Abstract
The calcium sensing receptor (CaSR) is a Family 3/C G protein-coupled receptor with slow and partial targeting to the plasma membrane in both native and heterologous cells. We identified cargo receptor family member p24A in yeast two-hybrid screens with the CaSR carboxyl terminus. Interactions were confirmed by immunoprecipitation of either p24A or CaSR in transiently transfected HEK293 cells. Only the immaturely glycosylated form of CaSR interacts with p24A. Dissociation likely occurs in the endoplasmic reticulum Golgi intermediate compartment (ERGIC) or cis-Golgi, since only the uncleaved form of a CaSR mutant sensitive to the trans-Golgi enzyme furin was coimmunoprecipitated with p24A. p24A and p24A(ΔGOLD) significantly increased total and plasma membrane CaSR protein but p24A(FF/AA) did not. The CaSR carboxyl terminus distal to T868 is required for differential sensitivity to p24A and its mutants. Interaction with p24A therefore increases CaSR stability in the ER and enhances plasma membrane targeting. Neither wt Sar1p or the T39N mutant increased CaSR maturation or abundance while the H79G mutant increased abundance but prevented maturation of CaSR. These results suggest that p24A is the limiting factor in CaSR trafficking in the early secretory pathway, and that cycling between the ER and ERGIC protects CaSR from degradation.
Keywords: calcium sensing receptor, membrane protein maturation, p24A, Sar1p GTPase, endoprotease cleavage
INTRODUCTION
The calcium sensing receptor (CaSR) is in Family C/3 of the G protein-coupled receptor superfamily [1]. Mutations in CaSR cause human diseases of calcium handling. Loss-of-function mutations increase the EC50 for activation by extracellular Ca2+, causing FHH (familial hypercalciuric hypercalcemia) and NSHPT (neonatal severe primary hyperparathyroidism) (reviewed in [1]). We [2,3] and others [4,5] have recently shown that some loss-of-function mutations cause intracellular retention of receptors, reducing CaSR-mediated signaling. Significant levels of intracellular CaSR are observed in both native cells and heterologous expression systems [6–9], suggesting that maturation of CaSR through the secretory pathway may be subject to regulation.
Although our understanding of the determinants of GPCR trafficking is incomplete, recent efforts have identified carboxyl terminal motifs which participate either in active ER export or retention/retrieval of nascent GPCRs [reviewed in 10]. GPCR exit from the ER requires incorporation into COPII vesicles, initiated by formation of a complex containing Sec23/24, and the small G protein Sar1p at specific ER exit sites. Activation of Sar1p by membrane-resident guanine nucleotide exchange factors initiates complex formation and budding while hydrolysis is required for release of Sar1p from budded vesicles, a prerequisite for fusion with Golgi membranes [reviewed in 11].
The mechanisms targeting GPCRs to ER exit sites for incorporation into transport vesicles have not been well defined. Some GPCRs may contain primary sequence determinants which initiate vesicle assembly, e.g., the carboxyl terminus of the D1 dopamine receptor interacts directly with γ-COP [12]. Other GPCRs may require interaction with cargo receptors for such targeting. The p24 family contains multiple type I transmembrane proteins of ≈24 kDa which interact with both cargo and COPI or COPII coatomers in the early secretory pathway [reviewed in 13]. Our understanding of p24 family functions is incomplete, although it is clear that members of the family form homo- and hetero-oligomers, which are a prerequisite for incorporation into transport vesicles [14]. Most members are ubiquitiously expressed and populate the early compartments of the secretory pathway in a subtype-biased manner [13,14]. The short carboxyl termini of p24 family members contain sequences which interact with γ-COP [15], as well as retrieval signals to permit cycling between adjacent secretory compartments [15]. Knockdown of individual subtypes with RNA interference and/or exogenous expression affects the stability of other family members, making identification of the unique role(s) of individual subtypes difficult, although this has not been observed for p24A (TEMED2, p24β1) [reviewed in 13]. Interaction of p24A was recently shown to contribute to resensitization of the protease-activated receptor-2 (PAR-2) [16]. The specificity of PAR-2 interaction with p24A was focused on the GOLD (Golgi dynamics) domain [17] which differs among p24 family members. ARF-1 activation releases PAR-2 from p24A to increase plasma membrane targeting [16]. In this report, we examine the role of p24A in CaSR maturation through the secretory pathway, and define a distinctly different role for p24A in protecting CaSR from ER associated degradation. Such divergent roles for p24A in maturation of distinct GPCRs suggests that the p24 family of cargo receptors may represent a significant contributor to the variation in the rates and extents of GPCR maturation through the secretory pathway.
