Abstract
Passive air sampling was undertaken using polyurethane foam passive air samplers at three types of locations, including indoors (six offices) at buildings in the central business district (CBD) and at a private suburban home (indoor and outdoor) located 9 km from the CBD in Brisbane, Queensland, Australia. Estrogenic (E-SCREEN—MCF7-BOS) and aryl hydrocarbon receptor (AhR) (CAFLUX— H4G1.1c2) activity were assessed for samples collected from each of these locations. The samples were tested either as crude extracts (“untreated”) or were subjected to H2SO4 silica gel (“treated”) for each location in order to determine whether chemicals, which are not resistant to this treatment like polycyclic aromatic hydrocarbons, potentially account for the observed activity. In most cases, H2SO4 treatment resulted in a statistically significant reduction of potency for both endpoints, suggesting that chemicals less resistant to treatment may be responsible for much of the detected biological activity in these locations. Estrogenic potency measurements (<0.22–185 pg m−3) were highest in the indoor offices, followed by the indoor suburban home and finally the outdoor suburban home (which was not estrogenic). Total AhR activity for crude extracts (1.3–10 pg m−3) however was highest for the outdoor suburban home site. Levels of polycyclic aromatic hydrocarbons were monitored indoors and outdoors at the suburban home. At that location, polycyclic aromatic hydrocarbon air concentrations were on average approximately two times higher outdoor than indoor, while AhR potency was five times higher outdoor than indoor. No significant correlation was found between the estrogenic and AhR activity (P=0.88) for the sites in this study.
Keywords: Arylhydrocarbon receptor activity, Estrogenicity, Bioanalytical methods, Indoor air, Passive air sampling
Introduction
Indoor environments present potentially enriched, highly variable and potentially distinct sources of chemical exposure [1]. Emerging pollutants of concern in indoor environments include substances with endocrine disrupting effects such as alkylphenols, phthalates and brominated flame retardants [2]. Many of these chemicals have been found to be present at elevated levels in indoor environments with respect to outdoor environments [2–6]. The risk presented by these indoor exposures is potentially further increased by the estimated proportion of time spent in these environments (90% or more) [7] increasing the likelihood of inhalation of these complex mixtures [8].
Effective indoor air monitoring requires relatively non-intrusive monitoring strategies. Ideally, these strategies should provide cost-effective monitoring over multiple locations and offer a time-integrated assessment of mixture toxicity and identify potential effects due to unknown or not routinely monitored compounds to preempt long-term health impacts. Passive sampling addresses many of the requirements for inexpensive and non-intrusive sampling in these environments. While estimating exposure (ambient concentration) from individual chemicals accumulated in these samplers is important, more information may be obtained about the combined effect of exposure to chemical mixtures (including uncharacterised compounds) using effect-based bioanalytical monitoring methods. These methods may include monitoring of specific receptor-mediated activity, which may include endocrine disrupting activity such as that mediated by the estrogen receptor (ER) or aryl hydrocarbon receptor (AhR)-mediated activity.
The AhR and ER are both transcription factors for signalling pathways. The disruption or activation of these pathways by xenobiotics are related to a multitude of effects in vivo, including immunosuppression, carcinogenesis and reproductive or developmental abnormalities [9]. The metabolic activation of important carcinogenic semi-volatile organic chemicals (SVOCs) such as certain polycyclic aromatic hydrocarbons (PAHs) to a more DNA reactive form and subsequent potential carcinogenesis may occur as a result of the induction of cytochrome P450 genes mediated by the AhR [10, 11]. A role in the initiation or exacerbation of inflammatory disorders in vivo has also been suggested for this receptor [12]. Considering the potential endocrine disrupting nature of many of the pollutants being measured at elevated levels indoors [2], the potential for cross-talk [13, 14] between the ER and AhR activity and a role for these interactions and receptors in carcinogenesis [15] and toxicity [16] an initial assessment of co-activity in indoor air is warranted.
Effect-based monitoring of exposures sampled by passive air samplers has been utilised previously in ambient air for monitoring mutagenicity, genotoxicity, AhR activity and cytotoxicity [17–22]. These studies have typically expressed the potency of effect on a per sampler basis. Controlled laboratory studies assessing individual industrial chemicals accumulated in passive air samplers with cytotoxicity measures has also been reported [23]. A related field of study has assessed the teratogenicity [24] and AhR activity [25] of complex organic films, which form on outdoor windows in urban environments. The receptor-mediated co-activity for estrogenicity and AhR have been investigated using both the vapour and particulate phases in ambient air [26, 27], PM10 particulate matter [28] and relevant sources such as vehicular emissions [29] and tobacco smoke [30] previously. The dioxin-like AhR activity of indoor house and office dust extracts (H2SO4 silica gel treated) have been quantified and ranged from 38 to 1,400 pg g−1 [31]. To our knowledge, however, the co-activity of these specific endpoints has not been assessed with air samples from indoor environments.
A preliminary field study was undertaken in order to evaluate the application of these techniques in indoor environments using passive air samplers. The passive air samplers used were a type of polyurethane foam (PUF) [32]. These samplers have been used effectively in indoor air studies [33–35] and globally for the monitoring of a range of SVOCs in ambient air [36]. The aim of this study was to assess the applicability of combining passive sampling with bioanalytical methods to assess indoor air exposures using estrogenicity (ER agonist activity) and AhR agonist activity as biological endpoints.
The toxic effect of more metabolically stable SVOCs like certain halogenated aromatic hydrocarbons (including polychlorinated dibenzodioxins/dibenzofurans and dioxin-like polychlorinated biphenyls) are mediated by the AhR receptor. Other non-halogenated SVOCs like PAHs do not exhibit dioxin-like toxic effects as they are more readily metabolised [37], but still induce a measurable response. In this case, the use of a clean-up step [31, 38, 39] may allow total AhR activity, including induction by PAHs (in the untreated sample), to be distinguished from an estimate of dioxin-like activity (in the H2SO4 silica gel treated sample). The activity of crude (untreated) and treated extracts was compared for both AhR and ER activity. The proportion of total AhR response accounted for by compounds, which are not resistant to this treatment, could then be quantified. Limited chemical analysis for PAHs was undertaken at specific locations in the untreated whole extracts, and the relative PAH profiles obtained were compared with the estimated total AhR activity. The relationship between ER and AhR activity across all locations was evaluated for crude extracts.
