Abstract
Background
Quantitative fiber tracking derived from diffusion tensor imaging (DTI) was used to determine whether white matter association, projection, or commissural tracts are affected in nondemented individuals with HIV infection and to identify the regional distribution of sparing and impairment of fiber systems.
Methods
DTI measured fractional anisotropy and diffusivity, quantified separately for longitudinal (λL) diffusivity (index of axonal injury) and transverse (λT) diffusivity (index of myelin injury), in 11 association and projection white matter tracts and six commissural tracts in 29 men and 13 women with HIV infection and 88 healthy, age-matched controls (42 men and 46 women).
Results
The total group of HIV-infected individuals had higher diffusivity (principally longitudinal) than controls in the posterior sectors of the corpus callosum, internal and external capsules, and superior cingulate bundles. High longitudinal diffusivity, indicative of axonal compromise, was especially prominent in posterior callosal sectors, fornix, and superior cingulate bundle in HIV with AIDS. Unmedicated patients had notably high transverse diffusivity, indicative of myelin compromise, in the occipital forceps, inferior cingulate bundle, and superior longitudinal fasciculus. Pontocerebellar projection fibers were resistant to HIV effects as were commissural fibers coursing through premotor and sensorimotor callosal sectors.
Conclusion
This quantitative survey of brain fiber tract integrity indicates that even nondemented HIV patients can have neuroradiological evidence for damage to association and commissural tracts. These abnormalities were vulnerable to exacerbation with AIDS and possibly mitigated by HAART.
Keywords: AIDS, brain, cognition, dementia, diffusion tensor imaging, HAART, HIV, MRI, white matter
Introduction
The HIV virus infects the brain through complex pathways affecting white matter before causing neuronal injury [1]. Postmortem evidence for the neurotoxic process of HIV infection includes diffuse white matter degradation [2]. In-vivo imaging has documented larger areas of white matter hyperintensities [3] and smaller volumes of cortical white matter [3,4] and corpus callosum in HIV-infected than in unaffected groups [5,6]. HAART has increased life expectancy of HIV-infected individuals, altering the pattern of brain pathology found in pre-HAART on autopsy [7] and reducing the incidence of HIV-related dementia [8]. In-vivo brain imaging shows improved or stable qualitative white matter disease indices in HIV patients with encephalopathy on protease inhibitor compared with those not so medicated [9].
Magnetic resonance diffusion tensor imaging (DTI) has been useful in identifying microstructural compromise of white matter in HIV infection even in regions appearing normal on conventional imaging [10]. Quantitative fiber tracking, however, has been used in only one other HIV study, which limited analysis to the genu and splenium of the corpus callosum and found neither anisotropy nor diffusivity differences in HIV-infected individuals relative to controls [11]. The present analysis expanded that fiber tracking study to quantify the integrity of 11 projection and association fiber bundles and the full extent of the corpus callosum to test the hypothesis that nondemented HIV-positive patients exhibit lower anisotropy and higher longitudinal and transverse diffusivity than controls for tracts coursing through frontostriatal regions known to show signs of HIV-related disturbance with magnetic resonance spectroscopy [12,13]. Medication status and history of AIDS were also considered.
Methods
Patients
We compared 42 HIV-positive patients from a naturalistic longitudinal study with 88 controls. Analyses of structural MRI [5] data and DTI genu and splenium data [11] from these HIV patients and controls and their clinical and demographic characteristics [14] have been published. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Characteristics of HIV patients and normal controls: mean (SD) or frequency count.
