Abstract
Chile’s gallbladder cancer rates are among the highest in the world, being the first cancer killer among Chilean women. To provide insights into the etiology of gallbladder cancer, we conducted an ecologic study examining the geographical variation of gallbladder cancer and several putative risk factors. The relative risk of dying from gallbladder cancer (relative to the national average mortality rate) between 1985 and 2003 was estimated for each of the 333 Chilean counties, using a hierarchical Poisson regression model, adjusting for age, sex, and geographical location. The risk of gallbladder cancer mortality was analyzed in relation to region (costal, inland, northern, and southern), poverty, Amerindian (Mapuche) population, typhoid fever, and access to cholecystectomy, using logistic regression analysis. There were 27,183 gallbladder cancer deaths, age-sex-adjusted county mortality rates ranging from 8.2 to 12.4 per 100,000 inhabitants, being higher in inland and southern regions; compare to the north-coastal, the northern-inland region had a 10-fold risk odds ratio (OR) (95% of confidence interval (95% CI): 2.4–42.2) and the southern-inland region had a 26-fold risk (OR 95%CI: 6.0–114.2). Independent risk factors for gallbladder cancer were: ethnicity (Mapuche) OR:3.9 (95%CI 1.8–8.7), typhoid fever OR:2.9 (95%CI 1.2–6.9), poverty OR:5.1 (95%CI 1.6–15.9), low access to cholecystectomy OR:3.9 (95%CI 1.5–10.1), low access to hospital care OR:14.2 (95%CI 4.2–48.7) and high urbanization OR:8.0 (95%CI 3.4–18.7). Our results suggest that gallbladder cancer in Chile may be related to both genetic factors and poor living conditions. Future analytic studies are needed to further clarify the role of these factors in gallbladder cancer etiology.
Keywords: gallbladder cancer, gallstones, Mapuche, typhoid, genetics
INTRODUCTION
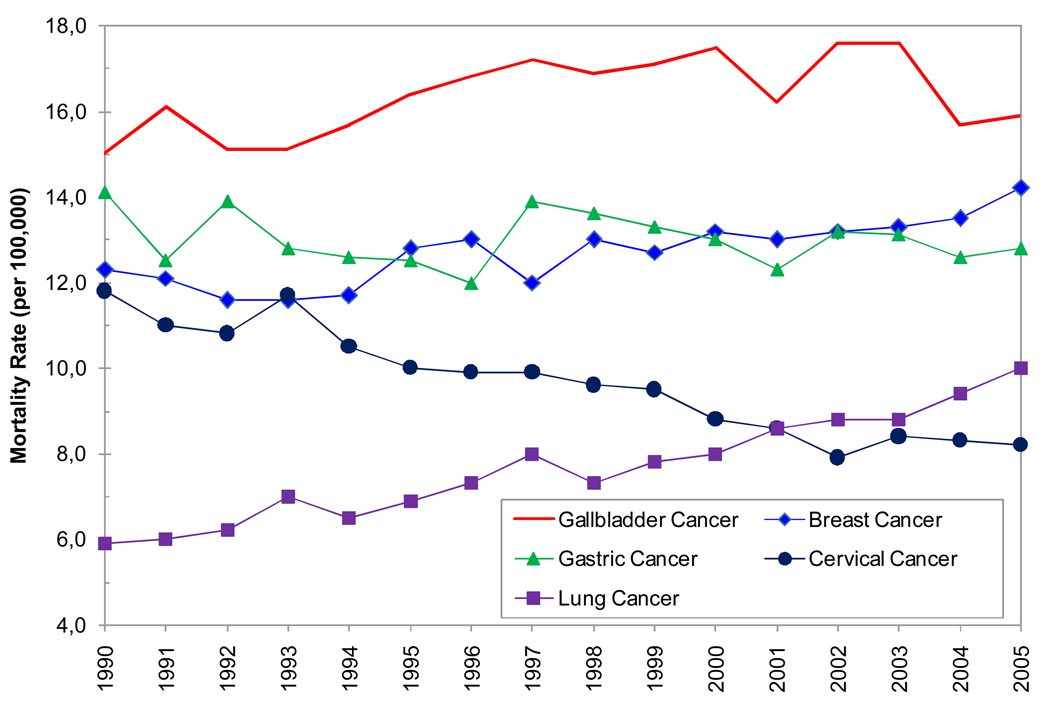
Chile’s gallbladder cancer incidence and mortality rates are among the highest in the world, with a mortality rate of 15.6 per 100,000 person-years in women and 7.0 per 100,000 person-years in men1. Gallbladder cancer is the first cause of cancer death among Chilean women, above breast, lung and cervical cancers (Figure 1). In Chile, the highest mortality rates are seen in Southern regions, especially in areas with high poverty, a large Amerindian (Mapuche) population, and insufficient access to health services, including access to surgery for gallbladder diseases (cholecystectomy)1,2.
