Much of what is known about the function of human rostrolateral prefrontal cortex (RLPFC; lateral Brodmann area 10) has been pieced together from functional magnetic resonance imaging (fMRI) studies over the past decade. Christoff and colleagues previously reported on an fMRI localizer task involving relational integration that reliably engages RLPFC in individual participants (Smith, Keramatian, & Christoff, 2007). Here, we report on a modified version of this task that better controls for lower-level processing demands in the relational integration condition. Using identical stimulus arrays for our experimental and control conditions, we find that right RLPFC is sensitive to increasing relational processing demands, without being engaged specifically during relational integration. By contrast, left RLPFC is engaged only when participants must consider the higher-order relationship between two individual relations. We argue that the integration of disparate mental relations by left RLPFC is a fundamental process that supports higher-level cognition in humans.
The anterior prefrontal cortex (aPFC) corresponds to the largest cytoarchitectonic area in humans: Brodmann area [BA] 10. This region has long been assumed to be important for higher cognitive function in humans, but its precise functions are not yet well understood (Ramnani & Owen, 2004). The bulk of what we know about this region comes from functional magnetic resonance imaging (fMRI) studies, along with a few anatomical studies in non-human primates (Burman, Palmer, Gamberini, & Rosa, 2006; Petrides & Pandya, 2007), and neuropsychological research from a small number of patients with BA 10 damage (Burgess, Veitch, de Lacy Costello, & Shallice, 2000). Single-unit recording data from aPFC are virtually non-existent; data have been reported recently for medial BA 10 (Tsujimoto, Genovesio, & Wise, 2008), but not for lateral BA 10, because this region is difficult to access with electrodes.
Anatomical studies indicate that aPFC receives and integrates large numbers of inputs from higher-order association cortices. Anterograde and retrograde tracer studies in monkeys indicate that aPFC neurons are interconnected primarily with other parts of prefrontal cortex and other association areas, rather than with low-level perceptual or motor areas (Burman, Palmer, Gamberini, & Rosa, 2006; ; Petrides & Pandya, 2007); the afferents and efferents of aPFC in humans are not yet known. Cytoarchitectonic research in adult humans reveal that aPFC neurons have extensive dendritic arborization and a high density of dendritic spines (Jacobs et al., 2001), rendering them ideal for integrating across multiple inputs from other neurons (Ramnani & Owen, 2004). Cell density in aPFC – particularly in the human – is characteristically low (Burman et al., 2006), which allows for more connections with supramodal areas.
Over the last decade, fMRI studies have yielded a number of hypotheses about aPFC function; several of these are beginning to converge on a few key ideas (Braver & Bongiolatti, 2002; Burgess, Scott, & Frith, 2003; Burgess, Simons, Dumontheil, & Gilbert, 2005; Christoff & Gabrieli, 2002; Christoff, Ream, Geddes, & Gabrieli, 2003; Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999; Koechlin, Ody, & Kouneiher, 2003; Ramnani & Owen, 2004). Lateral and medial aPFC are cytoarchitectonically distinct and have dissociable patterns of activation (Burgess et al., 2003; Gilbert, Spengler, Simons, Frith, & Burgess, 2006; Koechlin, Corrado, Pietrini, & Grafman, 2000). A meta-analysis of fMRI studies by Burgess and colleagues suggests that there are in fact three functionally distinct aPFC areas (Gilbert et. al. 2006). Here, we focus exclusively on lateral aPFC, also referred to as rostrolateral PFC (RLPFC), shown in blue in Supplementary Figure 1.
Neuropsychological research involving patients with frontotemporal dementia (FTD) initially laid the foundation for the relational integration hypothesis of RLPFC function, despite the fact that neuronal degeneration in this disorder is not limited to RLPFC. FTD patients with frontal but not temporal lobe damage showed a marked deficit in performance on two fluid reasoning tasks: transitive inference problems and the 2-relational trials of the Raven’s Progressive Matrices (RPM) (Waltz et al., 1999). On transitive inference problems like ‘Joe is taller than Hillary; Barack is taller than Joe’, it is necessary to jointly consider the two relations to figure out the correct ordering of the three names by height. On 2-relational RPM trials, it is necessary to jointly consider horizontal and vertical dimensions of change in a set of abstract figures arranged in a matrix. These results suggested that PFC is critical for the processing and/or integration of multiple mental relations (Waltz et al., 1999).