Experimental methods—passive samplers
Passive samplers were deployed indoors in two inner city office buildings in the Brisbane central business district (CBD) and both indoors and outdoors at a suburban home located 9 km from the CBD as a reference location (Table 1). Samplers were deployed for 40 days in the indoor offices (April–May 2007) and for 50 days at the suburban home (June–August 2007). One of the inner city office buildings and the suburban home reference site in this study have previously been monitored for polybrominated diphenyl ethers (PBDEs) levels [40], and a concentration gradient with offices ≫ suburban home was demonstrated for these locations. Other site-specific factors included the presence of an intermittent woodsmoke source for domestic-heating purposes at the suburban home during the sampling period.
Table 1.
E-SCREEN-derived (estrogenicity) relative proliferative effect and estradiol equivalent air concentration (pg m−3) and CAFLUX-derived (AhR activity) TCDD equivalent air concentration (pg m−3) for indoor offices, indoor suburban and outdoor suburban sites
| Description | E-SCREEN—estrogenicity |
CAFLUX–AhR activity |
||||||
|---|---|---|---|---|---|---|---|---|
| RPEa |
E Bio-Eq 50 (pg m−3)b |
TCDD Bio-Eq 20 (pg m−3)c |
||||||
| Untreated | Treated | Untreated | Treated | Untreated | Treated | |||
| Inner City Offices | ||||||||
| Building 1 | ||||||||
| Level 14 Office 1 | 1.3 | Full agonist | 0.95 | Full agonist | 8.9±0.72 | 41±7.4 | 3.8±0.59 | 1.4±0.14 |
| Level 14 Office 2 | 1.0 | Full agonist | 1.1 | Full agonist | 185±4.0 | 26±0.76 | 2.3±0.69 | 0.91±0.067 |
| Level 17 Photocopy room | 1.1 | Full agonist | 0.57 | Partial agonist | 5.4±1.1 | 0.88±0.044 | 1.3±0.028 | 1.3±0.14 |
| Level 17 Office | 0.93 | Full agonist | 0.12 | Not estrogenic | 59±11 | <0.25 | 6.1±0.023 | 1.1±0.09 |
| Building 2 | ||||||||
| Level 23 Office 1 | 1.0 | Full agonist | 0.30 | Not estrogenic | 99±34 | <0.25 | 7.2±1.4 | 1.3±0.15 |
| Level 23 Office 2 | 1.2 | Full agonist | 1.1 | Full agonist | 18±1.1 | 2.3±0.13 | 5.9±0.83 | 1.4±0.12 |
| Suburban home | ||||||||
| Indoor | 0.53 | Partial agonist | 0.16 | Not estrogenic | 1.5±0.22 | <0.17 | 2.1±0.17 | <0.21 |
| Outdoor | 0.14 | Not estrogenic | 0.12 | Not estrogenic | <0.22 | <0.86 | 10±1.3 | 1.3±0.15 |
| Average relative standard deviation (%) | 15 | 7.9 | 13 | 9.7 | ||||
Untreated whole extract with no H2SO4 treatment, treated extracts subjected to H2SO4 silica gel
RPE is the average relative proliferative effect of sample with respect to reference hormone control 17 β-estradiol (Eq. 1) classified with respect to activity ranges
E Bio-Eq is the average (±standard deviation) estradiol equivalent air concentration (Eq. 2)
TCDD Bio-Eq is the average (±standard deviation) 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalent air concentration (Eq. 3)
Samplers were deployed in a two-disc configuration per sampling chamber [41] in order to increase the sampling rate for the period. Indoor and outdoor samplers were deployed in typical indoor (single inverted stainless steel bowl) and outdoor (“flying saucer” two bowl) deployment chamber configurations. Performance reference compounds (PRCs) [42–46], including polychlorinated biphenyls covering a range of volatilities (PCBs 30>21>204), were loaded into PUF, which were co-deployed with the independent PUF samplers intended for effect-based monitoring at the suburban home. Deployment periods were extended at this location (from 40 to 50 days) to enable sufficient elimination from the PUF of at least one PRC to qualify for predicting the sampling rate (i.e. loss >20% to minimise influence of analytical uncertainty [47]). PRCs were loaded (50, 50 and 25 ng PUF−1 for PCBs 21, 30 and 204, respectively) using 20 mL of hexane PUF−1 as a solvent carrier and gently dried under purified nitrogen for 10 min.
PUF samplers deployed for bioanalytical assessment contained no PRCs since their presence may influence these assessments. Both PRC loaded (for chemical analysis) and non-PRC loaded PUF (for bioanalytical assessment) were deployed in identical chambers and in the same configuration for either indoor or outdoor sampling. The elimination of PRCs from the PRC loaded PUF sampler within the deployment period was used to make an in situ estimate of the volume of air sampled V A (m3) for both indoor and outdoor exposures (refer to Electronic supplementary material for further details). This air volume was then used to estimate the equivalent volume of air EqV A (m3) dosed into the individual bioassays by correcting for the proportion of extract injected during gel permeation chromatography (GPC) and finally the proportion of total extract volume dosed. In addition to chemical analysis for PRCs, limited chemical analysis for priority pollutant PAHs was undertaken with the PRC-loaded samplers at the suburban home reference site indoors and outdoors. Ambient concentrations (ng m−3) were estimated from the ratio of the amount accumulated in the passive sampler (ng) and the total volume of air sampled (m3).
All PUF (Tisch Environmental TE-1014 certified “flame retardant free”) were pre-extracted prior to deployment with HPLC grade acetone and then hexane using accelerated solvent extraction (high pressure; 75 °C; 1×5 min static cycle; 60% flush volume; 250 s purge time) and extracted after deployment using hexane (two static cycles). Each sample was comprised of two PUF from a single chamber and was subjected to GPC and calibrated for a range of SVOCs, including PAHs, PCBs, polychlorinated dibenzodioxins/furans, nitrated PAHs and organochlorine pesticides. Endocrine-disrupting compounds like bisphenol A and alkylphenols will also elute within this fraction. All samples were split 1:1 with one fraction being subjected to concentrated H2SO4/silica gel treatment (66.6 g:100 g) for 24 h. All samples were then solvent exchanged to a final volume of 60 µL in dimethylsulphoxide (DMSO) for bioassay or 100 µL in hexane for chemical analysis of PAHs (GC-MS full scan: Shimadzu QP2010; ZB5MS). Deuterated internal standards (D 8-naphthalene, D 10-acenaphthene, D 10-phenanthrene, D 12-chrysene and D 12-perylene) were used for the quantification of priority pollutant PAHs. All chemical analysis was performed by Queensland Health Scientific Services.
Experimental methods—bioanalytical
The bioassays used in this study include the E-SCREEN and CAFLUX assays in order to assess agonistic ER- and AhR-mediated activity, respectively. Further details for the E-SCREEN and CAFLUX assay procedures are provided in the Electronic supplementary material.