| Control | HIV | Group differences, χ2 or ANOVA |
|
|---|---|---|---|
| Sex: male/female | 42/46 | 29/13 | P = 0.02 |
| Age (years) | 44.5 (9.9) | 42.5 (9.8) | NS |
| Education (years) | 15.8 (2.3) | 13.8 (3.0) | P = 0.0001 |
| Handedness score (RH = 14–30; LH = 50–70) | 24.2 (13.7) | 25.2 (11.2) | NS |
| Body mass index | 25.8 (4.7) | 26.1 (3.9) | NS |
| Socioeconomic status (low score = high status) | 26.5 (13.0) | 34.8 (14.2) | P = 0.002 |
| Ethnicity (minority + hispanic/white) | 27/61 | 14/28 | NS |
| Peabody IQ | 103.7 (15.4) | 100.0 (15.0) | NS |
| Grooved Pegboard (left and right hands; sec.) | 68.2 (11.1) | 81.8 (27.5) | P = 0.0056 |
| Beck Depression Index | 2.6 (2.9) | 10.8 (8.6) | P = 0.0001 |
| Global Assessment of Functioning (GAF) | 81.6 (8.9) | 71.6 (11.7) | P = 0.0001 |
| Lifetime alcohol consumption (kg) | 42.0 (60.6) | 57.0 (55.4) | NS |
| aSmoker (never/ever) | 59/20 | 11/28 | P = 0.0001 |
| Hepatitis C positive | 0 | 7 | |
| History of substance abuse | 0 | 16 | |
| History of mood disorder | 0 | 12 | |
| History of anxiety disorder | 0 | 4 | |
| Self-reported neuropathy | 0 | 9 | |
| AIDS (CDC category C and/or CD4 cell count <200 cells/µl) | – | 11 | |
| Years infected with HIV | – | 8.4 (6.4) | |
| Currently unmedicated for HIV | 9 | ||
| Virally suppressed | 8 | ||
| CD4+ cell count | – | 546.6 (284.0) | |
| Log viral load | – | 3.0 (1.0) |
Smoking history available on 79/88 controls and 39/42 HIV patients.
Men and women infected with HIV were recruited from San Francisco Bay Area outpatient HIV/AIDS clinics by project staff to participate as a comparison group in a longitudinal study of the effects of alcohol on the progression of HIV infection on the brain. Controls were recruited by referral from patient participants, Internet posting, and flyers. Referrals and inquiries were followed up with a brief screening interview designed to identify exclusionary features. Those who met initial inclusion criteria were invited into the laboratory for a more detailed assessment after providing informed consent.
Clinical evaluation
All controls and HIV patients underwent a panel of blood tests. HIV patients with a CD4 cell count below 200 cells/µl at intake were excluded from the study, which was designed to be longitudinal. Structured interviews characterized history of HIV illness, treatment, and symptoms. Medication status was characterized as HAART [e.g., two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor, or two NRTIs and a nonnucleoside reverse transcriptase inhibitor (NNRTI)], non-HAART, or none. Patients entering the study had been on a stable regimen for at least 4 weeks. The Structured Clinical Interview for DSM-IV (SCID) [15] confirmed the absence of schizophrenia, bipolar disorder, alcohol abuse or dependence, or current (last 3 months) substance dependence or abuse in patients, and confirmed that prospective controls did not meet DSM-IV criteria for any Axis I disorder. No patient was clinically demented, but 11 (including one unmedicated person) had had an AIDS-defining event. A quantitative history of alcohol consumption yielded lifetime totals and enabled exclusion of heavy drinkers. Clinical assessment (Table 1) also included the Global Assessment of Functioning (GAF) from the SCID; Karnofsky score, a global rating of clinical disability; Beck Depression Index [16], a quantitative measure of depressive symptoms; socioeconomic status scale (SES), a two-factor scale based on education and occupation [17], and body mass index (height in cm/weight in kg2), an index of nutritional status. General cognitive status was assessed with the Peabody Picture Vocabulary Test (PPVT-III) [18].
Magnetic resonance image acquisition
Magnetic resonance imaging was performed on a 1.5T GE clinical whole-body system. A dual-echo fast spin-echo (FSE) coronal structural sequence was acquired (47 contiguous, 4 mm thick slices; TR/TE1/TE2=7500/14/98 ms; matrix=256 × 192). DTI was performed with the same slice location parameters as the dual-echo FSE, using a single-shot, spin-echo, echo-planar imaging technique (47 contiguous, 4 mm thick slices; TR/TE=10 000/103 ms; matrix=128 × 128; in-plane resolution =1.875 mm2; b=860 s/mm2). Diffusion was measured along six noncollinear directions (6 NEX) with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients [19]. For each slice, six images with no diffusion weighting (b=0 s/mm2) were also acquired.
Image processing
The structural data were passed through the FSL Brain Extraction Tool [20] to extract the brain. Eddy current-induced image distortions in the diffusion-weighted images for each direction were minimized by alignment with an average made of all 12 diffusion-weighted images using a two-dimensional six-parameter affine correction on a slice-by-slice basis [21]. The DTI data were then aligned using the FSE data by a nonlinear three-dimensional warp (third-order polynomial), which provided in-plane and through-plane alignment. On a voxel-by-voxel basis, fractional anisotropy and apparent diffusion coefficient, the latter decomposed into its longitudinal and (λL = λ1) and transverse (λT = [λ2 + λ3]/2) components, were computed. Fractional anisotropy ranged from 0 to 1 and diffusivity was expressed in units of 10−3 mm2/s.