MATERIALS AND METHODS
Materials
Human CaSR was from Dr. K. Seuwen, and Sar1p constructs from Dr. P. Wedegaertner. Human p24A cDNA was purchased from OriGene (#SC115869).
Plasmid construction
Human CaSR with an amino terminal FLAG epitope [18] was the template for all constructs. The furin cleavage site (ARRRKKRGLDV) was inserted between human CaSR amino acids D371 and T372 as described [3]. The truncation CaSRΔ868 (stop codon after T868) was generated by PCR. p24A was subcloned into pcDNA3.1myc-His vector (tag at carboxyl terminus) and used as template for mutants. p24A(ΔGOLD), generated by PCR mutagenesis, contained and internal deletion of residues 21-112 (numbering from the initiation methionine). p24A(FFAA) mutant (F194A/F195A) was generated by PCR. All constructs were verified by sequencing (GeneWiz, Inc.).
Cell transfection
HEK293 cells (ATCC), were cultured as per supplier recommendations in MEM plus 10% heat-inactivated fetal bovine serum, penicillin/streptomycin at 37 °C, 5% CO2, and transiently transfected with FuGENE HD (Roche) or Novafector (Venn Nova, Inc.) according to manufacturer’s protocols and cultured for indicated times prior to experiments.
Immunoprecipitation, SDS-PAGE, and Western blotting
Transfected HEK293 cells were lysed and proteins immunoprecipitated with M2 anti-FLAG antibody (Sigma) or anti-myc antibody (Invitrogen R950-25), resolved by SDS-PAGE on either 4–15% or 7.5% gels (Criterion, BioRad), transferred to nitrocellulose membranes, and probed with anti-CaSR polyclonal antibodies (LRG, 1:2000, Genemed Synthesis, Inc.) or anti-myc HRP-conjugated antibody (1:2000, Invitrogen R951-25), and visualized by enhanced chemiluminescence (SuperWest Pico Chemiluminescent substrate, Pierce).
Enzyme-linked immunoabsorbance assay
FLAG-CaSR was transfected as described in 6-well plates, and transferred to poly-lysine-coated 96 well plates (BD Biosciences) at 24 hrs after transfection. At 48 hrs, cells were fixed at 4 °C with either 4% paraformaldehyde or methanol, and probed with anti-FLAG M2 antibody conjugated to horseradish peroxidase (Sigma) according to the manufacturer’s protocol. Binding to untransfected HEK293 cells treated and fixed under identifical conditions was subtracted.