E-SCREEN (estrogenicity)
The E-SCREEN assay was conducted using the MCF7-BOS human breast cancer cell line (courtesy of Prof. A. Soto, Boston University, USA). These cells will proliferate in the presence of estrogenic compounds [48, 49]. Samples were tested in triplicate using a nine-point one-in-four dilution series at a maximum of 0.5% DMSO (1 µL sample; 200 µL assay volume). An estimate of viable cell number was obtained after a 6-day exposure period by adding cell titer 96®AQueous One Solution containing MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphen yl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) and incubating for a further 2 h. Viable cells will reduce the MTS in solution to a coloured formazan product, and the absorbance at 490 nm may be used as a measure of viable cell number. Results were classified in terms of estrogenicity with respect to the reference hormone control 17β-estradiol. The reference control was tested in triplicate in a nine-point, one-in-four dilution series (54 to 0.00083 pg; test volume 200 µL). Relative proliferative effect (RPE), used to compare the relative efficacy of response, was calculated as the ratio of sample to reference hormone control response (Eq. 1). The yEC95 and yEC5 are the absorbance readings at 490 nm for the 95% and 5% effective concentrations determined from the respective sample and reference compound dose response curves.
| (1) |
A sample showed full agonistic activity when RPE >0.8 and partial agonistic activity when RPE 0.5–0.8 and was deemed not estrogenic when RPE <0.5.
The relative potency of samples was quantified as an estradiol equivalent air concentration (E Bio-Eq (pg m−3)) using the relative EC50 values of the reference hormone (pg) to the sample (m3; Eq. 2).
| (2) |
The detection limit for the assay (pg µL−1 or pg m−3) was determined as the ratio of reference hormone EC50 (pg) to the maximum volume of sample dosed (µL) or this volume converted to equivalent air volume dosed (m3).
CAFLUX (AhR activity)
The Chemically Activated FLUorescent gene eXpression (CAFLUX [50]) cell bioassay utilises a recombinant rat hepatoma cell line (H4G1.1c2) that contains a stably transfected AhR-responsive enhanced green fluorescent protein (EGFP) reporter gene plasmid (pGreen1.1 [51, 52]). EGFP activity (expressed as relative fluorescent units (RFUs)) was measured in a microplate fluorometer with an excitation wavelength of 485 nm, an emission wavelength of 520 nm and a gain of 1,500. Cells grown in black clear bottom 96 well microplates were dosed in a five-point, one-in-ten dilution series in triplicate from two independent dilution series at a maximum of 1% DMSO (1 µL sample; 100 µL culture media). RFU readings were taken after 24-h exposure.
Solvent blank corrected sample RFU values were converted to a percentage of maximum reference compound effect. The reference compound for AhR activity was 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD equivalent air concentrations (TCDD Bio-Eq (pg m−3)) were determined as the ratio of the EC20 for TCDD (pg) and the equivalent air volume of sample dosed (m3), which had the equivalent inducing effect to 20% of TCDD max, as interpolated from the sample dose response curves (Eq. 3).
| (3) |
Detection limits for this induction level were determined as the ratio of reference compound EC20 (pg) to the maximum volume of sample dosed (µL) or this sample volume converted to an equivalent air volume (m3). Replicate field blanks were assessed on all assays. If significant induction effects at the levels outlined above were produced by the field blanks, detection limits were adjusted to the average field blank level plus three standard deviations. Equivalent air concentration estimates were blank-corrected in this case. Dose response curves for all assays were assumed to have a hill slope of 1 and were fitted to a three parameter logistic equation using Graph Pad Prism 5.
Results
Sampling rates estimated based on the elimination of the PRC PCB 30 (2,4,6-trichlorobiphenyl) ranged from 1.3 m3 day−1 (indoors) to 4.1 m3 day−1 (outdoors) per PUF disc. Based on these sampling rates, the EqVA dosing rates for bioanalytical assessment for indoor offices, indoor suburban and outdoor suburban environments were estimated at 0.63, 0.79 and 2.4 m3 µL−1 of sample extract, respectively. Results for E-SCREEN (estrogenicity) as RPE and E Bio-Eq50 (pg m−3) and CAFLUX (AhR activity) as TCDD Bio-Eq20 (pg m−3) are provided in Table 1. These potency or relative efficacy (RPE only) measures are reported as either untreated or treated, representing response measures without and with H2SO4 silica gel treatment of the sample, respectively. Average relative standards deviations were <15% for the different potency measures. Sampler-based equivalent concentrations (ng PUF−1) and equivalent accumulation rates (pg PUF day−1) are provided in the Electronic supplementary material (Table S1) due to the potential uncertainties associated with the use of a single PRC based air volume to derive equivalent air concentrations applied to a complex mixture of chemicals.
E-SCREEN (estrogenicity)
RPE values of the untreated samples show that indoor office air exposures contain chemicals with full agonistic activity, while the suburban indoor and outdoor exposures show partial activity or were not estrogenic, respectively. Treatment with H2SO4 generally resulted in a decrease in estrogenic efficacy; however, full agonistic activity was maintained for certain sites (Level 14 office 1 and 2 Level 23 office 2).
E Bio-Eq results of the untreated samples ranged from <0.22 to 1.5 pg m−3 for the suburban site and from 5.4 to 185 pg m−3 at the indoor offices. The effect of H2SO4 treatment caused in most cases a significant reduction in potency (two-way ANOVA; Bonferroni post-testing; P< 0.001) or loss of significant estrogenic activity, with <0.17 to <0.86 pgm−3 at the suburban home and <0.25 to 41 pg m−3 at the indoor offices. However, the effect of treatment for Level 14 office 1 was an increase in potency from 8.9 to 41 pg m−3. This may indicate the removal of anti-estrogenic or antagonistic determinants of response with the H2SO4 treatment. The dose response curves for samples from Level 14 office 2, which demonstrated equivalent efficacy (from 1.0 to 1.1) but reduced potency (from 185 to 26 pg m−3) with H2SO4 treatment, are provided in the Electronic supplementary material (Figure S1). There were significant differences found between sites in terms of E Bio-Eq both for untreated and treated samples (one-way ANOVA; Tukey post-testing; P<0.001). These differences were found not only between the indoor suburban site and inner city offices over multi-levels/buildings but also between and within levels of the same building.
