Nearly 65 million adults in the United States have hypertension and the age-adjusted prevalence of hypertension is higher in African Americans (38.8%) than in non-Hispanic whites (27.2%).1 African Americans with hypertension tend to have earlier onset, more severe hypertension, and they experience a disproportionate burden of adverse cardiovascular events.2 The relatively high cardiovascular morbidity and mortality in African Americans with hypertension has been attributed, in part, to their susceptibility to target-organ damage such as left ventricular (LV) hypertrophy and albuminuria.3,4 Moreover, African Americans with hypertension have higher pulse pressure and may have greater arterial stiffness than their non-Hispanic whites counterparts.5
Adrenomedullin (ADM) is a circulating 52-amino acid vasopeptide related to calcitonin gene related peptide, synthesized as part of a 185-amino acid precursor molecule, pre-pro-adrenomedullin (pre-proADM).6 ADM is secreted mainly by endothelial cells and widely distributed in various organs and tissues including the adrenal medulla, myocardium, kidney and lung.6 The physiological and pathophysiological role of ADM in humans is not established but animal studies indicate that it is a powerful vasodilator with antimitogenic, natriuretic, and diuretic effects, 6, 7 and that it may be involved in the regulation of ventricular remodeling.8 ADM increases endothelial nitric oxide production through phosphorylation of endothelial nitric oxide synthase, an increase in intracellular cAMP, a decrease in Ca2+ concentration, and activation of K+ channels in vascular smooth muscle cells.6 Reliable quantification of circulating ADM has been hindered by its short half life, its binding to complement factor H in the serum, and its tendency to adhere nonspecifically to surfaces.9 Recently, a midregional fragment of pro-ADM (MR-proADM), comprising amino acids 45–92 of pre-proADM has been identified in the circulation. This peptide is relatively stable and is produced in equimolar amounts to ADM, making it a surrogate for plasma levels of ADM gene products.10
Plasma ADM is higher in adults with hypertension than in normotensive individuals,11–13 correlates with the severity of hypertension13 and is associated with LV hypertrophy in multiple animal studies8,14,15 and in a small study (n =50) in humans.16 Little is known about the relation of ADM to pulse pressure, an index of arterial stiffness, LV mass, and albuminuria in adults with hypertension. In the present study, we investigated determinants of plasma MR-proADM in African American adults with hypertension and tested whether MR-proADM is associated with pulse pressure and measures of target-organ damage- LV mass and urine albumin:creatinine ratio (UACR).
Methods
This study was part of the Proteomic Markers of Arteriosclerosis Study which is investigating the association of multiple markers in various etiologic pathway of vascular disease with several phenotypes of arteriosclerosis.17 Participants belonged to the African American cohort of the Genetic Epidemiology Network of Arteriopathy Study (GENOA), a multicenter, community-based study that aims to identify genetic variants influencing blood pressure (BP) levels and the development of target-organ damage due to hypertension.18 Participants were enrolled in GENOA if two or more members of a sibship had hypertension before the age of 60 years. Subjects with a secondary cause of hypertension (such as documented renal artery stenosis or advanced renal insufficiency) were excluded. The Jackson, MS cohort of the Atherosclerosis Risk in Communities study, which had originally been a probability sample of persons with driver's licenses, was used to ascertain African-American sibships. If the eligible proband had at least one sibling with hypertension, all available full biologic siblings of the index hypertensive siblings were invited to participate in the study. The study was approved by the Institutional Review Board of the University of Mississippi Medical Center, Jackson MS. Written informed consent was obtained from each participant.
The present study included 1047 African Americans who had hypertension. Height measured by stadiometer and weight measured by electronic balance were used to calculate body mass index (BMI). Diabetes was considered present if the participant was being treated with insulin, or oral hypoglycemic agents, or had a fasting glucose level ≥126 mg/dL. Ever-smoking was defined as having smoked >100 cigarettes. Information about the use of medications was obtained from the participants at the time of the study visit. BP-lowering medications were classified as diuretics, beta-blockers, calcium-channel blockers, or renin-angiotensin-aldosterone system (RAAS) inhibitors.
Blood was drawn by venipuncture after an overnight fast. Serum total cholesterol and high-density lipoprotein (HDL) cholesterol, creatinine, and fasting plasma glucose, and were measured by standard enzymatic methods. Glomerular filtration rate was calculated (eGFR) using the abbreviated equation from the Modification of Diet in Renal Disease Study.19
Plasma levels of MR-proADM
Plasma was collected at the time of blood sampling in plastic vials containing ethylenediaminetetraacetic acid (EDTA). Samples were placed on ice and then centrifuged at 3000 × g and frozen at −80°C until assayed. MR-proADM was measured using a novel commercial assay in the chemiluninescence/ coated tube format (BRAHMS AG; Hennigsdorf, Germany).20 Tubes were coated with a purified sheep polyclonal antibody raised against a peptide representing amino acids 83–94 of pre-proADM. A purified sheep polyclonal antibody raised against a peptide representing amino acids 68–86 of pre-proADM was labeled with methylacridinium N-hydroxysuccinimide ester (InVent GmbH, Hennigsdorf, Germany) and used as a tracer. Dilutions of a peptide representing amino acids 45 to 92 of pre-proADM in normal horse serum served as standards. The immunoassay was performed by incubating 10-µl samples/standards and 200-µl tracer in coated tubes for 2 h at room temperature. Tubes were washed 4 times with 1-ml immunoassay wash solution (BRAHMS AG), and bound chemiluminescence was measured using an LB952T luminometer (Berthold, Bad Wildbad, Germany). The MR-proADM assay has been characterized in detail previously.20 The lower detection limit of the assay was 0.08 nmol/l; the functional assay sensitivity (defined as the lowest concentration detectable with an interassay coefficient of variation of 20%) is 0.12 nmol/l. The intra-assay coefficient of variation at 0.5 and 5 nmol/l is 3% and 3.5%, respectively; the interassay coefficient of variation at 0.5 and 5 nmol/l was 8.5% and 6.5%, respectively. We excluded 5 participants with plasma MR-proADM levels > 2 nmol/L.