RESULTS
CaSR interacts with p24A in the endoplasmic reticulum
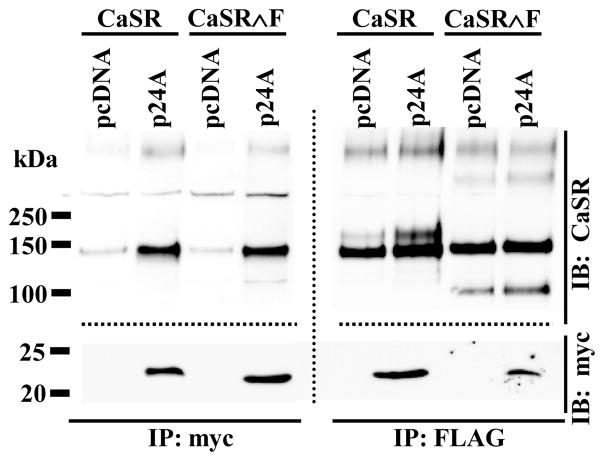
We isolated full length clones of p24A (p24β1, TEMED2, transmembrane emp24 domain trafficking protein 2) in several independent yeast two-hybrid screens of a human kidney library using the human calcium sensing receptor carboxyl terminus (residues 861-1078) as bait (data not shown). Since p24A has been implicated in resensitization of the PAR-2 receptor [16], we investigated whether CaSR and p24A interacted in mammalian cells. Figure 1 illustrates the results of heterologous expression of FLAG-CaSR and myc-p24A in HEK293 cells. Immunoprecipitation with anti-myc antibody (left panel) pulled down not only p24A (bottom portion of blot, probed with anti-myc-HRP antibody) but also CaSR (top portion of blot, probed with anti-CaSR antibody). Conversely, immunoprecipitation with anti-FLAG antibody (right panel) pulled down not only CaSR (top portion of blot) but also p24A (bottom portion of blot). Blots were cut just above the 50 kDa marker and probed for CaSR (upper portion) and p24A (lower portion). Comparison of CaSR immunoreactivity in the right and left panels suggests that p24A selectively interacts with the immaturely glycosylated form of CaSR (lower band, ≈140 kDa). To confirm this observation, we cotransfected HEK293 cells with myc-p24A plus FLAG-CaSR∧F, engineered to contain a cleavage site for the serine endoprotease furin within the extracellular domain. Furin, a member of the proprotein convertase family, is a TGN/endosome-resident type I membrane protein with its catalytic site facing the organelle lumen [19]. Furin has broad specificity for cleavage of arginine-rich sequences and activates prohormones [20], receptors [21] and ion channels [22] during their transit through the Golgi. We have shown that insertion of a furin cleavage sequence in the extracellular domain of CaSR does not affect trafficking or function at the plasma membrane, and that dissociation of the two cleavage fragments does not occur until CaSR∧F immunoprecipitation and solubilization in the presence of reducing agents [3]. Here we use cleavage of CaSR∧F as a biochemical marker of CaSR maturation, to determine whether the p24A CaSR interaction continues in the trans-Golgi compartment. Immunoprecipitation of FLAG-CaSR∧F from HEK293 cells coexpressing myc-p24A (right panel, Figure 1) and resolution under reducing conditions shows both the immature uncleaved form of FLAG-CaSR∧F (≈140 kDa) plus the larger furin cleavage fragment ≈ 108 kDa, as well as co-immunoprecipitated p24A (lower portion of right panel). In contrast, immunoprecipitation with anti-myc antibody (left panel) shows only the immature uncleaved form of FLAG-CaSR∧F (left panel, upper portion). The combined results with both FLAG-CaSR and FLAG-CaSR∧F indicate that p24A dissociates from CaSR prior to arrival at Golgi compartments mediating maturation of glycosylation and/or furin-mediated cleavage.
Figure 1. FLAG-CaSR and myc-p24A interact in the early secretory pathway.
HEK293 cells transfected with FLAG-CaSR or FLAG-CaSR∧F plus control vector (pcDNA) or myc-p24A were immunoprecipitated (after 24 hrs) with anti-myc (left panel) or anti-FLAG (right panel) antibodies and processed as described in Methods. Blots were probed with anti-CaSR antibody (upper portion) and anti-myc antibody conjugated with HRP. Dotted lines indicate different sections of the same blot processed separately with the indicated antibodies.