CAFLUX (AhR activity)
TCDD Bio-Eq air concentrations for the untreated samples ranged from 2.1 to 10 pg m−3 at the suburban home and from 1.3 to 7.2 pg m−3 at the indoor offices. After H2SO4 treatment, these ranged from <0.21 to 1.3 pg m−3 for the suburban home and from 0.91 to 1.4 pg m−3 at the indoor office sites. The dose response curves for the most potent site (outdoor suburban) with the wood smoke source are provided in the Electronic supplementary material (Figure S2).
The reduction in AhR activity with H2SO4 silica gel treatment averaged 75% and was statistically significant as a reduction in potency (two-way ANOVA; Bonferroni post-testing; P<0.05–0.001) or as a loss of activity at this induction level (<20% TCDDmax). The exceptions to this were for Level 14 office 2 (non-significant reduction with treatment) and the photocopy room where potency remained consistent at 1.3 pg m−3. In situ chemical oxidation may be occurring altering the chemical profile in favour of more stable chemicals since these types of office equipment can emit chemical-oxidising agents like ozone.
Multiple significant differences (one-way ANOVA; Tukey post-testing) were found between the different site types in terms of total AhR activity (P<0.01–0.0001), between different offices (P<0.01–0.001) and between indoor and outdoor suburban estimates (P<0.0001). Variation between sites declined with H2SO4 treatment with few significant differences in potency estimates.
Concentration of PAHs
Ambient PAH levels (ng m−3) for the suburban home reference site (indoor and outdoor) are provided in Table 2. All ambient concentrations were estimated from the levels of PAHs accumulated in the untreated proportion of extract from these locations. While splitting of the extracts for treatment has interfered with the detection of many priority pollutant PAHs, the outdoor/indoor ratio for those PAHs quantified indicates that the levels are on average a factor of 1.7 times higher outdoor than indoor at the suburban home site.
Table 2.
Ambient concentration estimates for polycyclic aromatic hydrocarbons (ng m−3) at the suburban home reference site
| Polycyclic aromatic hydrocarbons | Concentration (ng m−3) |
Ratio |
|
|---|---|---|---|
| Indoor | Outdoor | O/I | |
| Fluorene | <0.03 | 0.48 | |
| Phenanthrene | 1.2 | 1.8 | 1.5 |
| Fluoranthene | 0.45 | 0.68 | 1.5 |
| Pyrene | 0.25 | 0.42 | 1.7 |
| Benz[a]anthracene | <0.03 | 0.023 | |
| Chrysene | 0.030 | 0.069 | 2.3 |
| Benzo[b+k]fluoranthene | <0.03 | 0.028 | |
O outdoor ambient concentration estimate, I indoor ambient concentration estimate, benzo[b+k]fluoranthene benzo[b]fluoranthene and benzo[k]fluoranthene
Discussion
PCBs were chosen as a suitable class of reference chemicals for distinguishing between indoor and outdoor locations for complex mixture assessments due to the availability of calibration data for this class of chemicals in PUF [32]. PCB 30 (2,4,6-trichlorobiphenyl) was the only PRC with sufficient loss in both locations and was therefore used as the indicator PRC for determining sampling rates for both indoor (1.3 m3 day−1) and outdoor (4.1 m3 day−1) locations. These in situ PCB 30-based sampling rates show good agreement with the relative magnitude of indoor [35] and outdoor [53] sampling rates measured previously for SVOCs. In addition, the in situ PRC-derived outdoor estimate approximates the sampling rate range (3.5–4 m3 day−1) typically assumed in ambient monitoring studies for different classes of SVOCs, including PAHs [54–57]. The uptake of SVOCs is typically controlled by diffusion through the air side boundary layer [32, 58], and hence, sampling rates are relatively similar for many chemicals of interest. In this case, as a simplification, the approach used can be justified particularly as it allows us to attempt to account for the influence of the specific chambers [35] and the two-disc configuration used in this study.
The E-SCREEN assessment of passive air samples indicates that indoor air may potentially be a source of estrogenic activity. Whether exposure to indoor air in office buildings are a more significant source for these potential effects than indoor suburban homes requires further investigation across more sites as this initial study was limited to one indoor suburban reference site. Our finding of non-significant estrogenicity for the single outdoor air exposure site is consistent with more comprehensive outdoor seasonal monitoring across Australia using these techniques (Kennedy, unpublished data). Notably, a separate study sampling at different sites (indoor suburban, indoor offices and outdoor sites) in Australia using conventional active sampling systems (filter + sorbent) found higher estrogenicity (E-SCREEN MCF7) in indoor offices than outdoor air, with activity concentrated in the vapour phase in each case [59].
Estrogenic activity (human ovarian carcinoma BG1Luc4E2) has previously been reported for active air samples from an urban and rural location in Canada in both summer and winter seasons. This study found that induction was typically higher within the vapour phase than the particulate phase for each sample with volumes of air necessary to induce 50% of the maximal estradiol response ranging from 1.26 to 12.50 m3 [26]. By comparison, in this indoor air study, EC50 was achieved with air volumes ranging from 0.001 to 0.03 m3 for samples which showed full estrogenic activity (RPE > 0.8; indoor offices; untreated), which are several orders of magnitude lower. It is unlikely that these potency differences are due to uncertainties associated with the estimated volume of air sampled in our study as ambient concentration estimates made with active and passive sampling are typically within a factor of two to three [54, 56, 57]. Differences in potency may also arise through the assessment of both the vapour and particulate phases combined with passive sampling but also through differing sensitivities in the different bioanalytical methods (cell lines) used to assess estrogenicity in these studies.