Blood pressure indices
Resting systolic BP (SBP) and diastolic BP (DBP) were measured by random zero sphygmomanometer (Hawskley and Sons, London, UK) after participants had rested for at least 10 min in the supine position. Three measures at least 2 min apart were taken and the average of the second and third measurements was used. Pulse pressure was calculated as the difference between SBP and DBP. The diagnosis of hypertension was established based on BP levels measured at the study visit (≥140/90 mm Hg) or a prior diagnosis of hypertension and current treatment with antihypertensive medications.
Echocardiography
All participants were examined by 2-dimensional and Doppler echocardiography using an Acuson 128XP/10c (Acuson, Malvern, PA) with 2.5-, 3.5-, and 5.0-MHz transducers. The parasternal acoustic window was used to record at least 10 consecutive beats of 2D and M-mode recordings of the LV internal diameter and wall thicknesses at, or just below, the tips of the anterior mitral leaflet in long- and short-axis views. Correct orientation of planes for imaging and Doppler recordings was verified using standardized protocols. LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end diastole and end systole in 3 or more cardiac cycles. LVM was calculated according to the American Society of Echocardiography simplified cubed equation and indexed by height to the power 2.7 (LVMi).21
Albuminuria was assessed by UACR. The first voided urine was collected on the morning of the study visit and stored at −80°C until analyzed. Urine albumin and urine creatinine concentrations were measured by standard methods on a Hitachi 911 Clinical Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN), and UACR was expressed as milligrams of albumin per grams of creatinine. Subjects with creatinine >2.5 mg/dL (n = 9) or UACR >3000 mg/g (n = 6) were excluded from the analyses.
Statistical Methods
All of the statistical analyses were carried out using SAS v 9.1 (SAS Institute, Cary NC). Because of sibships in the sample, we used generalized estimating equations to account for intrafamilial correlations. Continuous variables were expressed as mean±SD and median (Q1, Q3). Categorical variables were expressed as number (percentage). Values for MR-proADM, LVMi, eGFR, and UACR were log transformed (after adding 1 in the case of UACR) to minimize skewness. Spearman correlation coefficients were used to assess the correlation between MR-proADM levels with pulse pressure, LVMi, and UACR. We used multivariable regression analyses to identify variables independently associated with plasma MR-proADM among age, sex, BMI, total and HDL cholesterol, smoking history, diabetes, previous history of myocardial infarction or stroke, and medication (BP-lowering and statin) use. Next, we constructed separate linear regression models to assess whether plasma MR-proADM was associated with pulse pressure, LVMi, and UACR. We assessed the associations in univariable models, in models that adjusted for age and sex, and finally in fully adjusted models that included conventional cardiovascular risk factors (age, sex, BMI, total and HDL cholesterol, smoking history, and diabetes), previous history of myocardial infarction or stroke, and medication (BP-lowering and statin) use. Statistical significance was determined at 2-sided P-value of <0.05.
Results
The characteristics of the study participants are shown in Table 1. Of the 1047 patients, 729 (72.5%) were women and 346 (33.5%) had diabetes. The mean plasma MR-proADM level was 0.60 nmol/L and the median was 0.55 nmol/L. Plasma MR-proADM increased with age, higher BMI, higher SBP, lower DBP, higher heart rate, lower eGFR, higher HDL, and levels were higher in women, diabetics, those with beta-blocker use, and history of smoking (Table 2). A significant proportion (40%) of the interindividual variation in plasma levels of MR-proADM could be explained by these clinical variables.
Table 1.
Characteristics of participants.
| Characteristics | (n = 1034) | |
|---|---|---|
| Mean±SD or n (%) | Median (Q1, Q3) | |
| Age, years | 64.8±8.6 | 64.9 (59.8, 70.5) |
| Women, n (%) | 729 (72.5) | |
| BMI, kg/m2 | 32.0±6.7 | 31 (27.4, 35.7) |
| Total cholesterol, mg/dL | 201±42 | 197.5 (172.5, 227) |
| HDL cholesterol, mg/dL | 57.5±17.9 | 55.2 (45, 66.8) |
| Systolic BP, mm Hg | 142±20 | 141 (128, 154) |
| Diastolic BP, mm Hg | 80±11 | 79 (72, 87) |
| Pulse pressure, mm Hg | 62±18 | 60 (50, 73) |
| Heart rate, bpm | 67±11 | 66 (59, 74) |
| eGFR, ml/min | 97.7±31.3 | 94.2 (77.2, 114.1) |
| Ever-smoker, n (%) | 418 (40.4) | - |
| Diabetes mellitus, n (%) | 346 (33.5) | - |
| Previous history of MI or stroke, n (%) | 135 (13.0) | - |
| BP-lowering medications, n (%) | 883 (85.4) | - |
| Beta-blocker, n (%) | 211 (20.4) | - |
| Calcium-channel blocker, n (%) | 367 (35.5) | - |
| Diuretic, n (%) | 585 (56.6) | - |
| RAAS inhibitor, n (%) | 506 (48.9) | - |
| Statin, n (%) | 222 (21.5) | - |
| Aspirin, n (%) | 371 (35.9) | - |
| MR-proADM, nmol/L | 0.60±0.23 | 0.55 (0.44, 0.72) |
| LVMi, g/m2.7 | 41.2±11.4 | 39.1 (33.7, 47.1) |
| UACR, mg/g | 57.3±208.8 | 6.9 (3.2, 20.8) |
Continuous variables are presented as mean ± standard deviation and inter-quartile range, whereas categorical variables are presented as counts and percentages.