Warping to common coordinates
To achieve common anatomical coordinates across patients, a population-average fractional anisotropy template [22] was constructed from the fractional anisotropy data of 120 controls (20 – 81 years old) with group-wise affine registration [23] followed by iterative nonrigid averaging. Each patient’s fractional anisotropy data set was registered to the population fractional anisotropy template with a nine-parameter affine transformation followed by nonrigid alignment using a multilevel, third-order B-spline, with 5-mm final control point spacing [24].
Fiber tracking
A more detailed description of our fiber tracking procedures appears elsewhere [22]. The fiber tracking routine [25,26] applies a target–source convention that restricts fibers to those originating in source voxels and passing through target voxels. Fiber tracking bundle targets were identified on the population fractional anisotropy template in several white matter locations [22]: fornix, internal capsule, external capsule, frontal forceps, occipital forceps, superior cingulate, inferior cingulate, superior longitudinal fasciculus, inferior longitudinal fasciculus, pontocerebellar tract, and cerebellar hemispheres. For the corpus callosum, six geometrically defined targets, modified to reflect documented [27] callosal anatomical projections [28] were identified on the midsagittal population fractional anisotropy template. Sources were perpendicular planes – anterior and posterior or superior and inferior to the targets – to the orientation of the fibers, or cubes surrounding the targets in the cases of the fornix, pontocerebellar tracts, and cerebellar hemispheres. For each patient the targets and sources were mapped from the population fractional anisotropy template to that patient’s native image space and passed to the fiber tracking routine [29]. Tracking parameters specified minimum fractional anisotropy (0.17), 37° maximum angular deviation between voxels, and minimum (11.25 mm) and maximum (45 mm) fiber lengths, with essentially no limit on the number of fibers (other than the number of source pixels). We refer hereafter to the group of fibers coursing through each target region as ‘fiber bundles’. For each fiber bundle, mean fractional anisotropy, λL, and λT were the units of analysis. After fiber detection, the fiber locations were transformed back to common coordinates (i.e., population fractional anisotropy template space) for display.
Statistical analysis
Hemisphere effects in DTI metrics were assessed with repeated measures analysis of variance (ANOVA) for laterality and group for each of the 10 bilateral fiber bundles. Group effects were assessed by two sets of repeated measures ANOVA for each DTI metric, that is, fractional anisotropy, λL, and λT: one ANOVA set included 11 association and projection bundles and the other set included six corpus callosum sectors. We predicted that HIV patients would have lower fractional anisotropy and higher diffusivity values than normal controls, prominent in fiber tracts involving frontostriatal systems. Medication effects were assessed with three-group Kruskal–Wallis nonparametric analyses (33 medicated HIV, nine unmedicated HIV, 88 controls) followed by Mann–Whitney tests because of the wide range of group sizes. Associations between DTI metrics and demographic, HIV indicators, motor performance, or comorbidities were tested with Pearson correlations, Spearman correlations, or χ2. Follow-up two-group differences with t-tests were considered significant if P was less than or equal to 0.02; three-group differences were significant with Scheffe tests (P=0.05).