FIGURE 1.
First five causes of cancer deaths among Chilean women. All of Chile, 1990–2005. Crude Mortality Rate per 100,000 women
Elaborated by the author from Chilean Ministry of Health Mortality database9
Apart from age, female gender, and a history of gallstones, the etiology of gallbladder cancer remains unclear. Reasons for the very high rates of gallbladder cancer in Chile are not known but both lifestyle and genetic factors have been suggested3–6. American Indians in the U.S. also have very high rates of gallstones and gallbladder cancer, like the Amerindians in Chile (Mapuche), suggesting a role of genetic susceptibility for both populations4,5,7. Chronic carriage of typhoid has been suggested as a risk factor for gallbladder cancer in India6, where the incidence of typhoid fever is high. Typhoid fever was endemic in Chile between 1960 and 1982, thus it is possible that typhoid may be contributing to the high risk of gallbladder cancer in Chile8.
To better understand the reasons for the high incidence and mortality of gallbladder cancer in Chile, we conducted a population-based ecologic study examining the relative risks of gallbladder cancer mortality between 1985 and 2002 for each of the 333 counties in Chile, as well as the relationships between several putative risk factors and the geographic variation of gallbladder cancer mortality.
METHODS
Study Population and Data Sources
Several national data sources were used for this analysis. Specifically, gallbladder cancer mortality rates for each of the 333 Chilean counties between 1985 and 2002 were calculated, using data from the Ministry of Health of Chile9. Chilean population estimates (approximately 15 million in total) by age, sex, race (self-declared), and county were obtained from the 2002 Census10. Data on the poverty index (the percent of the population in the county whose income does not cover minimum goods and services) was obtained from the 2003 survey Caracterización Socioeconómica Nacional (CASEN)11, and incidence rates of typhoid fever between 1999 and 2004 were based on data from Epidemiological Surveillance Statistics12.
Geographical Variations in Gallbladder Cancer Mortality
Since gallbladder cancer is a rare disease and many counties have small populations, we used a Poisson regression model to calculate spatially smoothed gallbladder cancer mortality risks for each county relative to the national average, adjusting for the population size and age-sex composition. We used a hierarchical Poisson regression model, which combines unstructured variability and spatial dependence (structured variability)13. This spatial variability was estimated by an a priori Intrinsic Conditional Autoregressive Distribution (Bayesian analysis)14. The model can be represented by:
In this model, α0 is the intercept that represents the logarithm of the national gallbladder cancer rate (baseline) throughout Chile; bi is the random area-specific effect in the logarithm of relative risk explained by the neighbors of the ith county and hi is the random area-specific unexplained residual. We conducted Bayesian analysis of these models with parameters obtained using Markov-Monte Carlo techniques employed in the program WinBUGS15. Convergences were evaluated based on standard criteria using the program BOA16. Comparison and selection of models were based on the Deviance Information Criterion17. Based on the results of this model, counties were dichotomized into high (RR >1) and low (RR<=1) gallbladder cancer mortality risk, using a ROC curve. Temporal trends for gallbladder cancer mortality between 1985 and 2002 were also calculated, using a Poisson regression model.
Risk Factors for Gallbladder Cancer Mortality
Multivariate logistic regression analyses were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for gallbladder mortality in relation to geographic location, percent living in poverty, urbanization (as stated by the National Institute of Statistics), typhoid incidence rate (1999–2004), percent of Amerindians (Mapuche) in the population, access to hospital care for gallbladder cancer patients (estimated as the ratio of the number of gallbladder diseases hospitalizations over the number of deaths from gallbladder cancer) and cholecystectomy (estimated from major surgery in patients with gallbladder disease). Gallbladder cancer mortality risk was evaluated comparing counties with a gallbladder cancer mortality relative risk (RR) greater than 1 with those with RR less than or equal to 1 (risk is relative to the national rate). Geographic location was assessed using four regions (north-coast, north-inland, south-coast, and south-inland). A county was considered coastal if it touched the coastline, while an inland county has no coastal borders. ROC curve analysis was used to dichotomize the proportion of the Mapuche population (≥ or < 3.7%), and typhoid fever incidence (≥ or < 7.2 per 100,000 population). Poverty level was categorized as: low (Poverty Index CASEN<20%), medium (Poverty Index CASEN between 20 and 40%), and high (Poverty Index CASEN > 40%) and urbanization as low (<40%), medium (between 40–75%), and high (> 75%). The ratio of gallbladder diseases hospitalizations over the number of deaths from gallbladder cancer was categorized as: high (> 60), medium (30–60), and low (less than 30). Access to cholecystectomy was categorized as: high (>80%). medium (between 50 and 80%) and low (< 50%).