BMI, body mass index ; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LVMi, left ventricular mass index; MR-proADM, midregional pro-adrenomedullin; RAAS, renin-angiotensin-aldosterone system.
Table 2.
Determinants of plasma MR-proADM concentrations.
| β±SD | P value | |
|---|---|---|
| Age, y | 0.008±0.01 | <0.0001 |
| Male sex | −0.13±0.02 | <0.0001 |
| BMI, kg/m2 | 0.015±0.01 | <0.0001 |
| SBP, per 10 mm Hg increase | 0.02±0.005 | 0.0004 |
| DBP, per 10 mm Hg increase | −0.03±0.01 | 0.0013 |
| Heart rate, per 10 bpm increase | 0.03± 0.001 | <0.0001 |
| eGFR, ml/min | −0.004±0.004 | <0.0001 |
| Diabetes | 0.045±0.02 | 0.02 |
| HDL, mg/dL | 0.001±0.006 | 0.012 |
| Beta-blocker use | 0.08±0.02 | 0.0002 |
| Ever-smoker | 0.07±0.02 | 0.0006 |
R2 of the model is 0.40
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SBP, systolic blood pressure.
Plasma MR-proADM was significantly correlated with pulse pressure, LVMi, and UACR (Fig. 1) and these measures increased with increasing quartiles of plasma MR-proADM levels (Fig. 2). Log MR-proADM was significantly associated with pulse pressure after adjustment for age and sex (P <0.007), and after additional adjustment for BMI, eGFR, smoking history, diabetes, history of myocardial infarction/stroke, and medication use (P =0.001). The association of plasma MR-proADM with pulse pressure was driven by separate associations of plasma MR-proADM with SBP and DBP as demonstrated by the significant associations of greater log MR-proADM with higher SBP (β±SE =5.31±1.57, P =0.0007) and lower DBP (β±SE =−2.05±0.82, P =0.0125) after using similar multivariable adjustment. Age, female sex, diabetes, higher HDL cholesterol, lower heart rate, and higher MR-proADM were all associated with higher pulse pressure while diuretic use and history of MI and/or stroke were associated with lower pulse pressure (Table 3). These variables and plasma MR-proADM levels explained 21% of the interindividual variation in pulse pressure.
Figure 1.
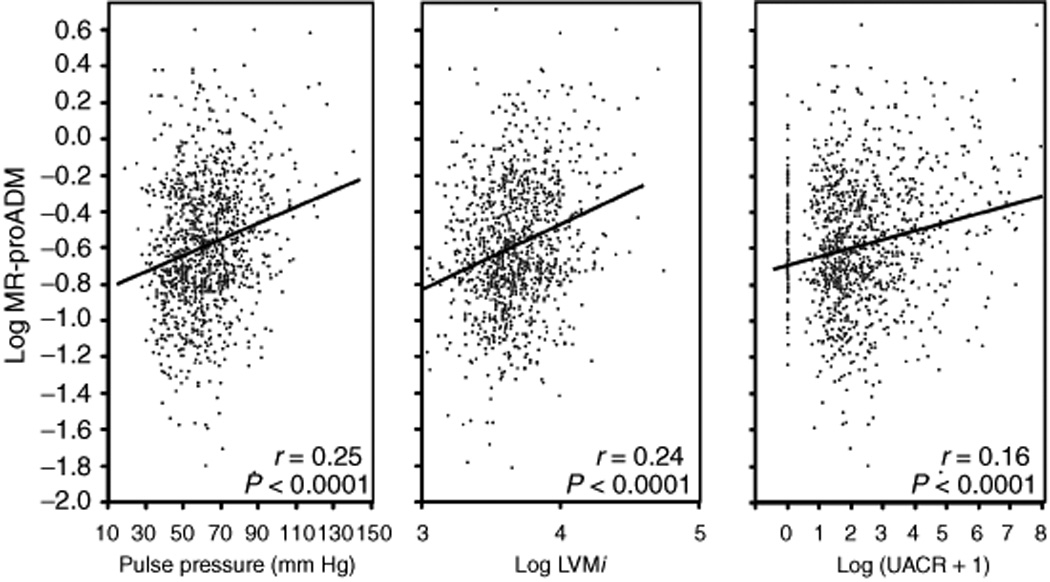
Scatter plots showing the correlation of plasma MR-proADM levels with pulse pressure, log LVMi, and log (UACR+1). The P values are derived from unadjusted Pearson product moment correlation coefficients.
Figure 2.