Subsequent fMRI studies have extended this work by showing that RLPFC is the region in PFC most closely linked to relational integration (Christoff et al., 2001; Kroger et al., 2002). In adults, this region is disproportionately engaged on 2-relational relative to both 1-relational and 0-relational RPM problems (Christoff et al., 2001; Crone et al., 2008). We have found that children aged 8-12, who have difficulty with 2-relational problems, engage RLPFC similarly for 1- and 2-relational RPM problems (Crone et al., 2008).
RLPFC has also been implicated in the integration of semantic relations, when participants are asked to evaluate propositional analogies such as “car is to road as sailboat is to water?” (Bunge, Wendelken, Badre, & Wagner, 2005; Wendelken, Nakhabenko, Donohue, Carter, & Bunge, 2008; Wright, Matlen, Baym, Ferrer, & Bunge, 2007). In an initial study we showed that RLPFC activation is greater when participants must evaluate whether two word pairs are related in the same way than when they are asked to consider relations among words separately for each of two word pairs (Bunge et al., 2005). Additionally, we showed that RLPFC is insensitive to demands placed on retrieval of individual semantic relations, which suggests that RLPFC plays a role in integrating across semantic relations subsequent to retrieval of these relations from long-term memory.
In a follow-up study, we showed that RLPFC supports evaluation of the concordance between two semantic relations, but not completion of a pair of relations (e.g. “shoe is to foot as glove is to hand?” but not “shoe is to foot as glove is to…?”) (Wendelken et al., 2008). Although completion problems take longer to solve than evaluation problems, the way these problems are posed encourages sequential semantic retrievals rather than simultaneous evaluation of two mental relations. Consistent with the idea that RLPFC is not involved in retrieving individual relations from long-term memory, we showed that this region is engaged equally strongly when participants must solve the following two types of problems: “shoe : foot :: glove : hand?” and “wear :: glove : hand?” In the former condition, participants must derive the semantic relationship between the first two words; in the latter condition, they are given a relational term and must simply determine whether it characterizes the relationship between the final two words. Thus, RLPFC – a region implicated in the highest levels of human cognition – is engaged even on a simple semantic task that involves comparison of a single relation (glove : hand) with a relational term (wear).
In contrast to RLPFC, other lateral PFC regions have shown sensitivity to other factors that affect task difficulty, rather than relational complexity per se (Kroger et al., 2002; Bunge et al., 2005). In summary, a number of studies suggest that RLPFC plays a unique role in the representation of relational structures, although other lateral PFC subregions also contribute to performance of reasoning tasks like those described above. Based on these and other findings, several researchers have argued that a basic task requirement that drives RLPFC is the need to jointly consider or integrate several distinct mental relations (Christoff et al., 2001; Wendelken et al., 2008) or the outcomes of two or more separate cognitive operations (Ramnani & Owen, 2004).
Building on her initial findings from the RPM task (Christoff et al., 2001), Kalina Christoff and colleagues devised a simple, elegant task that engaged RLPFC (Christoff et al., 2003), and later showed that this task engages RLPFC reliably in individual participants (Smith, Keramatian, & Christoff, 2007). In the change task condition, participants had to infer the dimension of change between the top pair of objects (texture or shape), and then determine whether the bottom pair of objects changed along the same dimension. In the feature task conditions, participants had to determine whether the bottom object matched either one of the top two objects along the specified dimension (texture or shape). For each of the 10 individuals studied by Smith et al., RLPFC was more active for the change task than the control tasks (Smith et al., 2007).