RESULTS
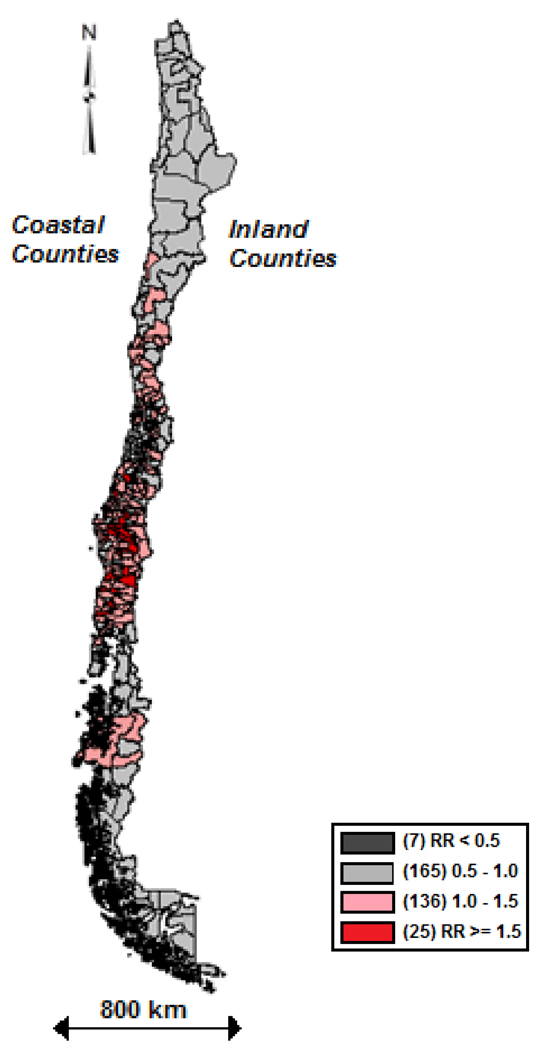
Geographic variation in gallbladder cancer mortality by county between 1985 and 2002, relative to average national mortality rates, adjusting for county-specific population size, and age and sex distributions, is shown in Figure 2. As shown, the highest gallbladder cancer mortality rates occurred in the southern part of the country, in particular the south-inland region.
FIGURE 2.
Geographic variation of Relative risk (RR) of gallbladder cancer in Chile (1985–2002) by county with reference to the national average, adjusted for county population size, age-sex distribution and spatially smoothed
Selected characteristics of the 333 Chilean counties by geographical location are shown in Table I. The Chilean population tended to cluster in the north-inland region, while the Mapuche population was higher in the south, representing 6.5% of the population. Southern regions, both inland and coastal, also had high levels of poverty, were more rural, had a high incidence of typhoid fever, and lower rates of gallbladder surgery; cholecistectomy ratio was highest in the north-inland counties and lowest in the south-inland counties (81% and 59% of gallbladder disease hospitalizations received surgery respectively). Gallbladder cancer mortality ranged from 8.22 to 12.44 per 100,000 inhabitants, with the south-inland region having the highest rates. Average relative risk for GBC mortality was lowest in the North-Coastal counties and higher in the South-Inland counties. The North inland and coastal counties have negative trend of the relative risk for GBC mortality, i.e. slightly decreases risk trend over the period. Both South coastal and inland counties have an upward trend.
TABLE I.
Selected Characteristics of the 333 Counties by Geographic Regions, Chile
| North-Coast | North-Inland | South-Coast | South-Inland | |
|---|---|---|---|---|
| No. counties | 28 | 101 | 67 | 137 |
| Characteristic | ||||
| Chilean pop, % | 12 | 49 | 14 | 24 |
| Mean percentage of Mapuche in pop (range) | 1.0% (0.3% – 3.0%) | 2.6% (0.0% – 10.6%) | 6.5% (0.2% – 64.7%) | 6.5% (0.0% – 59.5%) |
| Mean percentage of population living in poverty (range) |
16.7 (8.2 – 41.9) | 16.7 (1.4 – 42.0) | 26.5 (4.5 – 59.9) | 27.4 (10.9 – 59.5) |
| Mean percentage of urbanization (range) | 78% (0% – 99%) | 71% (0% – 100%) | 45% (0% – 99%) | 47% (0% – 97%) |
| Typhoid incidence rate 1999–2004, mean per 100,000 population (range) |
4.2 (0.0 – 10.5) | 5.2 (0.0 – 54.9) | 7.5 (0.0 – 38.3) | 7.0 (0.0 – 104.1) |
| Gallbladder disease hospital discharges. 1992– 2003, average rate per 100,000 inhab. (range) |
290.8 (50.5 – 757.1) | 316.1 (0.0 – 628.3) | 352.5 (0.0 – 747.2) | 441.1 (65.2 – 1,207.7) |
| Mean Cholecistectomy 1992–2003 a, (range) | 74% (39% – 100%) | 81% (47% – 100%) | 56% (22% – 100%) | 59% (17% – 100%) |
| Ratio of gallbladder diseases hospital discharges over gallbladder cancer death b(range) |
42 (19 – 78) | 37 (0 – 78) | 43 (0 – 102) | 37 (13 – 111) |
| Age-sex Adjusted Gallbladder cancer mortality rate per 100,000 inhab. (range) |
8.22 (0.0 – 11.3) | 8.53 (0.0 – 23.7) | 10.41 (0.0 – 38.3) | 12.44 (0.0 – 26.6) |
| RR for gallbladder cancer mortality (95% CI) | 0.83 (0.77 – 0.88) | 0.93 (0.89 – 0.98) | 0.98 (0.91 – 1.06) | 1.18 (1.14 – 1.23) |
| β factor (%) for the temporal trend of RR gallbladder cancer mortality, 1985–2002 (IC95%) |
−0.20% (−0.4 – 0.0) | −0.19% (−0.4 – 0.0) | 0.21% (0.0 – 0.4) | 0.30% (0.12 - 0.4) |
prepared by the authors from Ministry of Health hospital statistics, number of major surgery per 100 gallbladder diseases hospitalizations
a proxy for access to hospital care among gallbladder disease people.