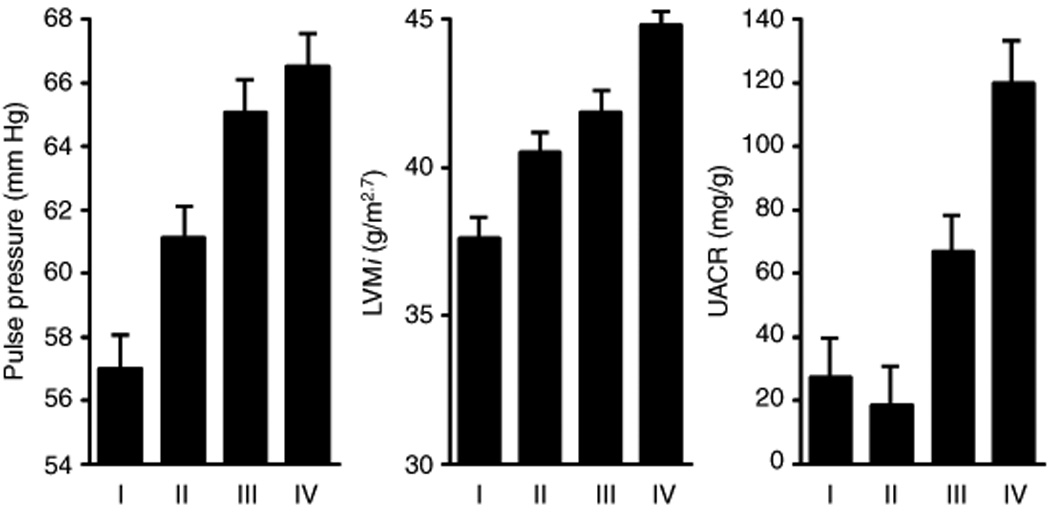
Mean±SE of pulse pressure, LVM index, and UACR in the quartiles of plasma concentration of MR-proADM.
Table 3.
Associations of plasma MR-proADM levels with pulse pressure, LVMi, and UACR in separate multivariable models.
| Pulse pressure | Log LVMi | Log (UACR+1) | ||||
|---|---|---|---|---|---|---|
| β±SE | P value | β±SE | P value | β±SE | P value | |
| Model 1 | 9.96±1.63 | <0.0001 | 0.18±0.02 | <0.0001 | 0.88±0.15 | <0.0001 |
| Model 2 | 4.27±1.58 | <0.007 | 0.16±0.02 | <0.0001 | 0.95±0.16 | <0.0001 |
| Model 3 (fully adjusted) | ||||||
| Age, year | 5.85±0.58 | <0.0001 | 0.05±0.01 | <.0001 | −0.09±0.05 | 0.10 |
| Male sex | −4.95±1.14 | <0.0001 | 0.04±0.02 | 0.016 | 0.29±0.10 | 0.003 |
| BMI, Kg/m2 | - | - | 0.01±0.001 | <.0001 | - | - |
| History of MI and/or stroke | −3.91±1.57 | 0.013 | 0.06±0.02 | 0.014 | 0.38±0.14 | 0.009 |
| Diabetes | 4.67±1.14 | <0.0001 | 0.03±0.02 | 0.04 | 0.75±0.10 | <.0001 |
| Total cholesterol, mg/dL | 0.99±0.54 | 0.069 | - | - | 0.10±0.05 | 0.03 |
| HDL cholesterol, mg/dL | 1.35±0.55 | 0.014 | - | - | - | - |
| SBP, mm Hg | - | - | 0.05±0.01 | <.0001 | 0.38±0.04 | <.0001 |
| DBP, mm Hg | - | - | - | - | - | - |
| Heart rate, bpm | −0.19±0.046 | <0.0001 | - | - | 0.02± 0.004 | 0.0004 |
| Log eGFR | - | - | - | - | −0.50±0.18 | 0.005 |
| Beta-blocker use | - | - | 0.06±0.02 | 0.001 | 0.19±0.10 | 0.074 |
| Calcium channel blocker use | - | - | 0.03±0.01 | 0.026 | 0.27±0.09 | 0.002 |
| Diuretics use | −5.34±0.94 | <0.0001 | - | - | - | - |
| Log MR-proADM | 5.08±1.59 | 0.0014 | 0.05±0.02 | 0.029 | 0.46±0.15 | 0.002 |
R2 of models for pulse pressure, LVMi, and UACR model are 0.21, 0.21, and 0.24 respectively.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LVMi, left ventricular mass index; MI, myocardial infarction; MR-proADM, midregional pro-adrenomedullin; SBP, systolic blood pressure; UACR, urine albumin:creatinine ratio.
Model 1 is unadjusted; model 2 is adjusted for age and sex; and model 3 is adjusted for age, sex, BMI, systolic BP, diastolic BP, eGFR, total cholesterol and HDL cholesterol, smoking history, diabetes, previous history of myocardial infarction or stroke) and the use of medications (BP-lowering and statin).
Higher log MR-proADM was significantly associated with higher log LVMi after adjustment for age and sex (P <0.0001), and after additional adjustment for BMI, eGFR, smoking history, diabetes, history of myocardial infarction/stroke, and medication use (P =0.029). Other variables associated with greater log LVMi included age, male sex, higher BMI, history of MI and/or stroke, diabetes, higher SBP, and use of beta-blockers and/or calcium channel blockers. These variables and plasma MR-proADM levels explained 21% of the interindividual variation in log LVMi.