Results
Commissural fiber integrity
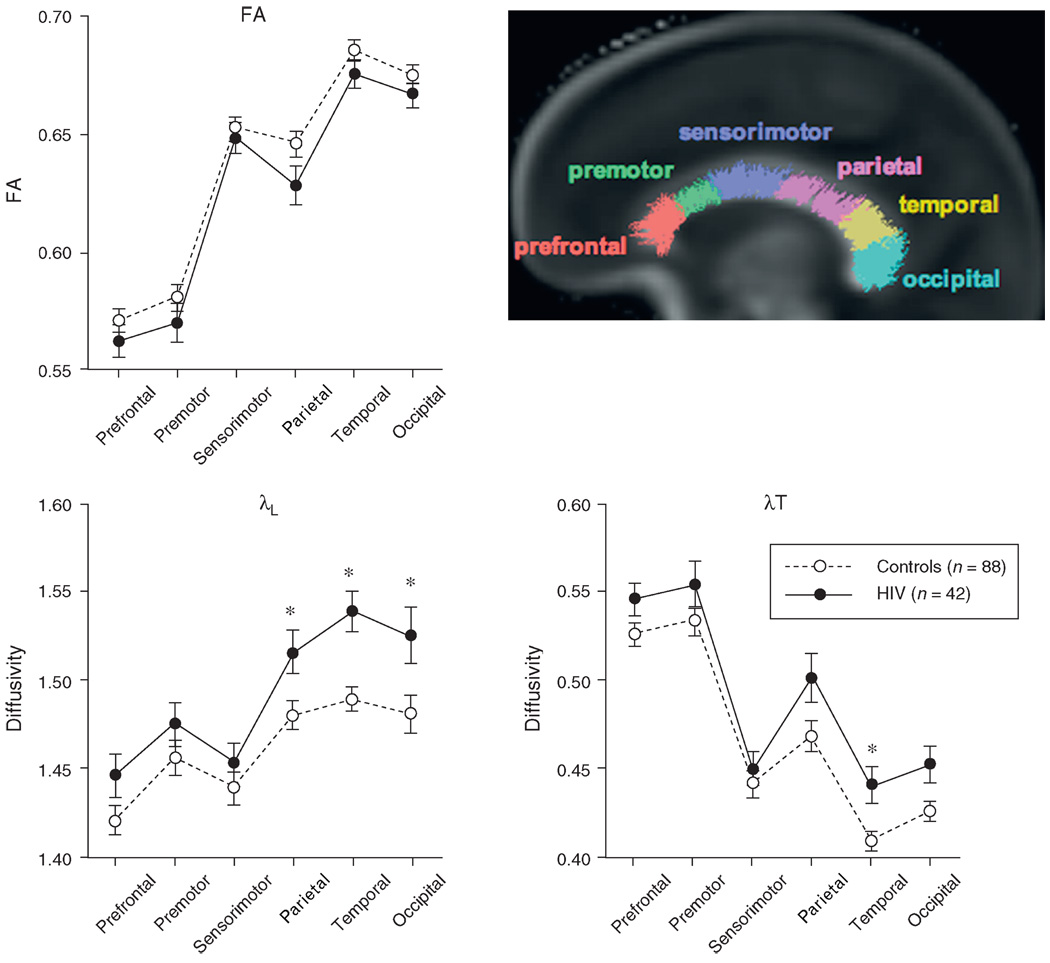
Repeated measures ANOVAs across the six callosal sectors were conducted for each DTI metric to seek simple and interaction effects involving group membership (HIV-infected vs. control). In no case was the group-by-sector interaction significant nor was the fractional anisotropy group effect [F(1, 128) = 2.483, P = 0.1176]. The group effects of the diffusivity ANOVAs, λL [F(1, 128) = 7.455, P = 0.0072] and λT [F(1, 128) = 5.145, P = 0.025], together with follow-up t-tests found higher diffusivity in HIV patients than in controls in the parietal [λL: t(128) = 2.449, P = 0.0157], temporal [λL: t(128)=3.659, P = 0.0004; λT: t(128) = 2.930, P = 0.004], and occipital [λL: t(128) = 2.469, P = 0.0149] sectors (Fig. 1).
Fig. 1. Mean ± SE fractional anisotropy, λL, and λT for each callosal sector of the control and HIV groups.
The sagittal brain image displays color-coded fiber tracts on a fractional anisotropy image for each of the six callosal sectors. *P<0.05.