p24A and mutants increase CaSR expression and plasma membrane targeting
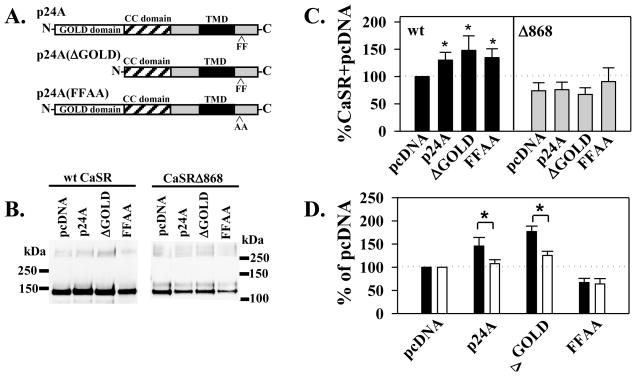
Close examination of the right panel of Figure 1 suggests that cotransfection with myc-p24A increases CaSR protein and maturation (compare pcDNA versus p24A lanes). To confirm this effect and dissect the domains of p24A which contribute to the CaSR p24A interaction, we generated several p24A mutants which have been shown to perturb p24A function (Figure 2A): (1) p24A(ΔGOLD), containing a deletion of the amino terminal GOLD domain (residues 21-112) required for interaction of p24A with the PAR-2 receptor [16]; and (2) p24A(FFAA), having mutations to alanine of a carboxyl terminal diphenylalanine motif (residues 194-195), part of the γ-COP interaction site required for assembly of p24A plus cargo into vesicles [15]. We expressed FLAG-CaSR with pcDNA, wt myc-p24A or the mutants in HEK293 cells and immunoprecipitated CaSR with anti-FLAG antibody after 24 hrs. Because we initially identified p24A as an interactor with the CaSR carboxyl terminus, we compared full length CaSR with the truncation CaSRΔ868, to determine whether the carboxyl terminus distal to T868 contributed to CaSR p24A interactions. A representative blot is illustrated in Figure 2B. Total CaSR protein was quantitated from western blots as in Figure 2B and normalized to wt FLAG-CaSR protein in the absence of added p24A (pcDNA) to permit comparison across multiple experiments. Figure 2C illustrates averaged results from 4-13 independent experiments. p24A and both p24A mutants significantly increased expression of FLAG-CaSR. Interestingly, truncation of the CaSR carboxyl terminus eliminated the differential effects of p24A and mutants on total FLAG-CaSRΔ868 protein, suggesting that determinants in the carboxyl terminus distal to T868 contribute to the p24A effects on CaSR protein abundance.
Figure 2. Consequences of FLAG-CaSR myc-p24A interactions.
A. Schematic illustration of wt p24A domains and mutations studied. GOLD (Golgi dynamics) domain; CC (coiled coil) domain; TMD (transmembrane domain); FF, diphenylalanine motif in carboxyl terminus. B. Western blots of FLAG-CaSR or FLAG-CaSRΔ868 (1 μg cDNA) coexpression with 1 μg cDNA of pcDNA, wt p24A, or the ΔGOLD or FFAA mutants illustrated in A. processed as described in Methods 24 hrs after transfection. C. Quantitation of western blots as illustrated in B. normalized to expression of FLAG-CaSR+pcDNA, plotted as average ± S.E.M. (n=4–13 independent experiments; *p< 0.05). D. Effect of p24A and mutants on plasma membrane targeting of FLAG-CaSR (48 hrs after transfection) by ELISA assay as described in Methods. Black bars, surface immunoreactivity, fixed in 4% paraformaldehyde; white bars, total immunoreactivity, fixed in methanol. Data are averages ± S.E.M. (n=7 independent experiments). A differential effect on membrane targeting was defined as a significant difference in surface versus total FLAG-CaSR (*p < 0.05).
