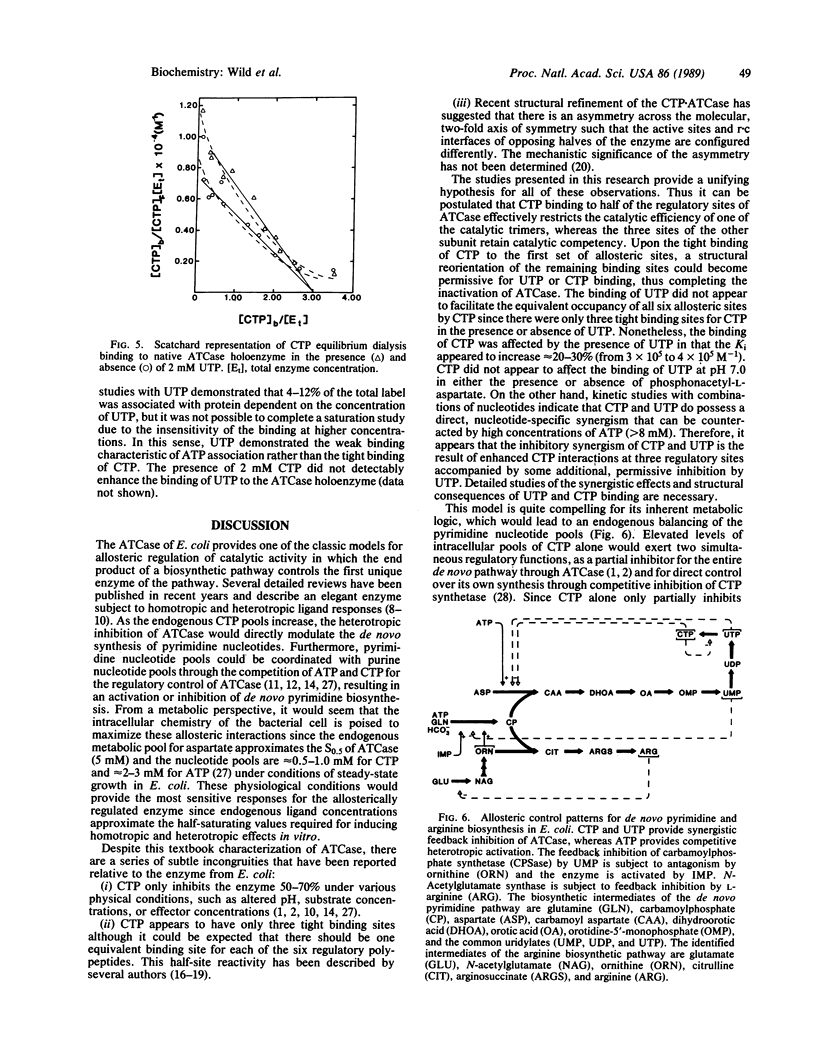
The allosteric control of aspartate transcarbamoylase (ATCase, EC 2.1.3.2) of Escherichia coli involves feedback inhibition by both CTP and UTP rather than just CTP alone. It has been known that CTP functions as a heterotropic inhibitor of catalysis; however, the inhibition by CTP alone is incomplete (50-70% at various aspartate concentrations) and there is only a partial occupancy of the allosteric binding sites by CTP at saturating concentrations. The logic of these allosteric characteristics can now be understood in that UTP is a synergistic inhibitor of ATCase in the presence of CTP even though UTP has no independent effect at pH 7.0. When saturating concentrations of CTP are present, the concentration of substrate required for half-maximal activity (S0.5) of the native holoenzyme for aspartate increases from 5 to 11 mM. When CTP and UTP are both present, the aspartate requirement increases further (S0.5 = 17 mM). At aspartate concentrations less than 5 mM, the heterotropic inhibition of ATCase is 90-95% in the presence of both pyrimidine nucleotides. UTP does enhance the binding of CTP to the holoenzyme but the number of tight binding sites does not change (n = 3). The binding of UTP is stabilized in the presence of CTP although its binding characteristics are not as strong as those of CTP. The recent crystallographic studies of Kim et al. [Kim, H.K., Pan, Z., Honzatko, R.B., Ke, H.M. & Lipscomb, W.N. (1987) J. Mol. Biol. 196, 853-875] have described a structural asymmetry across the molecular two-fold axis that is consistent with these CTP/UTP interactions. The synergistic inhibition of ATCase by both CTP and UTP provides a satisfying logic for ensuring a balance of endogenous pyrimidine nucleotide pools.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELSTEIN S. J. COENZYME BINDING BY X-IRRADIATED GLUTAMATE AND LACTATE DEHYDROGENASE. Biochemistry. 1965 May;4:891–896. doi: 10.1021/bi00881a015. [DOI] [PubMed] [Google Scholar]
- Allewell N. M., Friedland J., Niekamp K. Calorimetric analysis of aspartate transcarbamylase from Escherichia coli: binding of cytosine 5'-triphosphate and adenosine 5'-triphosphate. Biochemistry. 1975 Jan 28;14(2):224–230. doi: 10.1021/bi00673a005. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- Bethell M. R., Smith K. E., White J. S., Jones M. E. Carbamyl phosphate: an allosteric substrate for aspartate transcarbamylase of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1442–1449. doi: 10.1073/pnas.60.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltermann K. F., Beck D. A., Wild J. R. In vivo formation of hybrid aspartate transcarbamoylases from native subunits of divergent members of the family Enterobacteriaceae. J Bacteriol. 1986 Jul;167(1):285–290. doi: 10.1128/jb.167.1.285-290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gray C. W., Chamberlin M. J., Gray D. M. Interaction of aspartate transcarbamylase with regulatory nucleotides. J Biol Chem. 1973 Sep 10;248(17):6071–6079. [PubMed] [Google Scholar]
- Honzatko R. B., Monaco H. L., Lipscomb W. N. A 3.0-A resolution study of nucleotide complexes with aspartate carbamoyltransferase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5105–5109. doi: 10.1073/pnas.76.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuanyu Y. C., Wedler F. C. Effectors of Escherichia coli aspartate transcarbamoylase differentially perturb aspartate binding rather than the T-R transition. J Biol Chem. 1988 Mar 25;263(9):4172–4181. [PubMed] [Google Scholar]