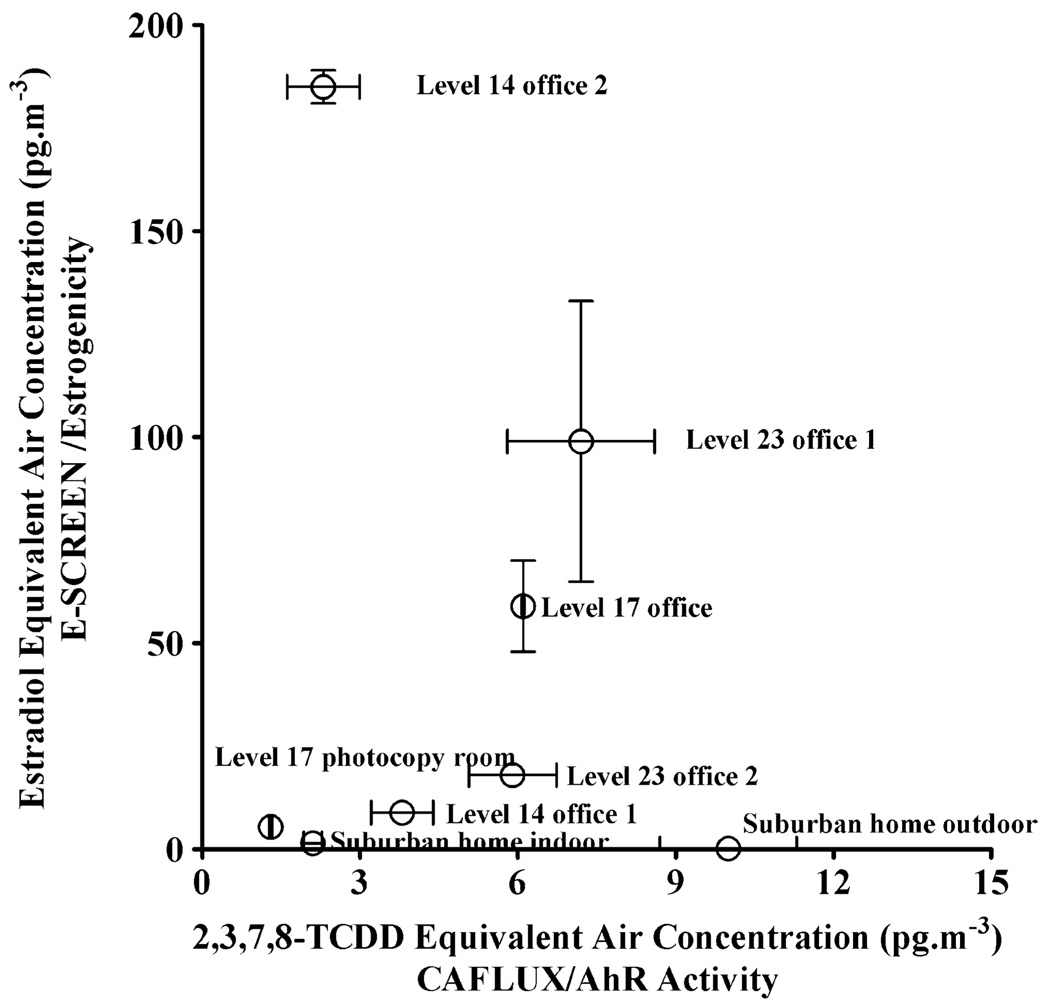
While typically activity is found to be higher in the vapour than particulate phases, estradiol equivalent air concentrations of 5 to 23 pg m−3 (MCF7) have been reported for fractionated PM10 extracts from an urban location in Canada [28]. Although only partial agonistic activity was observed, the activity of crude extracts was accounted for by non-polar fractions in this case. However, a recent study [27] of different regions within the Czech Republic has found anti-estrogenic but not estrogenic activity (human breast carcinoma MVLN) in both the vapour and particulate phases of ambient air except at a background site (no anti-/estrogenic activity). Interestingly, simultaneous measurements of AhR activity in that study found that the greater AhR activity observed in one of these regions was coincidental with greater anti-estrogenicity in both phases. In our study, the single outdoor site which had the highest AhR activity (10 pg m−3) showed no significant estrogenicity (<0.22 pg m−3). However, anti-estrogenicity assessments were not made in this case and should be considered for future assessments of these effects. Interestingly, the indoor air location with the highest AhR potency (Level 23 office 1; TCDD Bio-Eq20=7.2 pg m−3) was not significantly different to the AhR potency of the outdoor site, but showed full estrogenic activity (RPE=1.0) with an E Bio-Eq50 of 99 pg m−3. Furthermore, no significant correlation was found between untreated whole extract AhR and ER activity (Spearman rank correlation r=0.071; P= 0.88) as illustrated in Fig. 1. The relationship remains not significant when the outdoor site, which was not estrogenic (limit of detection of 0.22 pg m−3), is excluded from the correlation (r=0.61; P=0.17).
Fig. 1.
Plot of the estradiol equivalent air concentrations (E Bio-Eq (pg m−3)) versus the total TCDD equivalent air concentration (TCDD Bio-Eq (pg m−3)) for the locations in this study
It is important to note that PAHs can influence anti-estrogenicity/estrogenicity in MCF-7 cells through AhR-dependent gene expression [60, 61]. Interpretation will always be complicated by the fact that chemicals from the same class, including PAHs, may produce both anti-estrogenicity and estrogenicity through distinct mechanisms [28]. Many PBDEs for example have been assessed systematically through in vitro profiling for a range of endocrine disrupting effects and may be both agonists and antagonist for the ER [62] and are antagonistic for the AhR [63].
Despite the relatively high levels of PBDEs in these inner city office buildings, it is unlikely that the PBDEs quantified are accounting for the observed estrogenicity (weakly agonistic). The dominant congener determined previously at building 1 was BDE-47 with a concentration of (358 pg m−3) [40]. This concentration may be converted to an estradiol equivalent air concentration using a relative potency estimate for this congener (0.35×10−6) [62]. The equivalent concentration derived is approximately 0.13 fg m−3, which is several orders of magnitude lower than the E-SCREEN potency estimates determined at this location. In addition, PBDEs would be resistant to H2SO4 treatment, and in most cases, estrogenicity was reduced with treatment, suggesting the importance of other compounds for the observed effects. More comprehensive chemical analysis in combination with relative potency estimates for individual known xenoestrogens (bisphenol A, as well as certain phthalates, alkylphenols, pyrethroid and organochlorine pesticides [49, 64, 65] for example) would be required to determine the proportion of response potentially attributable to these compounds.
For most locations sampled, a significant proportion of the total AhR activity (average 75% of maximal response) observed was accounted for by chemicals not resistant to H2SO4 silica gel treatment. This result is consistent with previous findings [28, 66, 67] that most of the observed “total” AhR activity in air samples is unlikely to be determined by the more persistent halogenated aromatic hydrocarbons. In our study, the low air volumes sampled may also contribute to this observation since potent agonists like the polychlorinated dibenzodioxins are present in air at relatively low levels (i.e. fg m−3) [68].
Compounds which may account for a significant proportion of this untreated total AhR activity are polycyclic aromatic hydrocarbons, although the demonstration that the AhR can bind and be activated by structurally distinct chemicals [37] suggests that other chemicals can be involved. Environmental tobacco smoke (ETS) is typically a significant source for combustion by-products indoors; however, smoking has been banned in public buildings and within 4 m of building entrances in Queensland, Australia since January 2005 and 2006, respectively. In outdoor environments, vehicular emissions or woodsmoke are potential sources for AhR activity [69, 70]. Congested city streets external to the inner city office sites may be contributing to the observed effects, depending on the location of air intakes, treatment of incoming air and timing and volume of ventilation rates and infiltration effects. Spearman rank correlations for indoor/outdoor PAH levels and indoor/outdoor ratios suggest outdoor levels contribute significantly to measured indoor levels [5] in the absence of other combustion sources indoors like ETS.