Projection and association fiber bundle integrity
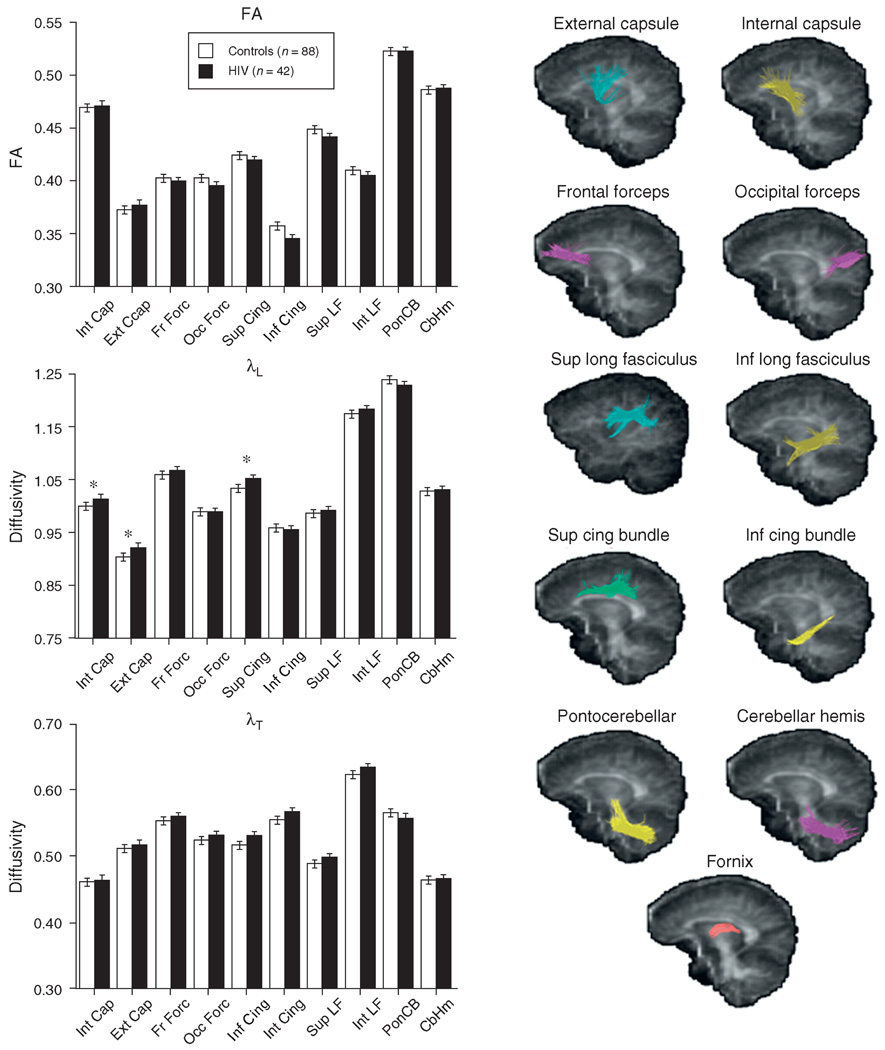
Absence of significant group-by-hemisphere interactions permitted subsequent analyses to use mean values from left and right hemispheres for fractional anisotropy and diffusivity. Repeated measures ANOVAs for fractional anisotropy across the 11 fiber bundles yielded neither a group effect nor a group-by-bundle interaction. By contrast, diffusivity measures were disproportionately higher in the HIV group than in the control group in certain fiber bundles [for λL: group F(1128) = 3.579, P = 0.0608; group-by-bundle (F(10, 1280) = 2.202, P = 0.0156); for λT: group F(1128) = 4.517, P = 0.0355; group-by-bundle (F(10, 1280) = 2.458, P = 0.0066)]. Fractional anisotropy and diffusivity values in the inferior cingulate bundle of one HIV-infected man were 3–7 SDs from the mean of the remaining patients; reanalysis excluding his DTI values had little effect on the pattern of significant findings. Follow-up t-tests identified higher diffusivity in the HIV than in the control group in internal capsule [t(128) = 2.437, P = 0.0162], external capsule [t(128) = 2.377, P = 0.0189], and superior cingulate bundle [t(128) = 2.591, P = 0.0107] for λL. Group values for fractional anisotropy, λL, and λT appear in Fig. 2.
Fig. 2. Mean±SE fractional anisotropy, λL, and λT for each of the 10 bilateral projection and association fiber bundles of the control and HIV groups.
The sagittal images provide examples of the location of each bundle tracked. Values of the fornix were not plotted because its diffusivity values were about twice that of the other tracts. *P<0.05.