Based on the geographical analysis, counties with RR > 1 for gallbladder cancer mortality were classified as high risk, while those with RR < 1 were classified as low risk. A multivariable logistic regression analysis of the gallbladder cancer risk is presented in Table II. Eleven percent of the counties in the north-coastal areas were classified as high-risk, while 67% of the south-inland counties were high-risk. Compared to the north-coast region, north-inland areas had a 10-fold (95% CI 2.4–42.2) risk of gallbladder cancer, and south-inland regions had a 26-fold risk (95% CI 6.0–114.2) of gallbladder cancer mortality (Table II). A large Mapuche population, high rates of typhoid fever, high poverty levels and high urbanization were risk factors for gallbladder cancer mortality, while high access to hospital care and high access to cholecystectomy were protective factors.
TABLE II.
Odds Ratios (OR) and 95% Confidence Intervals (95% CI) for Gallbladder Cancer Mortality Risk in Relation to Selected Factors in 333 Counties in Chile, 1985–2002. Multivariable Logistic Regression.
| Characteristics | No. Counties |
% of counties with RR>1a |
ORb (95% CI) | p | |
|---|---|---|---|---|---|
| Geographic Location | North-Coast | 28 | 11 | 1.0 | |
| North-Inland | 101 | 37 | 10.13 (2.4 – 42.2) | 0.001 | |
| South-Coast | 67 | 43 | 5.19 (1.1 – 24.5) | 0.04 | |
| South –Inland | 137 | 67 | 26.10 (6.0 – 114.2) | <0.001 | |
| Mapuche population | Low (<3.7%) | 227 | 41 | 1.0 | |
| High (≥3.7%) | 106 | 64 | 3.92 (1.8 – 8.7) | 0.001 | |
| Typhoid incidence rated |
Low (<7.2) | 281 | 44 | 1.0 | |
| High (≥7.2) | 52 | 69 | 2.92 (1.2 – 6.9) | 0.014 | |
| Poverty | Low | 105 | 25 | 1.0 | |
| Medium | 192 | 56 | 3.20 (1.6 – 6.4) | 0.001 | |
| High | 36 | 78 | 5.05 (1.6 – 15.9) | 0.006 | |
| Urbanization | Low (<40%) | 121 | 45 | 1.0 | |
| Medium (40–75%) | 101 | 67 | 5.29 (2.5 – 11.2) | <0.001 | |
| High (>75%) | 111 | 36 | 8.01 (3.4 – 18.7) | <0.001 | |
| Cholecistectomy c | High (>80%) | 115 | 31 | 1.0 | |
| Medium (50–80%) | 130 | 50 | 2.01 (1.0 – 4.0) | 0.048 | |
| Low (<50%) | 88 | 68 | 3.86 (1.5 – 10.1) | 0.006 | |
| Hospital care for gallbladder diseases d |
High | 34 | 21 | 1.0 | |
| Medium | 188 | 47 | 5.16 (1.7 – 15.8) | 0.004 | |
| Low | 111 | 59 | 14.25 (4.2 – 48.7) | <0.001 | |
RR: relative risk of gallbladder cancer > 1 from the Poisson Regression Analysis
Odds ratios for comparisons between two strata within each characteristics, adjusted for geographical location
number of major surgery per 100 gallbladder diseases hospital discharge
Ratio of gallbladder diseases hospital discharges over gallbladder cancer deaths
DISCUSSION
In this population-based ecological study of 333 counties in Chile, we show that there is a large geographic variation in gallbladder cancer risk not explained by chance, or age-sex distribution of the population. The highest mortality occurred in the southern regions, in particular the south-inland region. Our results also show that a high proportion of Mapuche Amerindians in the population, typhoid fever and poverty are important risk factors in the southern regions of Chile. It is not surprising that counties with large Mapuche population had higher risk for gallbladder cancer in Chile, since they have Amerindian origin and American Indians have one of the highest rates of gallstones and gallbladder cancer in the world5,6,18–20. The concomitant high rates of gallbladder cancer in Mapuches and American Indians suggest a possible role of genetic susceptibility. Similar