PAH air concentration estimates at the suburban home (Table 2) were on average 1.7 times higher outdoor than indoor, while total AhR activity (untreated) was five times higher outdoor than indoor at that same location. AhR activity assessed is a function of all contributors (and their interaction) to the observed effect rather than only those compounds, which we could detect at both locations. Several AhR agonists such as benzo[b]fluoranthene, benzo[k]fluoranthene and benz[a]anthracene were only detected in the outdoor sample. Several of the higher molecular weight USEPA priority pollutant PAHs are IARC human carcinogens (i.e. benzo[a]pyrene) or probable/possible human carcinogens [71] and have also been identified as agonists for the AhR (i.e. benzo[k]fluoranthene), benzo[a]pyrene, indeno[1,2,3-c,d]pyrene and dibenzo[a,h]anthracene [72, 73]. AhR activity is one possible biological response, which may be used as a marker for these types of compounds in exposure assessments. What is apparent in this study is that while chemical analysis was unable to detect all of these AhR active higher molecular weight PAHs as they are typically less abundant in air, we have observed detectable AhR activity using the CAFLUX assay.
Many of these priority pollutant PAHs identified as AhR agonists can be predominantly particle-bound in the more respirable size ranges in air [74]. The AhR activity of ambient PM10 [28, 67, 70, 75], total suspended particulate matter [66], and vapour and particulate phases [26, 67] and vapour plus particulate phases [76] have been previously assessed. Where vapour and particulate phases of ambient air have been assessed simultaneously, there is typically more activity detected in the particulate phase than in the vapour phase [26, 27, 67] although this may not be the case for all locations [26, 27].
The samplers used in this study (PUF) may have lower sampling rates for particle-bound contaminants than vapour phase contaminants [34, 77]. If AhR activity is concentrated within the particulate phase for these locations, this may result in an underestimation of the potential AhR activity. The TCDD Bio-Eq levels reported in this study (1.3–10 pg m−3) are consistent with but relatively low compared with levels found in the Czech Republic (70–130 pg m−3 [67] and 25– 86 pg m−3 [76] for vapour plus particulate phase samples) or for urban PM10 in Toronto, Canada (5–370 pg m−3 [28]). Lower sampling rates for particle-bound compounds in the PUF, lower PAH or particulate loadings and lower levels in indoor air, with respect to ambient air and the different bioanalytical methods used, may all be contributing factors to these differences.
Conclusion
No significant relationship was found between the co-activity of ER and AhR activity at these locations. Interestingly, in light of recent studies suggesting that endocrine disrupting chemicals may be present at relatively high levels in indoor air, we have identified estrogenicity associated with indoor air exposures sampled by PUF-based passive air samplers. A significant proportion of both estrogenicity and aryl hydrocarbon receptor activity may be associated with chemicals, which are not resistant to H2SO4 silica gel treatment, which provides further information for the prioritisation of further chemical analysis in these environments. Given recent studies indicating the potential for higher estrogenicity in the vapour phase and higher AhR in the particulate phases of air as well as the potential for interactions between these receptor systems, it may be important to assess these phases both separately and in combination. Passive air samplers can sample both phases of ambient air but there may be some discrimination introduced through lower sampling rates for predominantly particulate-bound compounds. The influence of this on potency estimates requires further study. This study has used an individual performance reference compound to derive the volume of air sampled and express results as equivalent air concentrations. This approach allows for greater comparability between studies (compared with sampler based estimates) where exposure times or sampling rates vary. Considerable improvement in these estimates would be made by the inclusion of more PRCs from more compound classes to better represent complex mixture exposures in the future.
Supplementary Material
Acknowledgements
EnTox is a partnership between Queensland Health and the University of Queensland. This work is funded by an ARC Linkage Grant (LP0560619) with industry partner support from Queensland Health Scientific Services, Queensland Environmental Protection Agency, South Australia Department of Health, Western Australia Department of Environment and Conservation, Australian Plague Locust Commission, National Measurement Institute, ERGO Forschungsgesellschaft, United Kingdom Environment Agency. The H4G1.1c2 cell line was developed through a grant from the United States National Institute of Environmental Health Sciences (ES04699).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-009-2825-6) contains supplementary material, which is available to authorized users.
Contributor Information
Karen Kennedy, Email: k.kennedy@uq.edu.au, EnTox (The National Research Centre for Environmental Toxicology), The University of Queensland, Brisbane, QLD 4108, Australia.
Miroslava Macova, EnTox (The National Research Centre for Environmental Toxicology), The University of Queensland, Brisbane, QLD 4108, Australia.
Frederic Leusch, Smart Water Research Facility, Griffith University, Gold Coast, QLD 4222, Australia.
Michael E. Bartkow, EnTox (The National Research Centre for Environmental Toxicology), The University of Queensland, Brisbane, QLD 4108, Australia
Darryl W. Hawker, School of Environment, Griffith University, Nathan, QLD 4111, Australia
Bin Zhao, Department of Environmental Toxicology, University of California, Davis, CA 95616, USA.