Role of HIV medication
Clinical and demographic characteristics of medicated and unmedicated HIV patients are summarized in Table 2. CD4 cell counts were not significantly lower [t(40) = 327, P = 0.192], but viral loads were higher [t(40) = 2.85, P = 0.0069] in unmedicated than in medicated groups. Although medication status had no differential effect on callosal metrics, three-group ANOVAs across the 11 fiber bundles (Fig. 3) yielded a group-by-region interaction for λL [F(20, 1270) = 3.608, P = 0.0001] and λT [F(20, 1270) = 2.780, P = 0.0001], whether or not the inferior cingulate DTI measures of the unmedicated group outlier were included. λT was higher (Scheffe tests) in inferior cingulate bundle, occipital forceps, and superior longitudinal fasciculus of the unmedicated group than in the control group. The inferior cingulate bundle difference was attenuated when the unmedicated outlier was excluded but endured a Fisher least significant difference test. In contrast to λT measures, Scheffe tests indicated that the medicated group had higher λL than controls in the internal capsule.
Table 2.
Characteristics of HIV patients on HAART or non-HAART and unmedicated HIV patients: mean (SD) or frequency count.
| Any HIV medications | No HIV medications | Group differences, χ2 or ANOVA |
|
|---|---|---|---|
| Sex: male/female | 23/10 | 6/3 | NS |
| Age (years) | 44.4 (8.4) | 35.4 (12.0) | P = 0.013 |
| Education (years) | 14.4 (2.7) | 11.6 (2.9) | P = 0.0082 |
| Socioeconomic status (lower score = higher status) | 32.3 (14.2) | 44.5 (9.7) | P = 0.0283 |
| Ethnic minority | 7/33 | 7/9 | P = 0.001 |
| Peabody IQ | 103.4 (14.3) | 87.5 (10.5) | P = 0.006 |
| Body mass index | 26.4 (4.3) | 25.1 (1.8) | NS |
| Beck Depression Index | 10.8 (9.3) | 10.9 (6.2) | NS |
| Global Assessment of Functioning (GAF) | 72.5 (11.9) | 68.2 (11.1) | NS |
| Karnofsky Scale | 95.8 (6.6) | 95.6 (5.3) | NS |
| Lifetime alcohol consumption (kg) | 59.5 (56.5) | 49 (54.3) | NS |
| Ever smoked cigarettes | 21/33 | 7/9 | NS |
| Years infected with HIV | 10.5 (6.2) | 2.2 (1.2) | P = 0.0006 |
| CD4+ cell count (cells/µl) | 576.7 (294.4) | 436.2 (221.7) | NS |
| Nadir CD4+ cell count (cells/µl) | 192.0 (129.7) | 342.4 (158.0) | P = 0.0128 |
| Viral load (copies/ml) | 5893.3 (17 977.0) | 26 984.8 (25 365.5) | P = 0.0069 |
| HCV positive | 5/33 | 2/9 | NS |
| History of substance abuse | 11/33 | 4/9 | NS |
| Self-reported neuropathy | 9/33 | 0/9 | P = 0.08 |
| HIV medication naïve | – | 6/9 | – |
| Detectable viral load | 27/33 | 8/9 | NS |
Fig. 3. Mean ± SE λL and λT for the projection and association bundles of the control group, medicated HIV group, and unmedicated HIV group.
*P < 0.05.
Clinical correlates
Viral load
Correlational analysis of patient descriptors in Table 1 with each fiber bundle metric revealed that patients with higher log viral load had higher λL (r = 0.439, P = 0.0033) in occipital forceps.
Peripheral neuropathy
Of the 33 participants in the medicated group, nine reported signs of peripheral neuropathy, but none in the unmedicated group expressed having such signs. Consistent with peripheral neuropathy was slow performance relative to the nonneuropathy group (Mann–Whitney: Z = 2.086, P = 0.037) and controls (Mann–Whitney: Z = 2.975, P = 0.0029) on Grooved Pegboard, a test of speed and manual dexterity. Patients with self-reported peripheral neuropathy had higher λL in the parietal sector of the corpus callosum than those without (Mann–Whitney: Z = 2.498, P = 0.0125).
AIDS
Of the 42 HIV participants, 11 had an AIDS-defining event (HIV/AIDS). Repeated measures ANOVA and follow-up Scheffe tests indicated that the HIV/AIDS group had higher λL, selective to the three posterior callosal sectors (three group-by-six sector ANOVA group effect: F(2, 127) = 6.398, P = 0.0023); the non-AIDS/HIV group also had higher λL in the temporal sector than in controls (Fig. 4). A similar but less robust pattern held for λT [F(2127) = 2.909, P = 0.0582]; specifically, relative to the control group, the HIV/AIDS group had higher λT in the temporal and occipital sectors, whereas this effect was limited to the temporal sector in the non-AIDS/HIV group. Although neither fractional anisotropy nor λT of the projection or association bundles distinguished the HIV/AIDS group from the other groups, λL did [group effect: F(2127) = 4.058, P = 0.0196], HIV/AIDS group had higher λL in the fornix and superior cingulate bundle than controls (Fig. 4).