to American Indians, Mapuches have high rates of obesity, diabetes, metabolic syndrome, and cholesterol gallstones7,20–23 all of which are risk factors for gallbladder cancer and are conditions with a genetic basis. Several observations have suggested that gallbladder cancer has a strong genetic component. For example, family history of gallstones has been shown to increase the risk of gallbladder cancer24 and certain genetic variants in estrogen metabolism (particularly among obese and diabetic individuals)25, inflammation, and lipid metabolism26–28 have been linked to an increased risk of gallstones and gallbladder cancer. Molecular studies of gallbladder cancer have also reported mutations of proto-oncogenes like K-ras and beta-catenin, alterations in some tumor suppressor genes, such as p53 and APC, and instability of microsatellites29–31. It is possible that the much higher risk of gallbladder cancer in the inland regions is related to a higher homogeneity of the Mapuche population due to a higher degree of inbreeding32. Although the percent of Mapuche in the population is similar that in inland and coastal southern counties (6.5% each), proximity to the sea has permitted for a higher genetic interchange32.
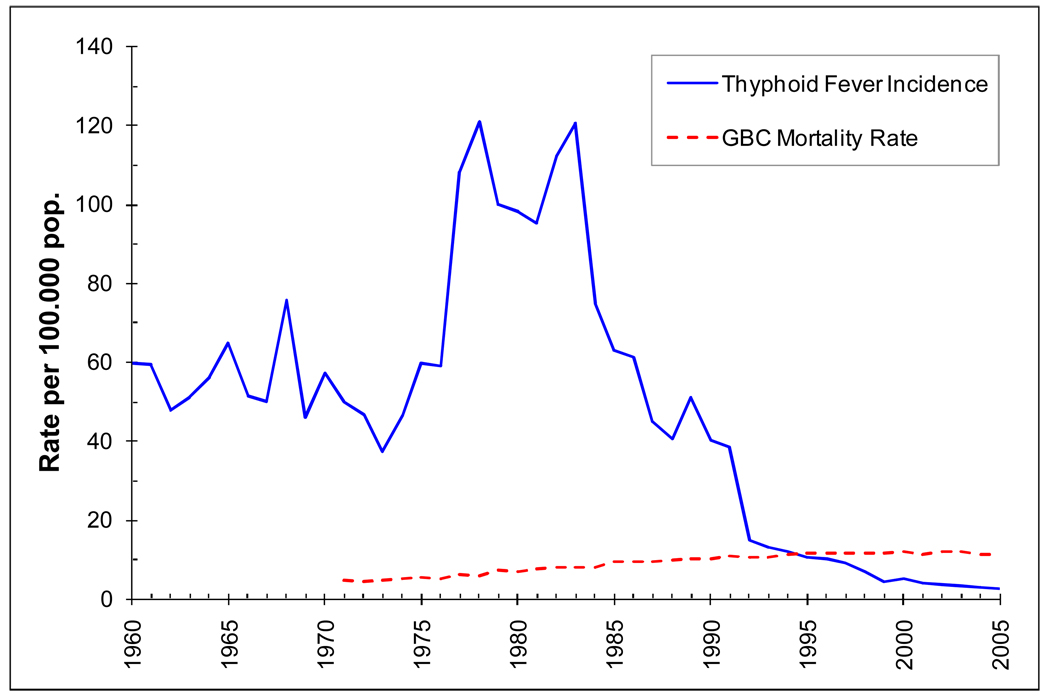
It is possible that the higher rates of typhoid contribute to the excess risk of gallbladder cancer. The observation that typhoid fever is a significant risk factor for gallbladder cancer in Chile is consistent with previous reports suggesting a link between chronic typhoid carriage and gallbladder cancer in typhoid-endemic area, such as India19,33,34. Chile has been a highly endemic area for typhoid fever since 1950, with an annual incidence rate of about 60 per 100,000 person-years until 19768. Between 1976 and 1985, an epidemic of typhoid surged in most urban areas of Chile due to the deteriorating living conditions, and the incidence increased to 121 per 100,000 person-years. However, in 1985, the incidence of typhoid started to decrease, and this trend continued until 1991, when typhoid incidence rate reached the current level of 3.3 per 100,000 person-years8 (Figure 3). The precipitous drop in typhoid incidence between 1985 and 1992 was the result of intensive intervention measures taken to avoid the expansion of the cholera epidemic that had been affecting Peru since 19918. Typhoid fever is caused by Salmonella typhi (S. typhi). Two to five percent of all individuals who develop clinical or subclinical infection with S. typhi become chronic carriers35. The propensity to become a chronic carrier after acute infection increases with age and is greater in women36. Previous studies in Chile have shown that chronic S. typhi carriers had a higher frequency of the erythrocite ABH non-secretor phenotype than non-carriers, suggesting that the carrier state may be influenced by genetic predisposition and that the ABH carbohydrates secreted in the bile may interfere with S. typhi colonization in the bile tract, resulting in increased gallstone formation and increased probability of becoming chronic carriers (OR=15)35,36. It is noteworthy that Chile has one the highest rates of gallstones and typhoid fever in the world. In Chile, during the hyperendemic period of typhoid (1976–1985), more than 90,000 people were exposed to S. typhi36. In 1980 the estimated carrier rate was 694 per 100,000 population, with unusually high rates estimated for middle-age-women (aged 30–39, 5586 per 100,000 and aged 40–49, 4575 per 100,000)36. Women