Christoff and colleagues originally designed this task to test the hypothesis that RLPFC is involved in the evaluation of internally generated information (Christoff et al., 2003). In the change condition, participants were required to evaluate internally generated information: the inferred dimension of change. In the feature conditions, participants had to evaluate external information: whether there was a match in texture or shape between two stimuli. On the surface, data from the relational matching task are consistent with idea that RLPFC is involved in evaluating internally generated information. However, based on other evidence from our laboratory, we do not believe that the internal vs. external distinction accounts for RLPFC engagement on the change task. In a verbal propositional analogy task described above (Wendelken et al., 2008), we found that RLPFC activation was lower for the two conditions that required greater internal generation of semantic representations relative to the two conditions that required comparison of two relations. Another way to interpret Christoff’s relational matching data is to note that the change task requires processing and integration of two relations, whereas the feature tasks merely require identification of a single relation.
The original relational matching task serves as an effective localizer for RLPFC in individuals, as intended. However, it does not – and nor was it intended to – provide an optimal test of the relational integration hypothesis. It is conceivable that participants engaged RLPFC more strongly in the change task than the feature task because they had to attend to relations among four rather than three items in the stimulus arrays, or because they had to judge a difference between stimuli for the change task, whereas they had to focus on a similarity between stimuli for the control tasks. Prior research indicates that the type of judgment a participant must make can influence RLPFC activation (e.g., Dobbins & Han, 2005). For example, in a prior study focused on the neural correlates of task rule representation, we found that RLPFC was strongly engaged when participants retrieved a non-match rule (pressing a left key to indicate the lack of a match between stimuli, and a right key to indicate a match) relative to a match rule with the opposite response contingencies, which they had learned first (Bunge et al., 2003).
To provide a more stringent test of the relational integration hypothesis, we created a new version of the relational matching task (Figure 1). Four patterned shapes were presented on every trial, and participants were asked on every trial to make a yes/no judgment regarding the presence or absence of a specific kind of match, by pressing one of two buttons on a button box. In the Shape task, participants were asked to determine whether there is a match in shape in either the top or bottom pair of stimuli. Likewise, in the Texture task, participants were asked to determine whether there is a match in texture (i.e. pattern) in either the top or bottom pair of stimuli. Both of these Feature tasks required lower-order relational judgments as to whether stimuli match along a given dimension. In contrast, on the Dimension task – our experimental condition – participants were asked to make a higher-order relational judgment: they indicated whether items within two pairs of stimuli were related to one another along the same dimension. Thus, although the stimuli appeared identical across conditions, only the Dimension task required subjects to integrate two relations in order to produce the correct response.
Figure 1.
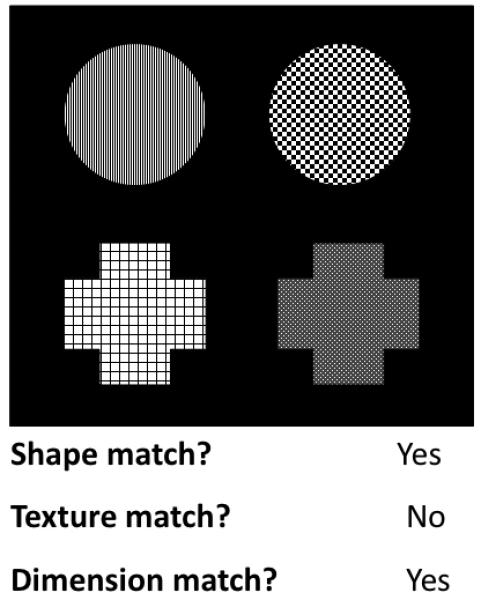
In our version of the relational matching task, each condition involved a yes/no judgment based on a stimulus array including four shapes with varying textures. On Shape trials, participants indicated whether there was at least one shape match present in any of the stimuli pairs. On Texture trials, participants indicated whether there was at least one texture match present in any of the stimuli pairs. On Dimension trials, participants indicated whether the stimuli in the bottom pair matched along the same dimension as the stimuli in the top pair. Shape and Texture trials required low-level relational processing, whereas Dimension trials required relational integration.