Fig. 4. Mean ± SE λL for each callosal sector (top) and for the fornix and superior cingulate bundle (bottom) of the control group, HIV only group, and HIV + AIDS group.
*P < 0.05.
Discussion
This report used quantitative fiber tracking to assess the microstructural integrity of major commissural, projection, and association fiber bundles in a naturalistic sample of nondemented HIV-infected men and women. Patients were not participating in treatment protocols and were heterogeneous with regard to HIV severity, treatment regimen, and comorbid conditions. Across all fiber bundles sampled, internal and external capsules (that course through the striatum), superior and inferior cingulate bundles (that link frontal to subcortical structures), and the posterior sectors of the corpus callosum showed effects of HIV, principally in longitudinal diffusivity, indicative of axonal compromise. Pontocerebellar projection fibers were particularly resistant to HIV effects as were commissural fibers coursing through premotor and sensorimotor callosal sectors.
Although most patients were on HAART (n = 26) or other HIV treatment regimen (n = 7), only eight were completely virally suppressed at scan. The unmedicated patients were comparable in CD4 cell count to medicated patients but were more recently infected and had higher current viral loads. Patients who were either drug-naive or not currently on a treatment regimen showed markedly higher transverse diffusivity than controls in inferior cingulate bundle, occipital forceps, and superior longitudinal fasciculus, indicative of myelin degradation.
By contrast, patients on an HIV treatment regimen had DTI values in the control range for these bundles. The mechanistic and functional significance of this finding is unclear, but it is tempting to speculate that active but subclinical inflammation, promoted by untreated HIV infection [30], contributed to high diffusivity. Case studies describe regression of white matter signal abnormalities in HIV patients with AIDS dementia after commencement of treatment with protease inhibitors or NNRTIs [9,31].
In general, HIV-related fiber tract abnormalities were detected with diffusivity measures rather than fractional anisotropy, regardless of medication or AIDS status. High longitudinal diffusivity in the parietal, temporal, and occipital callosal sectors, fornix, and superior cingulate bundle was prominent in the HIV group with AIDS, although AIDS did not fully account for the observed abnormalities. The observation by Chang et al. [32] that genu diffusivity was especially useful in longitudinally tracking HIV-related white matter degeneration is consistent with our observation that high diffusivity, notably λL rather than fractional anisotropy, serves as a marker of HIV-related neuropathology, possibly indicating an inflammatory process involving the axon.
Most previous DTI studies of HIV-infected patients have examined specific, geometrically defined regions of interest: corpus callosum [32–35], subcortical white matter,[32,33] basal ganglia [36], and frontal white matter [32,35,37,38]. Results are varied, depending on the region examined, measurement techniques, and clinical status of the patients. Our quantitative fiber tracking study identifying local disruption of microstructure in frontostriatal association and posterior commissural fiber systems, possibly selective to axons in patients with HIV infection compared with controls, extends earlier DTI studies. The limitations of the study are inherent in its naturalistic design, patient heterogeneity, and absence of formal treatment regimen. Thus, our inference for effects of HAART is based on the regional markers of myelin degradation seen in untreated but not in treated patients with comparable CD4 cell counts. The unmedicated group, however, was younger, had less education, lower socioeconomic status, and lower general intelligence scores than their medicated counterparts, factors that might have had unknown effects. To the extent that the untreated sample is representative of the untreated HIV-infected population, however, our observation raises the possibility that additional or tailored educational efforts need to be directed toward the unmedicated population. Further, the HIV-infected patients with self-professed signs of peripheral neuropathy exhibited abnormally high callosal longitudinal diffusivity, consistent with peripheral axonal damage associated with such signs [39]. If left untreated, further disruption of fiber systems could contribute to worsening of sensory signs and development of cognitive and motor dysfunction.