Higher log MR-proADM was significantly associated with higher log (UACR+1) after adjustment for age and sex, (P<0.0001), and after additional adjustment for BMI, eGFR, smoking history, diabetes, history of myocardial infarction/stroke, and medication use (P =0.002). Other variables associated with greater log (UACR+1) were male sex, history of MI and/or stroke, diabetes, higher SBP, higher heart rate, and use of calcium channel blockers. In separate analyses in non-diabetic subjects (n=696), log MR-proADM remained independently and significantly associated with log (UACR+1) (β±SD =0.47±0.15, P =0.002). These variables and plasma MR-proADM levels explained 24% of the interindividual variation in log (UACR+1).
Discussion
In a community-based sample of African Americans with hypertension, we found that higher plasma MR-proADM levels were associated with greater pulse pressure, LVMi, and UACR. These associations were independent of age, sex, eGFR, conventional risk factors, and medication use. To the best of our knowledge, the present study is the first to report the associations of plasma MR-proADM with pulse pressure and measures of target-organ damage in a relatively large sample of African American adults with hypertension.
The mean and median levels of plasma MR-proADM( 0.60 nmol/L/0.55 nmol/L) in our study were higher than previously reported levels in healthy individuals (0.33 nmol/L/~0.34 nmol/L).20 This is likely because study participants were hypertensive and a significant proportion were women and had diabetes. Our findings are consistent with previous studies that found plasma ADM levels to be higher in hypertensives,13,22 women,23 diabetics,24 older subjects,20,25 and subjects with higher BMI25 and renal impairment.11,24,26 In addition, we identified higher SBP, lower DBP, higher heart rate, higher HDL cholesterol, history of beta-blocker use, and history of smoking (Table 2) as additional independent predictors of higher plasma MR-proADM levels in African American adults with hypertension. Several small studies have reported that plasma ADM levels are higher in hypertensive patients than in normotensive controls11–13 and are associated with BP levels.12,13,22,27 However, in patients undergoing hemodialysis, particularly with cuprophane membranes, ADM accumulates in the plasma resulting in lower BP and an inverse association of plasma ADM with BP measurements has been noted in these patients.28,29
Pulse pressure is a surrogate of arterial stiffness and higher pulse pressure is associated with adverse cardiovascular outcomes.30 We noted that higher plasma MR-proADM levels were independently associated with greater pulse pressure and this association was due to separate independent associations of plasma MR-proADM with greater SBP as well as lower DBP. In the Framingham Heart Study,23 BP measurements and arterial tonometry parameters were obtained in 1962 participants of whom ~ 43% had hypertension. Plasma ADM was associated with mean arterial pressure only in men, but the associations with SBP, DBP, and pulse pressure were not reported.23 The association of plasma MR-proADM with pulse pressure in our study suggests that plasma MR-proADM may be a marker of arterial stiffness. However, pulse pressure is a crude measure of arterial stiffness and tonometry-derived indices of arterial stiffness, including pulse wave velocity and analysis of the arterial waveforms, have been proposed as better and more reproducible surrogates of arterial stiffness.31 Whereas the Framingham investigators found no significant association of plasma ADM with carotid-femoral pulse wave velocity,23 a small study of 126 Japanese subjects did demonstrate a significant association between plasma ADM and carotid-femoral pulse wave velocity.27 Further studies are needed to clarify the association of plasma ADM with measures of arterial stiffness.
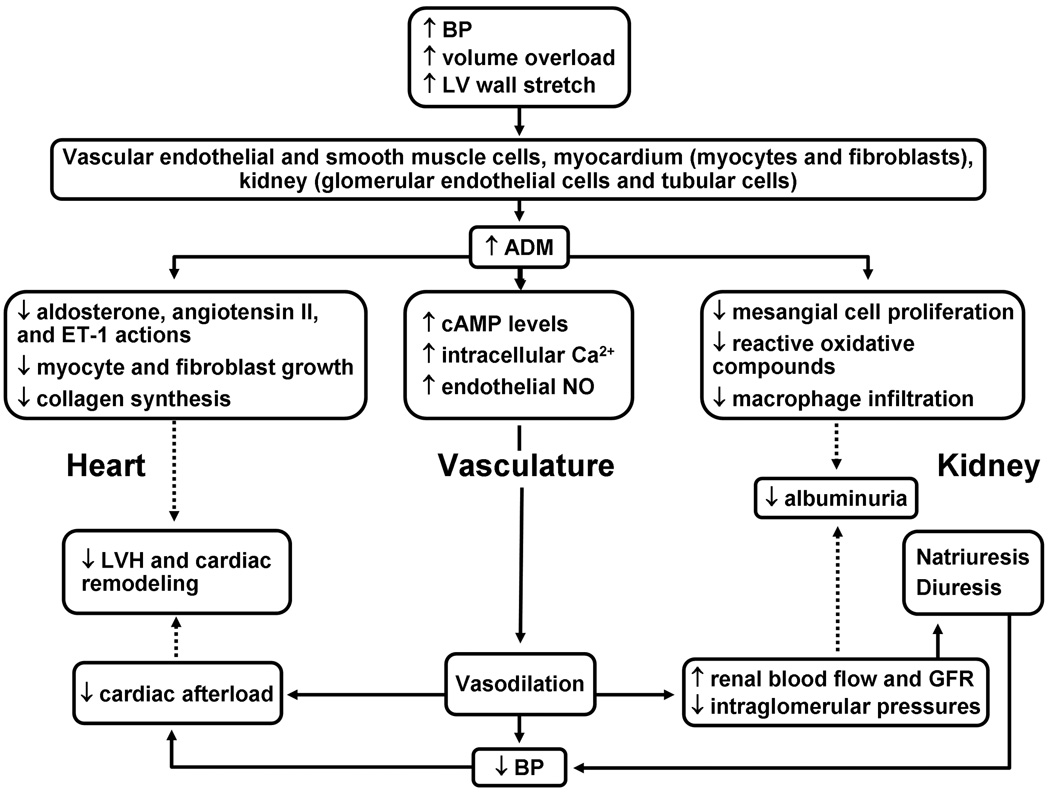
Plasma ADM levels were found to be higher in hypertensive patients with target organ damage including LVH and albuminuria, than in hypertensives without evident target organ damage.26 Our finding of an association of plasma MR-proADM levels with LVMi is consistent with the results of multiple animal studies8,14,15 and one16 of 2 small studies in humans.11,16 Using the partition value of ≥50 gm/m2.7 in both sexes to define LV hypertrophy, we found higher plasma MR-proADM to be associated with LV hypertrophy after multivariable adjustment (P =0.0076) (analyses not shown). LV hypertrophy is associated with increased cardiovascular morbidity and mortality in hypertensive adults and is more prevalent in African Americans.4 Previous studies suggest that the sympathetic nervous system32 and the renin-angiotensin aldosterone system33 contribute to the development and maintenance of LV hypertrophy by inducing myocardial hypertrophy and fibrosis. Myocardial myocytes and fibroblast may secrete ADM in response to pressure overload34 and mechanical wall stretching35 in an attempt to counteract the deleterious effects of hypertension given that ADM reduces BP36 and inhibits myocyte and fibroblast growth and collagen production.37