The maturely glycosylated 160 kDa form of CaSR has been considered synonymous with plasma membrane-localized receptors [eg. 23]. We next tested whether wt p24A or its mutants increased FLAG-CaSR maturation and targeting to the plasma membrane. We transiently transfected HEK293 cells with FLAG-CaSR plus pcDNA, wt or p24A mutants and determined plasma membrane (fixed with 4% paraformaldehyde) and total (fixed with methanol) expression by enzyme-linked immunoabsorbance assays at 48 hrs after transfection. All data from each experiment were normalized to FLAG-CaSR plasma membrane or total expression without added p24A (pcDNA), to permit quantitation across experiments. This analysis method highlights differential effects of cotransfected p24A and mutants on plasma membrane targeting, since surface and total immunoreactivity should vary in parallel if there are no additional effects of the coexpressed proteins on membrane trafficking and/or targeting. Results are plotted in Figure 2D. At 48 hrs after transfection, there was a significant increase in the surface localization of FLAG-CaSR upon cotransfection with either wt p24A or p24(ΔGOLD) compared to control (pcDNA), although total expression of FLAG-CaSR was comparable in all three conditions. In contrast, p24A(FFAA) reduced both surface and total expression of FLAG-CaSR relative to pcDNA, but had no selective effect on plasma membrane targeting. The combined results of Figure 2 suggest that enhanced FLAG-CaSR total expression (at 24 hrs) and surface localization (at 48 hrs) does not require the presence of the GOLD domain, but is dependent upon the diphenylalanine motif at the carboxyl terminus of p24A. These results suggest the possibility that packaging into transport vesicles and/or transit through the ERGIC is required for the p24A-mediated effects on FLAG-CaSR. Such transit may also contribute to a selective increase in plasma membrane targeting.
The Sar1p mutant H79G dissociates FLAG-CaSR stabilization from plasma membrane targeting
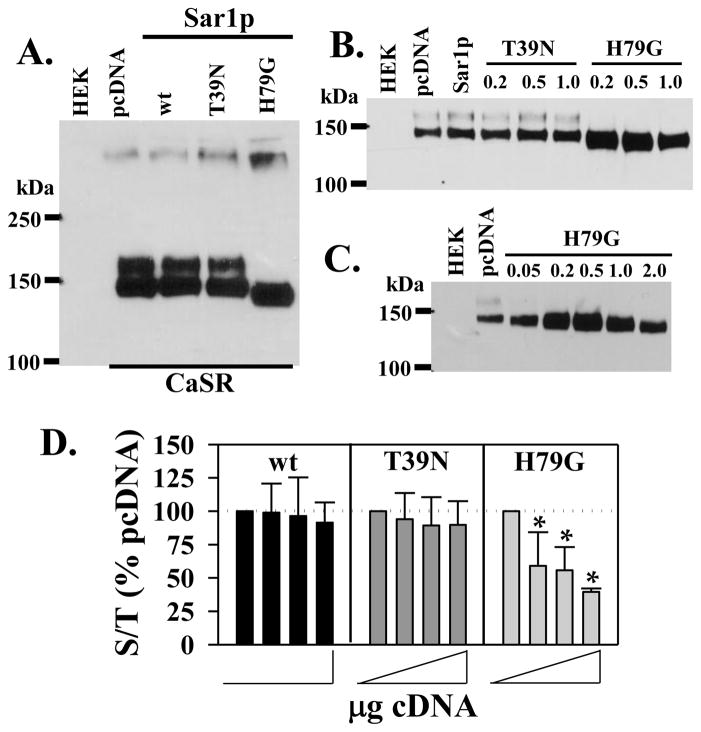
The phenylalanine residues in the p24A cytoplasmic tail are part of an extended motif (FFXXBB(X)n (where B=basic residues) through which p24 family members bind γ-COP coatomer during formation of transport vesicles [15]. Results of Figure 2 suggest that p24A-mediated transport may protect ER-localized immaturely glycosylated CaSR from degradation at early times, leading to enhanced plasma membrane abundance at later times. We sought to confirm this hypothesis using mutants of Sar1p to dissect the role of ER to ERGIC transport in CaSR stabilization. The H79G mutant has an extremely low GTP hydrolysis rate, effectively rendering it constitutively active [24], while the T39N mutant is inactive due to a low GDP release rate even in the presence of its cognate guanine nucleotide-exchange factor [25]. Both mutants have been used to block COPII-mediated vesicular trafficking from the ER to the ERGIC, although they act by distinct mechanisms [11]. Equivalent amounts of FLAG-CaSR and pcDNA, wt Sar1p, Sar1p(T39N) or Sar1p(H79G) cDNAs were transiently transfected in HEK293 cells, and immunoprecipitated FLAG-CaSR was quantified on western blots, Figure 3A. In contrast to results with wt p24A (Figures 2B and C), expression of wt Sar1p did not increase FLAG-CaSR abundance or maturation (Figures 3A and B), suggesting that endogenous