The same 60 stimulus arrays were used in each of the three conditions, and the correct answer for half of the trials in each condition was the ‘yes’ response. For example, an array with a shape match in the top and also a shape match in the bottom pair would elicit a ‘yes’ response on a dimension trial and also on a shape trial, but a ‘no’ response on a texture trial. 10 arrays included a shape match for one pair of items, 10 arrays included a texture match for one pair of items, and 10 arrays included neither a shape nor a texture match. 10 arrays included a texture match in both pairs as well as a shape match in both pairs, thereby adhering to the shape, texture and dimension rules. 10 arrays included texture matches for both pairs, thereby adhering to the texture and dimension rules, and the last 10 arrays included shape matches for both pairs, thereby adhering to the shape and dimension rules.
We sought to test whether RLPFC would be engaged more strongly on Dimension than Feature trials in our version of the relational matching task. To this end, we recruited 15 right-handed participants (9 males, mean age 23.8, range 19-35) from the UC Berkeley research subject volunteer program for an fMRI study. Informed consent was obtained in accordance with guidelines set by the Committee for Protection of Human Subjects at UC Berkeley. Participants were scanned on a Siemens 3Tesla Trio system at the UCSF Neuroscience Imaging Center.1 The experiment was run as a blocked design, with 12 trials per block, 5 blocks per scan, and 3 scans per session. At the beginning of each 50-second block, participants viewed one of three instruction cues for 2 seconds: “SHAPE”, “TEXTURE”, or ”DIMENSION”. Participants were then presented with a series of stimulus arrays at 4-second intervals. Each array was presented for a maximum of 3500 ms each, or until subjects had responded. There was a 10-second rest interval between successive blocks of trials. A total of 60 Dimension trials, 60 Shape trials, and 60 Texture trials were acquired per scan session. Block order was counterbalanced between participants. The proportion of yes and no responses was set to 50-50 for each of the three conditions.
As expected, participants were less accurate on the Dimension (79.32 ± 9.78 %) trials as compared with the Shape (91 ± 4.66 %; p = 0.001) and Texture (88.76 ± 6.48 %; p = 0.007) trials. They were also slower to respond on correctly performed Dimension (1199.11 ± 285 ms) than Shape (1020.19 ± 223 ms; p = 0.002) trials. Although participants made more errors on Dimension than Texture trials, there were no significant differences in response times (RT) for correctly performed trials in these conditions (Texture: 1121.36 ± 208 ms; p > .60). We posit that the judgment required on Texture trials was more difficult than on the Shape trials because the shape was a more salient feature of the stimuli than the pattern. Taking the accuracy and RT data together, we infer that relational processing demands increased from Shape to Texture to Dimension trials. However, the lack of a consistent difference in RTs for Dimension and Texture trials provided us with the opportunity to test for differences in activation between these conditions without the concern that greater activation on Dimension trials could be due, simply, to a significantly longer time on task.
As predicted by the relational integration hypothesis, a group fMRI analysis revealed left RLPFC activation for the Dimension > Feature contrast2 (Figure 2A; 57 voxels; cluster maximum at MNI coordinates of −36 57 9). This cluster of activation, observed in a whole-brain contrast at p<.0025, survived correction for multiple comparisons via small volume correction (pcor = .038; anatomically defined search space in RLPFC: middle and superior frontal gyrus AAL templates, anterior to y = 40 and ventral to z = 25; total volume 72,360mm3). A similarly located, but smaller, cluster in right RLPFC (19 voxels; cluster maximum at MNI coordinates of 39 54 18) was observed at a more liberal whole-brain threshold of p<.01 (Figure 2A, right panel). This cluster did not survive correction for multiple comparisons for the Dimension > Feature contrast (p>.50).
Figure 2.