in these age groups also have usually high rates of gallstones, ranging from 43% to 60%36. The combination of high rates of chronic typhoid carriage and gallstones is likely to result in an increase in incidence of gallbladder cancer in the future. Gallbladder cancer mortality in Chile has risen steadily and we could expect to see a future surge of incidence as a result of the typhoid epidemic between 1976–1985 typhoid endemic. However, since the latency period between typhoid and gallbladder cancer is long, most chronic carriers are still young, and not enough years have elapsed, it is still unclear when the 1976–1985 typhoid epidemic would impact the incidence of gallbladder cancer in Chile. Nevertheless, if cholecistectomy rates increase, particularly in high risk counties, the effect of the chronic S typhi carriers in gallbladder cancer incidence could be averted.
FIGURE 3.
Temporal trend of typhoid fever incidence rate and of gallbladder cancer (GBC) mortality rate in Chile between 1960–2005
Elaborated by the author from Chilean Ministry of Health Mortality database9
Poverty is an important risk factor for gallbladder cancer. Many factors, including typhoid infection, diet, and access to medical procedures, are related to poverty. In developing countries, cholecystectomy is associated with decreasing rates of gallbladder cancer incidence and mortality37. In Chile, although economic conditions have improved over time, we have not yet seen a large decrease in gallbladder cancer risk, due largely to the fact that most gallstone patients from high- risk areas do not have access to cholecystectomy procedures1,38,39. A study on the American Indians in New Mexico (a high risk populations for gallbladder cancer) showed that their incidence of gallbladder cancer has declined faster than other ethnic groups, presumably because of their better access to cholecystectomy40.
Reasons for the positive association between urbanization and gallbladder cancer are unknown but may be related to westernized lifestyles, better diagnosis or better reporting of gallbladder cancer. Surveys in Chile showed that people in rural areas had less risk factor for gallstone; in particular they had lower levels of cholesterol, triglycerides, and low-density lipoprotein cholesterol, than people in urban areas41. In addition, the prevalence of diabetes and obesity is higher in urban areas21. Together, these findings support the observation that more urbanized populations should have higher gallbladder cancer rates.
Certain nutritional factors may be related to the much higher rates of gallbladder cancer in the southern regions, but we do not have the data to evaluate their role. In an earlier study in Chile, consumption of red chili pepper, beef, and pork was linked to an increased risk of gallbladder cancer42. These types of foods are frequently eaten in the southern regions42,43. Inland counties have less access to seafood and vegetables, which have been associated with a reduced risk of gallbladder cancer42,44.
In summary, our ecologic study showed that there is a large geographic variation in gallbladder cancer mortality in Chile, with a much higher rate in the south-inland region. Future studies in Chile are needed to confirm the role of typhoid fever and genetic susceptibility to gallbladder cancer in Amerindians.
Acknowledgements
We want to thank Alejandro Jara and Alessandra Gederlini for their assistance in the statistical analysis and to Elizabeth Salinas for her assistance in mapping the data.