Michael S. Denison, Department of Environmental Toxicology, University of California, Davis, CA 95616, USA
Jochen F. Mueller, EnTox (The National Research Centre for Environmental Toxicology), The University of Queensland, Brisbane, QLD 4108, Australia
References
- 1.Weschler CJ. Atmos Environ. 2009;43:153–169. [Google Scholar]
- 2.Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Environ Sci Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- 3.Shoeib M, Harner T, Ikonomou M, Kannan K. Environ Sci Technol. 2004;38:1313–1320. doi: 10.1021/es0305555. [DOI] [PubMed] [Google Scholar]
- 4.Saito I, Onuki A, Seto H. Indoor Air. 2004;14:325–332. doi: 10.1111/j.1600-0668.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon L, Clayton A, Keever J, Perrit R, Whitaker D. Monitoring of phthalates and PAHs in indoor and outdoor air samples in Riverside California. Volume 2. Final Report. Durham, NC: California Environmental Protection Agency. Air Resources Board; 1992. [Google Scholar]
- 6.Rudel RA, Perovich LJ. Atmos Environ. 2009;43:170–181. doi: 10.1016/j.atmosenv.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Environment Australia. Commonwealth Government; State of Knowledge Report: Air Toxics and Indoor Air Quality in Australia. 2001
- 8.Nazaroff WW, Weschler GJ, Corsi RL. Atmos Environ. 2003;37:5451–5453. [Google Scholar]
- 9.Janošek J, Hilscherová K, Bláha L, Haloubek I. Toxicol in Vitro. 2006;20:18–37. doi: 10.1016/j.tiv.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Ide F, Kishi R, Akutagawa T, Sakai S, Nakamura M, Ishikawa T, Fujii-Kuriyama Y, Nakatsuru Y. Environ Sci Technol. 2007;41:3775–3780. doi: 10.1021/es062793g. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Nakatsura Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Proc Natl Acad Sci U S A. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauchi M, Hida A, Negishi T, Katsuoko F, Noda S, Mimura J, Hosoyo T, Yanaka A, Aburatani H, Fujii-Kuriyama Y, Motohashi H, Yamamoto M. Mol Cell Biol. 2005;25:9360–9368. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Smith R, Safe S. Arch Biochem Biophys. 1998;356:239–248. doi: 10.1006/abbi.1998.0782. [DOI] [PubMed] [Google Scholar]
- 14.Safe S, Wormke M. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 15.Safe S. Toxicol Lett. 2001;120:1–7. doi: 10.1016/s0378-4274(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 16.Safe S. Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- 17.Čupr P, Klánová J, Bartos T, Flegrováš Z, Kohoutek J, Holoubek I. Environ Pollut. 2006;144:406–413. doi: 10.1016/j.envpol.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 18.Isidori M, Ferrara M, Lavorgna M, Nardelli A, Parrella A. Chemosphere. 2003;52:121–126. doi: 10.1016/S0045-6535(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy K, Tang J, Bartkow ME, Denison M, Mueller JF. Organohalogen Compd. 2007;69:812–816. [Google Scholar]
- 20.Kennedy K, Tang JM, Bartkow ME, Hawker D, Macova M, Thier R, Mueller JF. Assessing toxic potency of ambient air using bioanalytical methods: a case study using passive air sampling. In: Doley D, editor. 14th International Union of Air Pollution Prevention and Environmental Protection Associations (IUAPPA) World Congress 2007, incorporating 18th Clean Air Society of Australia and New Zealand (CASANZ) Conference; Brisbane Australia: 2007. [Google Scholar]
- 21.Slapsyte G, Lastauskiene E, Mierauskiene J. Biologija. 2006;1:41–46. [Google Scholar]
- 22.Bonetta S, Carraro E, Bonetta S, Pignata C, Pavan I, Romano C, Gilli G. Chemosphere. 2009 doi: 10.1016/j.chemosphere.2009.02.039. doi:10.1016/j.chemosphere.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Rogers KR, Harper SL, Robertson G. Anal Chim Acta. 2005;543:229–235. [Google Scholar]
- 24.Diamond ML, Gingrich SE, Fertuck K, McCarry BE, Stern GA, Billeck B, Grift B, Brooker D, Yager TD. Environ Sci Technol. 2000;34:2900–2908. [Google Scholar]
- 25.Hodge EM, Diamond ML, McCarry BE, Stern GA, Harper PA. Arch Environ Contam Toxicol. 2003;44:421–429. doi: 10.1007/s00244-002-1272-6. [DOI] [PubMed] [Google Scholar]
- 26.Klein GP, Hodge EM, Diamond ML, Yip A, Dann T, Stern G, Denison M, Harper PA. Environ Health Perspect. 2006;114:697–703. doi: 10.1289/ehp.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novák J, Jálová V, Giesy JP, Hilscherová K. Environ Int. 2009;35:43–49. doi: 10.1016/j.envint.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Clemons JH, Allan LM, Marvin CH, Wu Z, McCarry BE, Bryant DW, Zacharewski TR. Environ Sci Technol. 1998;32:1853–1860. [Google Scholar]
- 29.Meek MD. Environ Res (Section A) 1998;79:114–121. doi: 10.1006/enrs.1998.3870. [DOI] [PubMed] [Google Scholar]
- 30.Meek MD, Finch GL. Environ Res. 1999;80:9–17. doi: 10.1006/enrs.1998.3872. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Takigami H, Nose K, Takahashi S, Asari M, Sakai S-I. Environ Sci Technol. 2007;41:1487–1493. doi: 10.1021/es061907l. [DOI] [PubMed] [Google Scholar]
- 32.Shoeib M, Harner T. Environ Sci Technol. 2002;36:4142–4151. doi: 10.1021/es020635t. [DOI] [PubMed] [Google Scholar]
- 33.Wilford BH, Harner T, Zhu J, Shoeib M, Jones KC. Environ Sci Technol. 2004;38:5312–5318. doi: 10.1021/es049260x. [DOI] [PubMed] [Google Scholar]
- 34.Harrad S, Abdallah MA-E. J Environ Monit. 2008;10:527–531. doi: 10.1039/b719638e. [DOI] [PubMed] [Google Scholar]
- 35.Hazrati S, Harrad S. Chemosphere. 2007;67:448–455. doi: 10.1016/j.chemosphere.2006.09.091. [DOI] [PubMed] [Google Scholar]
- 36.Pozo K, Harner T, Wania F, Muir DCG, Jones KC, Leonard A. Environ Sci Technol. 2006;40:4867–4873. doi: 10.1021/es060447t. [DOI] [PubMed] [Google Scholar]
- 37.Denison MS, Heath-Pagliuso S. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 38.Brown DJ, Van Overmeire I, Goeyens L, Chu MD, Denison MS, Clark GC. Organohalogen Compd. 2002;58:401–404. [Google Scholar]
- 39.Van Wouwe N, Windal I, Vanderperren H, Eppe G, Xhrouet C, De Pauw E, Goeyens L, Baeyens W. Talanta. 2004;63:1269–1272. doi: 10.1016/j.talanta.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Toms L-M, Mueller JF, Bartkow M, Symons RK. Australian Government Department of the Environment and Heritage; Assessment of concentrations of polybrominated diphenyl ether flame retardants in indoor environments in Australia. 2006