Albuminuria in systemic hypertension may occur with or without a decline in GFR, is a marker of target-organ damage, and is independently associated with cardiovascular morbidity and mortality.38 We found plasma MR-proADM to be associated with UACR even after adjustment for eGFR and regardless of diabetes status. Using the partition value of ≥17 mg/g to define abnormal UACR,39 we found higher plasma MR-proADM to be associated with abnormal UACR after multivariable adjustment (P =0.004) (analyses not shown). The association of plasma ADM with albuminuria was investigated in two prior small studies of diabetic subjects22,24 and was found to be significant in only one of the studies.22 Albuminuria in hypertension is likely due to endothelial dysfunction resulting in the increased permeability of the glomerular endothelial cells, rather than alterations in glomerular pressure or filtration rate alone.40 In addition, defective proximal tubular handling of albumin, possibly mediated by cytokines,41 may be another important mechanism of proteinuria. The effect of ADM on the glomerular pressure varies in different animal models.42–44 In diabetic rats, ADM may contribute to glomerular hypertension by dilating the afferent arterioles more than the efferent arterioles.42 However, intra-renal administration of ADM in anesthetized dogs resulted in a dose-dependent increase in GFR,43 and intravenous injection of ADM into unilaterally nephrectomized rats increased the diameters of both afferent and efferent arterioles,44 suggesting that ADM may decrease glomerular pressure, in these animal models, by equally dilating the glomerular afferent and efferent arterioles.43,44 ADM gene delivery45 and chronic ADM infusion46 to hypertensive rats resulted in a long term and stable reduction of BP and a decrease in albuminuria suggesting that ADM may play a protective role in hypertensive glomerular sclerosis and albuminuria by regulating renal hemodynamics,43,44 and inhibiting mesangial cell proliferation, reactive oxygen species generation, and macrophage infiltration.47
Our findings suggest that plasma MR-proADM may be a marker of arterial stiffness and target-organ damage in African American adults with essential hypertension and motivate further investigation of this peptide as a biomarker for early detection and evaluation of progression of target-organ damage in African American adults with hypertension. The cross-sectional design of our study precludes interpretations about causality. A probable explanation of the associations of MR-proADM with pulse pressure and target-organ damage measures is that ADM is secreted by vascular endothelial and smooth muscle cells, myocardial myocytes and fibroblasts, renal tubular cells, and glomerular endothelial cells in response to elevated BP as a couterregulatory mechanism to protect against hypertension-related damage (Fig. 3). The generalizability of our findings to other racial/ethnic groups is not established. Although plasma levels of MR-proADM were significantly associated with indices of target-organ damage, its ability to identify the presence or absence of target-organ damage was modest. Using only plasma level of MR-proADM to predict the presence of target-organ damage, the area under the receiver operating characteristics curve was 0.63 for LV hypertrophy and 0.62 for abnormal UACR (analyses not shown), in models adjusted for age and sex. Most participants were on BP-lowering medications and although we adjusted for medication use in our analyses, an effect of BP-lowering medications on the observed associations cannot be completely ruled out. Strengths of our study include the relatively large sample of African American adults with hypertension included in our study, using uniform protocols including questionnaires, anthropometric and laboratory measurements, and the novel assay used to measure plasma MR-proADM.
Figure 3.
Summary of some protective actions of ADM on the heart, kidney, and the vascular system.
*Solid arrows indicate ‘increase or stimulate’; dotted arrows indicate ‘decrease or inhibit’
*BP, blood pressure; MR-proADM, midregional fragment of pro-ADM; LV, left ventricle; LVH, left ventricular hypertrophy; NO, Nitric oxide.)