- Ke H. M., Honzatko R. B., Lipscomb W. N. Structure of unligated aspartate carbamoyltransferase of Escherichia coli at 2.6-A resolution. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4037–4040. doi: 10.1073/pnas.81.13.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Pan Z. X., Honzatko R. B., Ke H. M., Lipscomb W. N. Structural asymmetry in the CTP-liganded form of aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1987 Aug 20;196(4):853–875. doi: 10.1016/0022-2836(87)90410-4. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. Structure at 2.9-A resolution of aspartate carbamoyltransferase complexed with the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1643–1647. doi: 10.1073/pnas.82.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisinger T., Haas D. N-Acetylglutamate synthase of Escherichia coli regulation of synthesis and activity by arginine. J Biol Chem. 1975 Mar 10;250(5):1690–1693. [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- Matsumoto S., Hammes G. G. An equilibrium binding study of the interaction of aspartate transcarbamylase with cytidine 5'-triphosphate and adenosine 5'-triphosphate. Biochemistry. 1973 Mar 27;12(7):1388–1394. doi: 10.1021/bi00731a019. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan S. F., Kantrowitz E. R. Superproduction and rapid purification of Escherichia coli aspartate transcarbamylase and its catalytic subunit under extreme derepression of the pyrimidine pathway. J Biol Chem. 1985 Nov 25;260(27):14712–14716. [PubMed] [Google Scholar]
- Pastra-Landis S. C., Evans D. R., Lipscomb W. N. The effect of pH on the cooperative behavior of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1978 Jul 10;253(13):4624–4630. [PubMed] [Google Scholar]
- Piérard A. Control of the activity of Escherichia coli carbamoyl phosphate synthetase by antagonistic allosteric effectors. Science. 1966 Dec 23;154(3756):1572–1573. doi: 10.1126/science.154.3756.1572. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Roof W. D., Foltermann K. F., Wild J. R. The organization and regulation of the pyrBI operon in E. coli includes a rho-independent attenuator sequence. Mol Gen Genet. 1982;187(3):391–400. doi: 10.1007/BF00332617. [DOI] [PubMed] [Google Scholar]
- Suter P., Rosenbusch J. P. Asymmetry of binding and physical assignments of CTP and ATP sites in aspartate transcarbamoylase. J Biol Chem. 1977 Nov 25;252(22):8136–8141. [PubMed] [Google Scholar]
- Tauc P., Leconte C., Kerbiriou D., Thiry L., Hervé G. Coupling of homotropic and heterotropic interactions in Escherichia coli aspartate transcarbamylase. J Mol Biol. 1982 Feb 25;155(2):155–168. doi: 10.1016/0022-2836(82)90442-9. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Gasser F. J. Modes of modifier action in E. coli aspartate transcarbamylase. Arch Biochem Biophys. 1974 Jul;163(1):69–78. doi: 10.1016/0003-9861(74)90455-x. [DOI] [PubMed] [Google Scholar]
- Wild J. R., Johnson J. L., Loughrey S. J. ATP-liganded form of aspartate transcarbamoylase, the logical regulatory target for allosteric control in divergent bacterial systems. J Bacteriol. 1988 Jan;170(1):446–448. doi: 10.1128/jb.170.1.446-448.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. R., Kirschner M. W., Schachman H. K. Aspartate transcarbamoylase (Escherichia coli): preparation of subunits. Methods Enzymol. 1978;51:35–41. doi: 10.1016/s0076-6879(78)51007-0. [DOI] [PubMed] [Google Scholar]