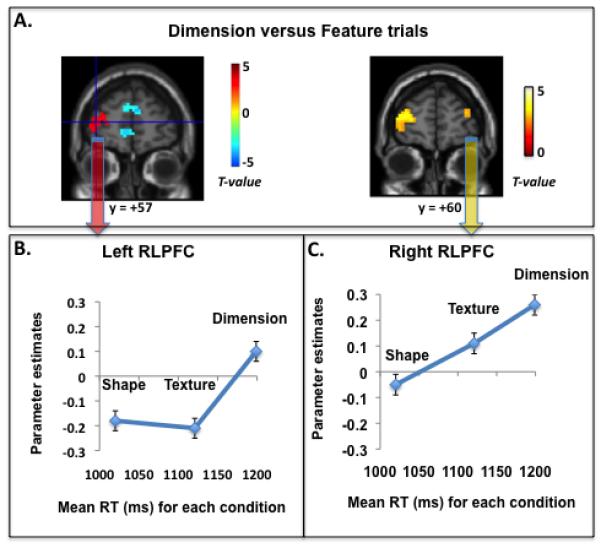
fMRI results for our relational matching task (N=15). A. A whole-brain comparison of Dimension versus Feature tasks (p < .005 uncorrected, >10 voxels) revealed that left RLPFC was relatively more engaged by Dimension trials, whereas rostromedial PFC was relatively more engaged by Feature trials. At a liberal statistical threshold (p < .01 uncorrected), right RLPFC was also engaged by Dimension relative to Feature trials. Shown in B. and C. are plots of RLPFC parameter estimates for each condition relative to the resting baseline. The x-axis features the mean RT for each condition; Shape RTs were faster than Texture RTs, which in turn were numerically but not statistically faster than Dimension RTs. Left RLPFC was specifically engaged when relational integration was required, whereas right RLPFC was more generally engaged as a function of relational task demands.
We performed analyses of variance (ANOVAs) for these bilateral RLPFC regions of interest (ROIs) to examine their activation profile across the three conditions. Left RLPFC was engaged specifically by the Dimension trials and did not discriminate between Texture and Shape trials (F < 1), even though Texture trials were associated with longer RTs than Shape trials (Figure 2B). Consistent with this ROI result, a whole-brain contrast of Texture > Shape revealed widespread activation in right RLPFC but no involvement of left RLPFC, even at a liberal uncorrected threshold of p<.05. This pattern is strongly consistent with a role in relational integration. In contrast with left RLPFC, right RLPFC exhibited a numerically graded pattern of activation across the three conditions, with stronger activation for Texture than Shape trials (F(1,13) = 5.9, p = .03), but no significant differences between Dimension and Texture trials (F(1,13) = 1.6, p > .20) (Figure 2C). An ANOVA comparing the left and right ROIs revealed a significant interaction between side and condition (F(2,13) = 4.0, p = .03).
To test for relationships between individual differences in RLPFC activation and performance, we conducted simple regression analyses on the ROIs in left and right RLPFC (Table 1). Positive brain-behavior correlations across participants were observed for RTs but not for accuracy, most likely because of the larger dynamic range in values of RT. The patterns of between-subjects correlations provided further evidence of the differences between left and right RLPFC noted above. That is, left RLPFC exhibited a stronger correlation between activation and performance on Dimension trials than either of the Feature conditions, with no difference between Texture and Shape trials, whereas right RLPFC exhibited a graded pattern of correlation across the three conditions (R2 for Shape < Texture < Dimension; Table 1).
Table 1.
Correlations between RLPFC activation and RTs across participants
| Strength of correlation (R2) between activation and RTs |
|||
|---|---|---|---|
| Region | Shape | Texture | Dimension |
| Left RLPFC (−36 57 9) |
.29 | .29 | .62 |
| Right RLPFC (39 54 18) |
.08 | .36 | .43 |
Thus, both the ANOVAs and the simple regression analyses indicated that left RLPFC was particularly involved on Dimension trials, for which the relationship between two relations needed to be evaluated. Right RLPFC did not distinguish between Dimension and Texture trials; rather, this region was more generally sensitive to relational processing demands, with activation levels on correctly performed trials mirroring average RTs. Christoff and colleagues’ original relational matching task yielded strong bilateral RLPFC activation, as appropriate for a localizer task. In contrast, our modified relational matching task reveals that left but not right RLPFC meets a more stringent test of the relational integration hypothesis. Our current observations converge with Christoff’s RPM study (2001), in which differential engagement for 2-relational vs. 1-relational problems endured for left but not right RLPFC after correcting for differences in time-on-task. These observations may shed light on the laterality differences observed in other studies.