REFERENCES
- 1.Andia M, Ferreccio C, Gederlini A. Gallbladder cancer: trend and risk distribution in Chile. Rev Med Chile. 2006;134:565–574. doi: 10.4067/s0034-98872006000500004. [DOI] [PubMed] [Google Scholar]
- 2.Ferreccio C, Chianale J, González C, Nervi F. Epidemiología Descriptiva del Cáncer Digestivo en Chile (1982–1991): Una Aproximación desde la Mortalidad. Santiago: Edit Alfa Beta; 1995. p. 147. [Google Scholar]
- 3.Pandey M. Risk factors for gallbladder cancer: a reappraisal. Eur J Cancer Prev. 2003;12:15–24. doi: 10.1097/00008469-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 5.Lazcano-Ponce E, Miquel J, Muñoz N, Herrero R, Ferreccio C, Wistuba I, Alonso P, Aristi G, Nervi F. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 6.Strom BL, Soloway RD, Rios-Dalenz JL, Rodríguez-Martínez HA, West SL, Kinman JL, Polansky M, Berlin JA An international collaborative case-control study. Risk factors for gallbladder cancer. Cancer. 1995;76:1747–1756. doi: 10.1002/1097-0142(19951115)76:10<1747::aid-cncr2820761011>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Galman C, Miquel JF, Perez RM, Einarsson C, Stahle L, Marshall G, Nervi F, Rudling M. Bile acid synthesis is increased in Chilean Hispanics with gallstones and in gallstone high-risk Mapuche Indians. Gastroenterology. 2004;126:741–748. doi: 10.1053/j.gastro.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Laval E, Ferreccio C. Fiebre Tifoidea: emergencia, cúspide y desaparición de una enfermedad infecciosa en Chile. Invited review. Rev Chil Infect. 2007;24:435–440. [PubMed] [Google Scholar]
- 9.MINSAL. [accessed on October 2, 2007];Ministerio de Salud de Chile, Departamento de Estadísticas (DEIS) www.minsal.cl.
- 10.INE, Instituto Nacional de Estadística de Chile. [accessed on June 2, 2007];Estadísticas Demográficas y Vitales. http://www.ine.cl/ine/canales/chile_estadistico/demografia_y_vitales/demo_y_vita.php.
- 11.MIDEPLAN. [accessed on June 2, 2006];Ministerio de Planificacióny Co operación, Encuesta CASEN. www.mideplan.cl/casen.
- 12.MINSAL. [accessed on October 2, 2007];Ministerio de Salud de Chile, Departamento de Epidemiología. www.minsal.cl.
- 13.Wakefield JC, Best NG, Waller LA. Spatial Epidemiology: Methods and Applications. Oxford University Press; 2000. Bayesian Approaches to Disease Mapping; pp. 104–127. [Google Scholar]
- 14.Besag J, York J, Mollie A. Bayesian image restoration, with two applications in spatial statistics. Annals of the Institute of Statistical Mathematics. 1991;43:1–59. [Google Scholar]
- 15.Spiegelhalter D, Best N. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modeling. Stat Med. 2003;22:3687–3709. doi: 10.1002/sim.1586. [DOI] [PubMed] [Google Scholar]
- 16.Smith BJ. Bayesian Output Analysis Program (BOA), Version 0.5.0 for S-PLUS and R. 2000 Available at http://www.public-health.uiowa.edu/BOA.
- 17.Best N, Cowles MK, Vines K. Coda: Convergence diagnosis and output analysis software for Gibbs sampling output, Version 0.30. 1995. MRC Biostatistics Unit; Technical Report.
- 18.Carey MC, Paigen B. Epidemiology of the American Indians’ burden and its likely genetic origins. Hepatology. 2002;36:781–791. doi: 10.1053/jhep.2002.36545. [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Rashid A, Devesa SS, Fraumeni JF., Jr. Biliary tract cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3rd ed. Oxford University Press; 2006. pp. 787–800. [Google Scholar]
- 20.Miquel JF, Covarrubias C, Villaroel L, Mingrone G, Greco AV, Puglielli L, Carvallo P, Marshall G, Del Pino G, Nervi F. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937–946. doi: 10.1016/s0016-5085(98)70266-5. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco EP, Perez FP, Angel BB, Albala CB, Santos JL, Larenas G, Montalvo D. Prevalence of type 2 diabetes and obesity in two Chilean aboriginal populations living in urban zones. Rev Med Chil. 2004;132:1189–1197. doi: 10.4067/s0034-98872004001000005. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer E. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 23.Perez F, Santos JL, Albala C, Calvillan M, Carrasco E. Obesity and leptin association in three Chilean aboriginal populations. Rev Med Chil. 2000;128:45–52. [PubMed] [Google Scholar]