levels of p24A, rather than Sar1p are limiting. FLAG-CaSR abundance was likewise unaffected by Sar1p(T39N), while Sar1p(H79G) reduced the molecular mass and increased the abundance of immature FLAG-CaSR. To confirm these effects, we varied the ratio of CaSR:Sar1p cDNAs. Figure 3B illustrates the results of cotransfection of FLAG-CaSR (0.5 μg cDNA) with pcDNA or wt Sar1p (0.5 μg cDNA) or 0.2, 0.5 or 1.0 μg of either T37N or H79G Sar1p mutants. As observed with a 1:1 ratio (Figure 3A), wt Sar1p or the T39N mutant of Sar1p had minimal effects on FLAG-CaSR abundance or maturation, while the H79G mutant of Sar1p caused an increase in abundance of CaSR even at 0.2 μg cDNA. Figure 3C demonstrates that even at the lowest cDNA amount tested (0.05 μg), the H79G mutant increased FLAG-CaSR abundance and altered glycosylation (note the absence of the ≈160 kDa band and reduced molecular mass of the immature band). Since the H79G mutant of Sar1p does not block transport vesicle formation [11], these results are consistent with the effects of wt p24A and the p24A(ΔGOLD) mutant illustrated in Figure 2, i.e., facilitation of CaSR movement from the ER protects CaSR from degradation and increases CaSR abundance. GTP hydrolysis is required for uncoating of transport vesicles preparatory to fusion with ERGIC membranes, and the H79G mutant of Sar1p is hydrolysis-deficient [24]. Incorporation of FLAG-CaSR into Sar1p(H79G)-containing transport vesicles should not only prevent degradation of FLAG-CaSR but also prevent plasma membrane targeting. To confirm this, HEK293 cells were transfected with 0.5 μg cDNA of FLAG-CaSR and various amounts of the cDNA for Sar1p, Sar1p(T39N) or Sar1p(H79G) (0.5 μg to 2 μg; total transfected cDNA was kept constant with pcDNA3.1). Surface localization and total expression of FLAG-CaSR was evaluated as in Figure 2D at 72 hrs after transfection, and plotted as the ratio of surface/total abundance normalized to FLAG-CaSR + pcDNA (no added Sar1p), Figure 3D. Neither Sar1p nor the T39N mutant had effects on the ratio of surface to total expression of FLAG-CaSR, while the H79G mutant decreased the ratio of surface/total FLAG-CaSR at concentrations which increased total CaSR abundance (recall Figures 3B and C). Overall, the results of Figure 3 suggest that the H79G mutant of Sar1p permits sequestration of FLAG-CaSR from the degradation compartment but prevents plasma membrane targeting.
Figure 3. Effect of Sar1p and mutants on CaSR maturation.
A. Western blot of FLAG-CaSR (0.5 μg) plus pcDNA, Sar1p or the T39N or H79G mutants (0.5 μg) immunoprecipitated with anti-FLAG and probed with anti-CaSR antibody after 48 hrs transfection. B. Experiment as described in A. over a wider range of T39N or H79G cDNA (0.2 - 1.0 μg), at 0.5 μg FLAG-CaSR cDNA. pcDNA or Sar1p were transfected at 0.5 μg cDNA. C. Effects of a broader range of Sar1p(H79G) mutant cDNA at 0.5 μg CaSR cDNA; pcDNA control was 0.5 μg cDNA. D. Surface expression of FLAG-CaSR in the presence of Sar1p and mutants, analyzed by ELISA assay as described in Methods. FLAG-CaSR (0.5 μg cDNA) plus 0, 0.5, 1 or 2 μg of wt, T39N or H79G Sar1p cDNA. Data are plotted as surface immunoreactivity (PFA-fixed)/total immunoreactivity (MeOH-fixed), normalized to 0 added Sar1p for each experiment (dotted line, 100%), and plotted as average of 3–5 independent experiments ± S.D. (*p < 0.05 relative to no addition).