Acknowledgements
We would like to thank our diligent research assistants (Jeffrey Eisen, Donna Murray, Marya Schulte, Andrea Spadoni, Carla Raassi, Daniel J. Pfefferbaum, Ted Sullivan, Alexander Jack, Julia Sandler, Carrie McCloskey, Shara Vinco, Marissa Huang, Shannon Muir, and Suzanne Franklin) and research clinicians (Julia Buss,RN, Crystal Caldwell, Stephanie A. Sassoon, PhD, Anne O’Reilly, PhD, Anjali Deshmukh, MD) for their invaluable work in participant recruitment, clinical evaluation, medical examination, scheduling, screening, contact maintenance, data collection, and data entry. This work was supported by NIAAA grants AA012999 and AA017347.
References
- 1.Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- 2.Budka H. Neuropathology of myelitis, myelopathy, and spinal infections in AIDS. Neuroimag Clin N Am (Neuroimaging AIDS II) 1997;7:639–650. [PubMed] [Google Scholar]
- 3.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 4.Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, et al. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, et al. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31:12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Everall IP, Hansen LA, Masliah E. The shifting pattern of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 9.Filippi CG, Sze G, Farber SJ, Shahmanesh M, Selwyn PA. Regression of HIV encephalopathy and basal ganglia signal intensity abnormality at MR imaging in patients with AIDS after the initiation of protease inhibitor therapy. Radiology. 1998;206:491–498. doi: 10.1148/radiology.206.2.9457204. [DOI] [PubMed] [Google Scholar]
- 10.Pfefferbaum A, Sullivan EV. Diffusion MR imaging in neuropsychiatry and aging. In: Gillard J, Waldman A, Barker P, editors. Clinica MR neuroimaging: diffusion, perfusion, spectroscopy. 2nd ed. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 11.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fiber tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 13.Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbloom MJ, Sullivan EV, Sassoon SA, O′Reilly A, Fama R, Kemper CA, et al. Alcoholism, HIV infection and their comorbidity: factors affecting self-rated health-related quality of life. J Stud Alcohol Drugs. 2007;68:115–125. doi: 10.15288/jsad.2007.68.115. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 17.Hollingshead A, Redlich F. Social class and mental illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn LM, Dunn ES. Peabody Picture Vocabulary Test – Third Edition. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 19.Neeman M, Freyer JP, Sillerud LO. A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med. 1991;21:138–143. doi: 10.1002/mrm.1910210117. [DOI] [PubMed] [Google Scholar]
- 20.Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration. I: General methods and intra-subject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Learned-Miller EG. Data driven image models through continuous joint alignment. IEEE Trans Pattern Anal Machine Intell. 2006;28:236–250. doi: 10.1109/TPAMI.2006.34. [DOI] [PubMed] [Google Scholar]
- 24.Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans Inf Technol Biomed. 2003;7:16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Mori S, Solaiyappan M, van Zijl PC, Davatzikos C. A framework for callosal fiber distribution analysis. Neuroimage. 2002;17:1131–1143. doi: 10.1006/nimg.2002.1285. [DOI] [PubMed] [Google Scholar]
- 27.Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres – one brain: functions of the corpus callosum. New York: Alan R. Liss Inc.; 1986. pp. 47–74. [Google Scholar]
- 28.Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- 29.Gerig G, Corouge I, Vachet C, Krishnan KR, MacFall JR. Quantitative analysis of diffusion properties of white matter fiber tracts: a validation study; 13th Proceedings of the International Society for Magnetic Resonance in Medicine; Miami, FL. 2005. [Google Scholar]
- 30.Rostasy KM. Inflammation and neuroaxonal injury in multiple sclerosis and AIDS dementia complex: implications for neuroprotective treatment. Neuropediatrics. 2005;36:230–239. doi: 10.1055/s-2005-865864. [DOI] [PubMed] [Google Scholar]
- 31.Thurnher MM, Post MJ, Rieger A, Kleibl-Popov C, Loewe C, Schindler E. Initial and follow-up MR imaging findings in AIDS-related progressive multifocal leukoencephalopathy treated with highly active antiretroviral therapy. AJNR Am J Neuroradiol. 2001;22:977–984. [PMC free article] [PubMed] [Google Scholar]
- 32.Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. Am J Neuroradiol. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- 34.Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. Am J Neuroradiol. 2005;26:2275–2281. [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. Am J Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- 36.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 39.Hahn K, Robinson B, Anderson C, Li W, Pardo CA, Morgello S, et al. Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Exp Neurol. 2008;210:30–40. doi: 10.1016/j.expneurol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]