We speculate that RLPFC in each hemisphere may have privileged access to certain types of representations, due to a slight temporal lead in the inputs from ipsilateral versus contralateral cortex. Perhaps right RLPFC plays a more active role in processing visuospatial relations than left RLPFC, but left RLPFC assists with relational integration as needed. Conversely, perhaps left RLPFC plays a more active role in processing verbal or semantic relations, but right RLPFC assists as needed. Such an account might explain laterality effects observed in relational integration studies involving visuospatial relations (e.g. this relational matching task; Raven’s Progressive Matrices) and those involving verbal or semantic relations (e.g. propositional analogy tasks involving words or nameable objects). A technique with higher temporal resolution than fMRI is required to test this hypothesis.
These data provide strong evidence that left RLPFC processes higher-order relations between relations, rather than low-level relations between individual representations. Unlike other tasks used to probe RLPFC function, identical stimulus displays and type of judgment (decision regarding the presence or absence of a match) were used here for the relational integration and non-integration conditions, making this a stronger test of the integration hypothesis.
A challenge for studies examining RLPFC function is that the conditions that tend to engage RLPFC also tend to be the most difficult for participants to solve. There is evidence that RLPFC is engaged when participants are alerted to the fact that an upcoming task will be demanding (Dobbins & Han, 2006), and that this region exhibits sustained activation that varies as a function of difficulty of a block of trials (Velanova et al., 2003; Braver, Reynolds, & Donaldson, 2003). In the present study, as in the original RLPFC localizer task, the conditions were presented in blocks, as we sought to minimize RLPFC contributions to rule retrieval (Bunge et al., 2003). It is likely that participants perceived the Dimension blocks to be more difficult than the Shape and Texture blocks. However, mitigating concerns about a possible difficulty confound, we have found here that left RLPFC was no more active on Texture than Shape trials, despite the fact that the Texture condition was more difficult as measured by RTs. Also, we have previously shown in our propositional analogy paradigm that RLPFC activation was stronger for the easier conditions, which relied more heavily on relational integration than the more difficult conditions (Wendelken et al., 2008).
It has been argued that it is the ability to represent higher-order mental relations, investigated here, that sets humans apart from non-human primates (Penn, Holyoak, & Povinelli, 2008). It is certainly tempting to speculate that the emergence of RLPFC over evolution and over the course of individual human development made possible the complex mentation that humans are capable of (Bunge and Preuss, in press).
Supplementary Material
Supplementary Figure 1. A meta-analysis conducted by Gilbert and colleagues (Gilbert, Spengler, Simons, Steele et al., 2006) provided evidence for functional dissociations within rostral PFC. Their classification algorithm correctly determined based on the absolute x and y coordinates of activation in rostral PFC, for 92% of studies reviewed, whether the task that had yielded the activation involved episodic retrieval (blue), mentalizing (red) or multitasking (green). The results are plotted on an axial slice of a normalized T1-weighed image (z=0). The region shown in blue, referred to as RLPFC, is the focus of our research.
Supplementary Figure 2. Sample stimuli and group results from Christoff’s (Smith et al., 2007) RLPFC localizer task. A. On the Change task, participants had to infer the dimension of change between the top pair of objects (texture or shape) and then determine whether the bottom pair of objects changed along the same dimension. On the Feature tasks, participants had to determine whether the bottom object matched either one of the top two objects along the specified dimension (texture or shape). The correct answers to the examples shown here are (a) “no” and (b) “yes”. B. Group level analysis of the Change task versus the two Feature tasks (N=10). The contrast map shows the number of participants for whom a voxel was engaged by Change vs. Feature tasks at p < .05 uncorrected.