Acknowledgments
This work was supported by grants HL-81331 and M01 RR00585 from the National Institutes of Health.
Dr. Morgenthaler, Dr. Struck and Dr. Bergmann are employed by BRAHMS AG, which developed the assay that we used for the measurement of MR-proADM in this study.
Footnotes
Disclosure
No other author has a conflict of interest.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK, Rautaharju P, Crow R, Folsom AR, Ekelund LG, Hutchinson R, Tyroler HA, Heiss G. Black-white differences in electrocardiographic left ventricular mass and its association with blood pressure (the ARIC study). Atherosclerosis Risk in Communities. Am J Cardiol. 1994;74:247–252. doi: 10.1016/0002-9149(94)90365-4. [DOI] [PubMed] [Google Scholar]
- 4.Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, Oberman A, Kitzman DW, Hopkins PN, Liu JE, Devereux RB. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–1188. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 5.Agyemang C, Bhopal R, Redekop WK. Does the pulse pressure in people of European, African and South Asian descent differ? A systematic review and meta-analysis of UK data. J Hum Hypertens. 2007;21:598–609. doi: 10.1038/sj.jhh.1002191. [DOI] [PubMed] [Google Scholar]
- 6.Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480–2487. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- 7.Jougasaki M, Wei CM, Aarhus LL, Heublein DM, Sandberg SM, Burnett JC., Jr Renal localization and actions of adrenomedullin: a natriuretic peptide. Am J Physiol. 1995;268:F657–F663. doi: 10.1152/ajprenal.1995.268.4.F657. [DOI] [PubMed] [Google Scholar]
- 8.Niu P, Shindo T, Iwata H, Iimuro S, Takeda N, Zhang Y, Ebihara A, Suematsu Y, Kangawa K, Hirata Y, Nagai R. Protective effects of endogenous adrenomedullin on cardiac hypertrophy, fibrosis, and renal damage. Circulation. 2004;109:1789–1794. doi: 10.1161/01.CIR.0000118466.47982.CC. [DOI] [PubMed] [Google Scholar]
- 9.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 10.Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25:1369–1372. doi: 10.1016/j.peptides.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Kohno M, Hanehira T, Kano H, Horio T, Yokokawa K, Ikeda M, Minami M, Yasunari K, Yoshikawa J. Plasma adrenomedullin concentrations in essential hypertension. Hypertension. 1996;27:102–107. doi: 10.1161/01.hyp.27.1.102. [DOI] [PubMed] [Google Scholar]
- 12.Nishikimi T, Horio T, Kohmoto Y, Yoshihara F, Nagaya N, Inenaga T, Saito M, Teranishi M, Nakamura M, Ohrui M, Kawano Y, Matsuo H, Ishimitsu T, Takishita S, Matsuoka H, Kangawa K. Molecular forms of plasma and urinary adrenomedullin in normal, essential hypertension and chronic renal failure. J Hypertens. 2001;19:765–773. doi: 10.1097/00004872-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kato J, Kitamura K, Matsui E, Tanaka M, Ishizaka Y, Kita T, Kangawa K, Eto T. Plasma adrenomedullin and natriuretic peptides in patients with essential or malignant hypertension. Hypertens Res. 1999;22:61–65. doi: 10.1291/hypres.22.61. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto A, Nishikimi T, Yoshihara F, Horio T, Nagaya N, Matsuo H, Dohi K, Kangawa K. Ventricular adrenomedullin levels correlate with the extent of cardiac hypertrophy in rats. Hypertension. 1999;33:1146–1152. doi: 10.1161/01.hyp.33.5.1146. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Nishikimi T, Akimoto K, Tadokoro K, Mori Y, Minamino N. Upregulation of ligand, receptor system, and amidating activity of adrenomedullin in left ventricular hypertrophy of severely hypertensive rats: effects of angiotensin-converting enzyme inhibitors and diuretic. J Hypertens. 2003;21:1171–1181. doi: 10.1097/00004872-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Sumimoto T, Nishikimi T, Mukai M, Matsuzaki K, Murakami E, Takishita S, Miyata A, Matsuo H, Kangawa K. Plasma adrenomedullin concentrations and cardiac and arterial hypertrophy in hypertension. Hypertension. 1997;30:741–745. doi: 10.1161/01.hyp.30.3.741. [DOI] [PubMed] [Google Scholar]
- 17.Granger CB, Van Eyk JE, Mockrin SC, Anderson NL. National Heart, Lung, And Blood Institute Clinical Proteomics Working Group report. Circulation. 2004;109:1697–1703. doi: 10.1161/01.CIR.0000121563.47232.2A. [DOI] [PubMed] [Google Scholar]
- 18.Boerwinkle E. Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39:3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Honda K, Ishikawa S, Kitamura K, Eto T, Saito T. Plasma adrenomedullin levels in patients with non-insulin dependent diabetes mellitus: close relationships with diabetic complications. Endocr J. 1998;45:241–246. doi: 10.1507/endocrj.45.241. [DOI] [PubMed] [Google Scholar]
- 23.Levy D, Hwang SJ, Kayalar A, Benjamin EJ, Vasan RS, Parise H, Larson MG, Wang TJ, Selhub J, Jacques PF, Vita JA, Keyes MJ, Mitchell GF. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation. 2007;115:3079–3085. doi: 10.1161/CIRCULATIONAHA.106.652842. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Unzueta MT, Montalban C, Pesquera C, Berrazueta JR, Amado JA. Plasma adrenomedullin levels in type 1 diabetes. Relationship with clinical parameters. Diabetes Care. 1998;21:999–1003. doi: 10.2337/diacare.21.6.999. [DOI] [PubMed] [Google Scholar]