DISCUSSION
Little is know regarding the critical determinants of CaSR trafficking through the secretory pathway during biosynthesis, despite the identification of trafficking mutants which can cause human diseases of calcium handling [1,2]. In this report, we demonstrate that p24A (p24β1, TEMED2) interacts with the immaturely glycosylated form of CaSR in the ER and early secretory pathway and contributes to the cargo receptor required for packaging of CaSR into transport vesicles. p24A interacts with neither the maturely glycosylated CaSR nor the cleaved form of CaSR∧F, suggesting that p24A mediates transit of CaSR to/from the ERGIC/cis-Golgi. Despite the documented importance of the GOLD domain in interactions with PAR-2 [16], the wt and ΔGOLD mutant had comparable effects on CaSR stabilization and maturation. Packaging of CaSR into transport vesicles (which is blocked by the p24A(FFAA) mutant) and transit of CaSR to a post-ERAD compartment is required for stabilization and ultimately enhanced targeting to the plasma membrane. Knockdown of p24A causes disruption of the Golgi apparatus in HEK293 cells [16], so we chose to test for p24A involvement in CaSR maturation through the secretory pathway with exogenous expression of wt or mutant p24A. Increasing cellular levels of wt p24A significantly enhanced CaSR abundance and selectively increased plasma membrane targeting, suggesting that endogenous p24A levels limit CaSR stabilization and trafficking to the plasma membrane. In contrast, CaSR abundance and maturation was unaffected by increased levels of wt Sar1p, suggesting endogenous levels are in excess with respect to packaging of CaSR into transport vesicles. Unknown at present is whether p24A functions as a homodimer-oligomer or in concert with additional p24 family members in regulating CaSR.
The current results raise interesting questions regarding the potential role(s) for p24 family members in sorting and stabilizing GPCRs. First, do the majority of GPCRs require cargo receptors for transit through the secretory pathway, and as a corollary, does the diversity of p24 family members provide a sufficient range of compartmental targeting capabilities to account for the diversity of regulated and unregulated transit times to the plasma membrane? Both PAR-2 [16] and CaSR interact with p24A albeit through distinct domains and with distinct functional consequences. Second, does p24-mediated transit through the ERGIC/cis-Golgi followed by COPI-mediated return to the ER allow for sorting of nascent GPCRs away from the ERAD compartment to a storage compartment? Evidence is mounting for ER subcompartments with specific, non-redundant functions in biosynthesis, degradation and signaling [26,27]. Finally, do p24 family members represent a means for biochemical assessment of compartment-specific protein interactions during GPCR biosynthesis? Recent studies have demonstrated pre-assembly and co-trafficking of GPCR signaling complexes within the biosynthetic pathway as a means of generating signaling specificity and fidelity at the plasma membrane [eg. 28,29]. Isolation of GPCRs bound to compartment-specific cargo receptors may reveal early steps in the complex assembly process.
CONCLUSIONS
We demonstrate that abundance and biosynthetic maturation of CaSR through the early secretory pathway is facilitated by the cargo receptor p24A and a mutant having a deletion of the GOLD domain, p24A(ΔGOLD), but not by the p24A carboxyl terminal mutant p24A(FFAA). The interaction between p24A and CaSR occurs at the ER and/or ERGIC/cis-Golgi, but does not persist within the Golgi. Of interest is that interaction of CaSR with transport-competent p24A stabilizes receptor protein, as does interaction with the Sar1p mutant H79G, suggesting that interaction with cargo receptors and associated protein partners protects cargo from ERAD. An intriguing possibility, therefore, is that CaSR which has passed all quality control checkpoints must interact with p24A, be transported to and be retrieved from the ERGIC, to enter a fully competent ‘releasable’ pool of ER-localized receptor. The p24A(FFAA) mutant, which does not support incorporation into transport vesicles, increases CaSR degradation. It remains to be determined whether the p24 family of cargo receptors regulates trafficking and/or stability of other G protein-coupled receptors.