Acknowledgments
The authors thank Kalina Christoff and Ian Dobbins for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Participants viewed visual stimuli projected onto a mirror above their heads, and responded by pressing buttons on a response pad held in the right hand. Stimulus presentation and response acquisition were controlled by the Presentation software system (http://nbs.neuro-bs.com). Thirty-three 3.45mm axial slices (3mm plus .45mm gap) were collected for the fMRI scans, with the following specifications: TR=2000ms, TE=25ms, Field Of View=230mm, and 128×128 acquisition matrix.
Standard SPM5 procedures were used to preprocess and analyze the fMRI data (Wellcome Department of Cognitive Neurology, London).
References
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15(3):523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39(4):713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90(5):3419–28. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15(3):239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Preuss TM. Evolutionary and developmental issues in cognitive neuroscience. In: George Koob, Richard F., editors. Encyclopaedia of Behavioral Neuroscience. Thompson & Michel Le Moal; (in press) in press. [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan LPJ, McLeod P, editors. Measuring the Mind: Speed, Control, and Age. Oxford University Press; Oxford: 2005. pp. 217–248. [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Palmer SM, Gamberini M, Rosa MG. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol. 2006;495(2):149–172. doi: 10.1002/cne.20837. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2002;28(2):168–186. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14(5):1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117(6):1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl R, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Developmental Science. 2008 doi: 10.1111/j.1467-7687.2008.00743.x. Online version published Oct 17. DOI: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating Rule- versus Evidence-Based Prefrontal Activity during Episodic and Lexical Discrimination: a Functional Magnetic Resonance Imaging Investigation of Detection Theory Distinctions. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating rule- versus evidence-based prefrontal activity during episodic and lexical discrimination: a functional magnetic resonance imaging investigation of detection theory distinctions. Cereb Cortex. 2006;16(11):1614–1622. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain-behavior associations. Cereb Cortex. 2006;16(12):1783–1789. doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele D, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, et al. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb Cortex. 2001;11(6):558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399(6732):148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A. 2000;97(13):7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12(5):477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31(2):109–130. doi: 10.1017/S0140525X08003543. discussion 130-178. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K. Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage. 2007;36(4):1387–1396. doi: 10.1016/j.neuroimage.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. Dev Neurosci. 2005;27(5):277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Transient Neuronal Correlations Underlying Goal Selection and Maintenance in Prefrontal Cortex. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23(24):8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz J, Knowlton B, Holyoak K, Boone KB, Mishkin FS, Santos M, et al. A system for relational reasoning in human prefrontal cortex. Psychol Sci. 1999;10:119–125. [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20(4):682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wright SB, Matlen BJ, Baym CL, Ferrer E, Bunge SA. Neural correlates of fluid reasoning in children and adults. Frontiers in Human Neuroscience. 2007;1:1–8. doi: 10.3389/neuro.09.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A meta-analysis conducted by Gilbert and colleagues (Gilbert, Spengler, Simons, Steele et al., 2006) provided evidence for functional dissociations within rostral PFC. Their classification algorithm correctly determined based on the absolute x and y coordinates of activation in rostral PFC, for 92% of studies reviewed, whether the task that had yielded the activation involved episodic retrieval (blue), mentalizing (red) or multitasking (green). The results are plotted on an axial slice of a normalized T1-weighed image (z=0). The region shown in blue, referred to as RLPFC, is the focus of our research.
Supplementary Figure 2. Sample stimuli and group results from Christoff’s (Smith et al., 2007) RLPFC localizer task. A. On the Change task, participants had to infer the dimension of change between the top pair of objects (texture or shape) and then determine whether the bottom pair of objects changed along the same dimension. On the Feature tasks, participants had to determine whether the bottom object matched either one of the top two objects along the specified dimension (texture or shape). The correct answers to the examples shown here are (a) “no” and (b) “yes”. B. Group level analysis of the Change task versus the two Feature tasks (N=10). The contrast map shows the number of participants for whom a voxel was engaged by Change vs. Feature tasks at p < .05 uncorrected.