- 25.Kato J, Kitamura K, Uemura T, Kuwasako K, Kita T, Kangawa K, Eto T. Plasma levels of adrenomedullin and atrial and brain natriuretic peptides in the general population: their relations to age and pulse pressure. Hypertens Res. 2002;25:887–892. doi: 10.1291/hypres.25.887. [DOI] [PubMed] [Google Scholar]
- 26.Ishimitsu T, Nishikimi T, Saito Y, Kitamura K, Eto T, Kangawa K, Matsuo H, Omae T, Matsuoka H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94:2158–2161. doi: 10.1172/JCI117573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kita T, Kitamura K, Hashida S, Morishita K, Eto T. Plasma adrenomedullin is closely correlated with pulse wave velocity in middle-aged and elderly patients. Hypertens Res. 2003;26:887–893. doi: 10.1291/hypres.26.887. [DOI] [PubMed] [Google Scholar]
- 28.Cases A, Esforzado N, Lario S, Vera M, Lopez-Pedret J, Rivera-Fillat F, Jimenez W. Increased plasma adrenomedullin levels in hemodialysis patients with sustained hypotension. Kidney Int. 2000;57:664–670. doi: 10.1046/j.1523-1755.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki H, Nagake Y, Akagi S, Sugimoto T, Ichikawa H, Makino H. Plasma adrenomedullin levels in patients on hemodialysis. Nephron. 2001;89:20–25. doi: 10.1159/000046038. [DOI] [PubMed] [Google Scholar]
- 30.Smulyan H, Safar ME. The diastolic blood pressure in systolic hypertension. Ann Intern Med. 2000;132:233–237. doi: 10.7326/0003-4819-132-3-200002010-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49:1413–1426. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 33.Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 34.Romppanen H, Marttila M, Magga J, Vuolteenaho O, Kinnunen P, Szokodi I, Ruskoaho H. Adrenomedullin gene expression in the rat heart is stimulated by acute pressure overload: blunted effect in experimental hypertension. Endocrinology. 1997;138:2636–2639. doi: 10.1210/endo.138.6.9106. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruda T, Kato J, Kitamura K, Imamura T, Koiwaya Y, Kangawa K, Komuro I, Yazaki Y, Eto T. Enhanced adrenomedullin production by mechanical stretching in cultured rat cardiomyocytes. Hypertension. 2000;35:1210–1214. doi: 10.1161/01.hyp.35.6.1210. [DOI] [PubMed] [Google Scholar]
- 36.Chao J, Jin L, Lin KF, Chao L. Adrenomedullin gene delivery reduces blood pressure in spontaneously hypertensive rats. Hypertens Res. 1997;20:269–277. doi: 10.1291/hypres.20.269. [DOI] [PubMed] [Google Scholar]
- 37.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Effects of adrenomedullin on cultured rat cardiac myocytes and fibroblasts. Eur J Pharmacol. 1999;382:1–9. doi: 10.1016/s0014-2999(99)00559-2. [DOI] [PubMed] [Google Scholar]
- 38.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 39.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 40.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714–725. doi: 10.1007/s00125-008-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002;39:899–919. doi: 10.1053/ajkd.2002.32764. [DOI] [PubMed] [Google Scholar]
- 42.Hiragushi K, Wada J, Eguchi J, Matsuoka T, Yasuhara A, Hashimoto I, Yamashita T, Hida K, Nakamura Y, Shikata K, Minamino N, Kangawa K, Makino H. The role of adrenomedullin and receptors in glomerular hyperfiltration in streptozotocin-induced diabetic rats. Kidney Int. 2004;65:540–550. doi: 10.1111/j.1523-1755.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 43.Ebara T, Miura K, Okumura M, Matsuura T, Kim S, Yukimura T, Iwao H. Effect of adrenomedullin on renal hemodynamics and functions in dogs. Eur J Pharmacol. 1994;263:69–73. doi: 10.1016/0014-2999(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 44.Hirata Y, Hayakawa H, Suzuki Y, Suzuki E, Ikenouchi H, Kohmoto O, Kimura K, Kitamura K, Eto T, Kangawa K, et al. Mechanisms of adrenomedullin-induced vasodilation in the rat kidney. Hypertension. 1995;25:790–795. doi: 10.1161/01.hyp.25.4.790. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Zhao C, Jiang J, Li J, Xiao X, Wang DW. Adrenomedullin gene delivery alleviates hypertension and its secondary injuries of cardiovascular system. Hum Gene Ther. 2005;16:372–380. doi: 10.1089/hum.2005.16.372. [DOI] [PubMed] [Google Scholar]
- 46.Yoshihara F, Suga S, Yasui N, Horio T, Tokudome T, Nishikimi T, Kawano Y, Kangawa K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in Dahl salt-sensitive rats. Regul Pept. 2005;128:7–13. doi: 10.1016/j.regpep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 47.Chini EN, Chini CC, Bolliger C, Jougasaki M, Grande JP, Burnett JC, Jr, Dousa TP. Cytoprotective effects of adrenomedullin in glomerular cell injury: central role of cAMP signaling pathway. Kidney Int. 1997;52:917–925. doi: 10.1038/ki.1997.413. [DOI] [PubMed] [Google Scholar]