Acknowledgments
We thank Drs. Klaus Seuwen and Paul Wedegaertner for cDNA clones, Elissa White for technical support, and the Breitwieser lab for helpful discussions. Supported by GM 077563 and Geisinger Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol. 2008;22:129–148. doi: 10.1016/j.berh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 3.White EA, McKenna J, Cavanaugh A, Breitwieser GE. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol. 2009;23:1115–1123. doi: 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- 5.Pidasheva S, Canaff L, Simonds WF, Marx SJ, Hendy GN. Impaired cotranslational processing of the calcium-sensing receptor due to signal peptide missense mutations in familial hypocalciuric hypercalcemia. Hum Mol Genet. 2005;14:1679–1690. doi: 10.1093/hmg/ddi176. [DOI] [PubMed] [Google Scholar]
- 6.Bruce JIE, Yang X, Ferguson CJ, Elliott AC, Steward MC, Case RM, Riccardi RMD. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 7.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 8.Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J Invest Dermatol. 2007;127:1074–1083. doi: 10.1038/sj.jid.5700633. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay N, Légrádi G, Bai M, Kifor O, Ye C, Vassilev PM, Brown EM, Lechan RM. Calcium-sensing receptor in the rat hippocampus: a developmental study. Dev Brain Res. 1997;100:13–21. doi: 10.1016/s0165-3806(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2006;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Nakano A. Mechanisms of COPII vesicle formation and protein sorting. FEBS Letters. 2007;581:2076–2082. doi: 10.1016/j.febslet.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 12.Bermak JC, Li M, Bullock C, Weingarten P, Zhou QY. Interaction of γ-COP with a transport motif in the D2 receptor C-terminus. Eur J Cell Biol. 2002;81:77–85. doi: 10.1078/0171-9335-00222. [DOI] [PubMed] [Google Scholar]
- 13.Strating JRPM, Martens GJM. The p24 family and selective transport processes at the ER-Golgi interface. Biol Cell. 2009;101:495–509. doi: 10.1042/BC20080233. [DOI] [PubMed] [Google Scholar]
- 14.Emery G, Rojo M, Gruenberg J. Coupled transport of p24 family members. J Cell Science. 2000;113:2507–2516. doi: 10.1242/jcs.113.13.2507. [DOI] [PubMed] [Google Scholar]
- 15.Bethune J, Kol M, Hoffmann J, Reckmann I, Brugger B, Wieland F. Coatomer, the coat protein of COPI transport vesicles, discriminates endoplasmic reticulum residents from p24 proteins. Mol and Cell Biol. 2006;26:8011–8021. doi: 10.1128/MCB.01055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo W, Wang Y, Reiser G. p24A, a type I transmembrane protein, controls ARF1-dependent resensitization of protease-activated receptor-2 by influence on receptor trafficking. J Biol Chem. 2007;282:30246–30255. doi: 10.1074/jbc.M703205200. [DOI] [PubMed] [Google Scholar]
- 17.Anantharaman V, Aravind L. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 2002;3(5):research0023.1–0023.7. doi: 10.1186/gb-2002-3-5-research0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 19.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends in Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 21.Moriguchi T, Haraguchi K, Ueda N, Okada M, Furuya T, Akiyama T. DREG: a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Genes to Cells. 2004;9:549–560. doi: 10.1111/j.1356-9597.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 22.Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC. Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: role of furin-mediated endogenous proteolysis. J Biol Chem. 2008;283:7455–7463. doi: 10.1074/jbc.M707399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization: implications for function of monomeric Ca(2+) receptor. J Biol Chem. 1999;274:27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- 24.Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura S, Yamamoto A, Misumi Y, Sohda M, Barr FA, Fujii G, Shakoori A, Ohno H, Mihara K, Nakamura N. Dynamics of Golgi matrix proteins after the blockade of ER to Golgi transport. J Biochem. 2004;135:1–16. doi: 10.1093/jb/mvh024. [DOI] [PubMed] [Google Scholar]
- 29.Dupre DJ, Robitaille M, thier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281:34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]