- 41.Thompson J, Kennedy K, Hawker DW, Mueller JF, Bartkow ME. Organohalogen Compd. 2007;69:1034–1037. [Google Scholar]
- 42.Gouin T, Harner T, Blanchard P, Mackay D. Environ Sci Technol. 2005;39:9115–9122. doi: 10.1021/es051397f. [DOI] [PubMed] [Google Scholar]
- 43.Gouin T, Wania F, Ruepert C, Castillo LE. Environ Sci Technol. 2008;42:6625–6630. doi: 10.1021/es8008425. [DOI] [PubMed] [Google Scholar]
- 44.Pozo K, Harner T, Shoieb M, Urrutia R, Barra R, Parra O, Focardi S. Environ Sci Technol. 2004;38:6529–6537. doi: 10.1021/es049065i. [DOI] [PubMed] [Google Scholar]
- 45.Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alvarez DA, Cranor WL, Clarck RC, Mogensen BB. Environ Sci Technol. 2002;36:85–91. doi: 10.1021/es010991w. [DOI] [PubMed] [Google Scholar]
- 46.Moeckel C, Harner T, Nizzetto L, Strandberg B, Lindroth A, Jones KC. Environ Sci Technol. 2009;43:3227–3232. doi: 10.1021/es802897x. doi:10.1021/es802897x. [DOI] [PubMed] [Google Scholar]
- 47.Huckins JN, Petty JD, Prest HF, Clarck RC, Alvarez DA, Orazio CE, Lebo JA, Cranor WL, Johnson BT. A Guide for the Use of Semipermeable Membrane Devices (SPMDs) as Samplers of Waterborne Hydrophobic Organic Contaminants (Draft) Columbia Environmental Research Center, U.S.G.S. California Analytical Division Agilent Technologies Inc; 2002. [Google Scholar]
- 48.Soto AM, Sonnenschein C. J Steroid Biochem. 1985;23:87–94. doi: 10.1016/0022-4731(85)90265-1. [DOI] [PubMed] [Google Scholar]
- 49.Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. Environ Health Perspect. 2005;103 Supplements:113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aarts JMMJG, Jonas A, Van den Dikkenberg LL, Brouwer A. Organohalogen Compd. 1998;37:85–88. [Google Scholar]
- 51.Zhao B, Denison M. Organohalogen Compd. 2004;66:3332–3336. [Google Scholar]
- 52.Nagy SR, Sanborn JR, Hammock BD, Denison MS. Toxicol Sci. 2002;65:200–210. doi: 10.1093/toxsci/65.2.200. [DOI] [PubMed] [Google Scholar]
- 53.Chaemfa C, Barber JL, Gocht T, Harner T, Holoubek I, Klanova J, Jones KC. Environ Pollut. 2008;156:1290–1297. doi: 10.1016/j.envpol.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Gouin T, Harner T, Daly GL, Wania F, Mackay D, Jones KC. Atmos Environ. 2005;39:151–166. [Google Scholar]
- 55.Harner T, Shoeib M, Diamond ML, Ikonomou MG, Stern G. Chemosphere. 2006;64:262–267. doi: 10.1016/j.chemosphere.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Environ Toxicol Chem. 2004;23:1355–1364. doi: 10.1897/03-420. [DOI] [PubMed] [Google Scholar]
- 57.Klánová J, Kohoutek J, Hamplová L, Urbanová P, Holoubek I. Environ Pollut. 2006;144:393–405. doi: 10.1016/j.envpol.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 58.Bartkow M, Booij K, Kennedy KE, Müller J, Hawker D. Chemosphere. 2005;60:170–176. doi: 10.1016/j.chemosphere.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 59.Bartkow M, Macova M, Tang J, Kennedy K, Mueller JF. Final Report to the Department of the Environment, Water, Heritage and Arts. National Research Centre for Environmental Toxicology. The University of Queensland; 2008. Towards the Integration of Bioanalytical Tools into Air Pollution Assessment, Regulation and Management. Clean Air Research Programme. [Google Scholar]
- 60.Chaloupka K, Krishnan V, Safe S. Carcinogenesis. 1992;13:2233–2239. doi: 10.1093/carcin/13.12.2233. [DOI] [PubMed] [Google Scholar]
- 61.Gozgit JM, Nestor KM, Fasco MJ, Pentecost BT, Arcaro KF. Toxicol Appl Pharmacol. 2004;196:58–67. doi: 10.1016/j.taap.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 63.Peters AK, Nijmeijer S, Gradin K, Backlund M, Bergman A, Poellinger L, Denison MS, Van den Berg M. Toxicol Sci. 2006;92:133–142. doi: 10.1093/toxsci/kfj186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Go V, Garey J, Wolff MS, Pogo GT. Environ Health Perspect. 1999;107:173–177. doi: 10.1289/ehp.99107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumoto H, Adachi S, Suzuki Y. Arch Environ Contam Toxicol. 2005;48:459–466. doi: 10.1007/s00244-003-0243-x. [DOI] [PubMed] [Google Scholar]
- 66.Hamers T, van Schaardenburg MD, Felzel EC, Murk AJ, Koeman JH. Sci Total Environ. 2000;262:159–174. doi: 10.1016/s0048-9697(00)00600-8. [DOI] [PubMed] [Google Scholar]
- 67.Ciganek M, Neca J, Adamec V, Janosek J, Machala M. Sci Total Environ. 2004;334–335:141–148. doi: 10.1016/j.scitotenv.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 68.Gras J, Mueller JF. Dioxins in ambient air in Australia-Technical report No. 4. Australia: Department of Environment and Heritage; 2004. [Google Scholar]
- 69.Mason GGF. Environ Health Perspect. 1994;102 Supplement 4:111–116. doi: 10.1289/ehp.94102s4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arrieta DE, Ontiveros CC, Li W-W, Garcia JH, Denison MS, McDonald JD, Burchiel SW, Washburn BS. Environ Health Perspect. 2003;111:1299–1305. doi: 10.1289/ehp.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.IARC. Agents reviewed by the IARC Monographs Volumes 1–99. International Agency for Research on Cancer. 2008 Updated 12th May 2008. [Google Scholar]
- 72.Behnisch PA, Hosoe K, Sakai S-i. Environ Int. 2003;29:861–877. doi: 10.1016/s0160-4120(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 73.Machala M, Vondracek J, Blaha L, Ciganek M, Neca J. Mutat Res. 2001;497:49–62. doi: 10.1016/s1383-5718(01)00240-6. [DOI] [PubMed] [Google Scholar]
- 74.Kaupp H, McLachlan M. Atmos Environ. 2000;34:73–83. [Google Scholar]
- 75.Brown LE, Trought KR, Bailey CI, Clemons JH. Sci Total Environ. 2005;349:161–174. doi: 10.1016/j.scitotenv.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Skarek M, Janosek J, Cupr P, Kohoutek J, Novotna-Rychetska A, Holoubek I. Environ Int. 2007;33:859–866. doi: 10.1016/j.envint.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Klánová J, Èupr P, Kohoutek J, Harner T. Environ Sci Technol. 2008;42:550–555. doi: 10.1021/es072098o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.