- 24.Hsing AW, Bai Y, Andreotti G, Rashid A, Deng J, Chen J, Han TQ, Wang BS, Zhang BH, Shen MC, Fraumeni JF, Jr, Gao YT. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based Study. Int J Cancer. 2007;121:832–838. doi: 10.1002/ijc.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou L, Xu J, Gao YT, Rashid A, Zheng SL, Sakoda LC, Shen MC, Wang BS, Deng J, Han TQ, Zhang BH, Meyers DA, et al. CYP17 MspA1 polymorphism and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2006;118:2847–2853. doi: 10.1002/ijc.21708. [DOI] [PubMed] [Google Scholar]
- 26.Sakoda LC, Gao YT, Chen BE, Chen J, Rosenberg P, Rashid A, Deng J, Shen MC, Wang BS, Han TQ, Zhang BH, Cohen-Webb H, et al. Prostaglandin–endoperoxide synthase 2 (PTGS2) gene polymorphisms and risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Carcinogenesis. 2006;27:1251–1256. doi: 10.1093/carcin/bgi314. [DOI] [PubMed] [Google Scholar]
- 27.Hsing AW, Gao YT, McGlynn KA, Niwa S, Zhang M, Han TQ, Wang BS, Chen J, Sakoda LC, Shen MC, Zhang BH, Deng J, et al. Biliary tract cancer and stones in relation to chronic liver conditions: A population-based study in Shanghai, China. Int J Cancer. 2007;120:1981–1985. doi: 10.1002/ijc.22375. [DOI] [PubMed] [Google Scholar]
- 28.Hsing AW, Sakoda LC, Chen J, Rashid A, Chu L, Deng J, Wang BS, Shen MC, Chen E, Rosenberg P, Zhang M, Andreotti G, et al. Variants of inflammation-related genes and the risk of gallstones and biliary tract cancer: a population-based study in China. Cancer Res. (Submitted) [Google Scholar]
- 29.Saetta AA. K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder cancer. J Surg Oncol. 2006;93:644–649. doi: 10.1002/jso.20532. [DOI] [PubMed] [Google Scholar]
- 30.Roa J, Roa I, De Aretxabala X, Melo A, Faría G, Tapia O. Mutación del gen K-ras en el cáncer de la vesícula biliar. Rev Med Chile. 2004;132:955–960. [PubMed] [Google Scholar]
- 31.Rashid A, Ueki T, Gao Y, Houlihan P, Wallace C, Wang B, Shen M, Deng J, Hsing AW. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 32.Llop E, Harb Z, Moreno R, Rothhammer F. Genetic marker variation in coastal populations of Chile. Homo. 2002;53:170. doi: 10.1078/0018-442x-00044. [DOI] [PubMed] [Google Scholar]
- 33.Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784–787. doi: 10.1111/j.1572-0241.2000.01860.x. [DOI] [PubMed] [Google Scholar]
- 34.Mager D. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;28:4–14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann E, Chianale J, Rollan A, Pereira J, Ferreccio C, Sotomayor V. Blood group antigen secretion and gallstone disease in the Salmonella typhi chronic carrier state. J Infect Dis. 1993;167:993–994. doi: 10.1093/infdis/167.4.993. [DOI] [PubMed] [Google Scholar]
- 37.Levi F, Lucchini F, Negri E, La Vecchia C. The recent decline in gallbladder cancer mortality in Europe. Eur J Cancer Prev. 2003;12:265–267. doi: 10.1097/00008469-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Ayuso RM, Hernandez V, Gonzalez R, Carvacho C, Navarrete C, Alvarez M, Gonzalez R, Marshall G, Miquel JF, Nervi F. Natural history of cholelithiasis and incidence of cholecystectomy in an urban and a Mapuche rural area. Rev Med Chil. 2002;130:723–730. [PubMed] [Google Scholar]
- 39.Chianale J, Valdivia G, Del Pino G, Nervi F. Mortalidad por cáncer vesicular en Chile y su relación con las tasas de colecistectomía. Análisis de la última década. Rev Med Chile. 1990;118:1284–1288. [PubMed] [Google Scholar]
- 40.Bakarat J, Dunkelberg J, Ma T. Changing patterns of gallbladder carcinoma in New Mexico. Cancer. 2006;106:434–440. doi: 10.1002/cncr.21620. [DOI] [PubMed] [Google Scholar]
- 41.Ministerio de Salud de Chile, Encuesta Nacional de Salud. 2003 www.minsal.cl.
- 42.Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, Watanabe H, Tajima K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer. 2002;102:407–411. doi: 10.1002/ijc.10716. [DOI] [PubMed] [Google Scholar]
- 43.Pandey M, Shukla V. Diet and gallbladder cancer: a case-control. Eur J Cancer Prev. 2002;11:365–368. doi: 10.1097/00008469-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Matsuba T, Qiu D, Kurosawa M, Lin Y, Inaba Y, Kikuchi S, Yagyu K, Motohashi Y, Tamakoshi1 A. Overview of epidemiology of bile duct and gallbladder cancer focusing on JACC Study. J Epidemiol. 2005;15 Suppl 2:S150–S156. doi: 10.2188/jea.15.S150. [DOI] [PMC free article] [PubMed] [Google Scholar]