Abstract
Previous studies have suggested the existence of a gender bias in repair after demyelination. Here we report the existence of gender dimorphism for the regulation of cell number in the subventricular zone (SVZ), an area that has been studied for its repair potential. The number of Sox2+ multipotential cells in the SVZ of young adult female mice was greater than in age-matched male siblings, but this difference was not evident prior to the surge of sex hormones (i.e., in prepubertal mice). To begin asking whether hormonally derived signals were responsible for these gender-related differences, we analyzed proliferation and survival of cultured male-and female-derived SVZ cells. Estrogen, but not testosterone treatment increased cell proliferation and survival of cultured cells after IFN-γ treatment or after UV irradiation, regardless of the gender of origin. Because apoptosis in UV-irradiated SVZ cells correlated with the expression of the proapoptotic molecule p53, we postulated that this molecule could be responsible for the gender dimorphism in the SVZ. In agreement with this prediction, no difference in the SVZ cell number was detected in male and female p53 null mice. Together with previous reports, these results implicate p53 as an important component of the mechanism regulating gender dimorphism in the SVZ.
Keywords: estrogen, subventricular zone, testosterone, apoptosis, cell cycle
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS), and in MS patients gender dimorphism is well characterized in the progression, risk, and recovery of the disease. Women are more susceptible than men to contracting the disease (Duquette et al., 1992, 1993; Hawkins and McDonnell, 1999; Whitacre et al., 1999); they show a greater number of active lesions on MRI (Pozzilli et al., 2003), but they also tend to have a more favorable clinical course of disease than men (Duquette et al., 1992). These results are paralleled by a greater susceptibility of female mice to develop experimental autoimmune encephalomyelitis (EAE; Voskhul and Palaszynski, 2001). In contrast, men are more often affected by progressive forms of MS (Duquette and Girard, 1993), and they tend to show a greater number of hypointense MRI lesions, indicative of greater tissue damage than in females (Pozzilli et al., 2003).
Multiple factors can contribute to the explanation of gender dimorphism, including a differential response to sex steroids or intrinsic differences in immune response (Dalal et al., 1997; Wilcoxen et al., 2000; Pelfrey et al., 2002). The reduction in the severity of disease and the decreased relapse rate during pregnancy have further supported the notion that a relationship exists between the hormonal status of the patient and the disease progression (Confavreux et al., 1998; Lorenzi and Ford, 2002; El-Etr et al., 2005). The protective role of estrogens was supported by in vitro studies in cultured rat Schwann cells (Zhu and Glaser, 2008) and rodent (Takao et al., 2004) and human oligodendrocytic lines (Cantarella et al., 2004), whereas testosterone was shown to potentiate death pathways (Caruso et al., 2004).
In vivo studies further revealed gender dimorphism in disease progression and repair and possibly in the response to therapeutic intervention (Pelfrey et al., 2002). The most significant change observed in female mice after ovariectomy was a shift to a more acute progressive EAE course, and gonadectomy did not alter the course of the disease in males (Fillmore et al., 2004). This suggested a protective effect of estrogens that was further supported by the amelioration of the clinical symptoms in male EAE mice after administration of estriol (Palaszynski et al., 2004). The existence of gender-related differences in repair was further suggested by studies on remyelination in old rats with chemically induced demyelination, which revealed a more rapid repair response in females compared with males (Li et al., 2006).
To begin understanding the cellular and molecular substrate of gender differences in repair, we have focused our analysis on cells of the adult subventricular zone (SVZ). These neural stem cells have the rare ability to divide in the adult brain and generate cells of all three lineages, neurons, astrocytes, and oligodendrocytes (Lois and Alvares-Buylla, 1993). SVZ cells have been shown to migrate toward the lesioned areas of the brain (Sundholm-Peters et al., 2005; Kim and Szele, 2008) and affect the efficiency of remyelination in demyelinated lesions (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002). Previous studies in zebra finch (Lee et al., 2007), mice (Suzuki et al., 2007), and rats (Saravia et al., 2004) suggested that administration of estrogens enhances (Saravia et al., 2004; Lee et al., 2007; Suzuki et al., 2007), whereas ovariectomy suppresses, the proliferation of SVZ cells after injury (Lee et al., 2007; Suzuki et al., 2007). Our study validates previous reports and defines the existence of a gender-based difference in the number of SVZ cells that is associated with a differential response to hormonal treatment and is dependent on the expression levels of the proapoptotic molecule p53.
METHODS AND MATERIALS
Animals
All experiments were performed in postnatal day 8 and postnatal day 60 C57BL/6J mice. We obtained p53−/− mice from Trp53Tm1Tyj C57BL/6J heterozygous breeding pairs. Mouse genotypes were confirmed by tail clipping and PCR using the primers 5′-ACAGCGTGGTGGTACCTTAT-3′ (ImRo36), 5′-TATACTCAGAGCCGGCCT-3′ (ImRo37), and 5′-TCCTCGTGCTTTACGGTATC-3′ (neo), yielding a fragment of 375 bp for p53+/+ and 525 bp for p53−/− mice. Mice were maintained under sterile, pathogen-free conditions and in compliance with policies of the Institutional Animal Care and Use Committee of Robert Wood Johnson Medical School/UMDNJ.
Neurosphere Cultures, Treatments, and UV Irradiation
SVZ were dissected from wild-type C57BL/6J mice in Leibovitz’s L-15 medium (Gibco, Carlsbad, CA) on postnatal day 60. After digestion with papain (Sigma, St. Louis, MO) at 37°C, cells were mechanically dissociated by pipetting and resuspended in proliferation medium: DMEM/F12 (catalog No. 11039-021; all cultures were maintained in phenol-red-free media; Gibco) containing 2 mM L-glutamine (Gibco); 25 mM glucose; 2 μg/ml heparin (Sigma); hormone mix including apotransferrin, insulin, putrescine, progesterone, and sodium selenite; and 20 ng/ml epidermal growth factor (EGF). An equal number of cells was plated in uncoated 24-well plates and proliferated in a 37°C, 5% CO2 incubator for 7 days. During the proliferation, fresh EGF (Gibco) was added every other day.
For hormones and IFN-γ treatment, primary neurospheres were rinsed in 0.1 M PBS and mechanically dissociated by pipetting. The same number of cells was plated in uncoated 48-well plate in proliferation medium with 100 nM of cyclodextrin-encapsulated 17β-estradiol (catalog No. E4389; water soluble; Sigma) or cyclodextrin-encapsulated testosterone (catalog No. T5035; water soluble; Sigma). The hormonal concentration for estrogen or testosterone was calculated on the basis of the actual weight (mg) of the hormone in the preparation. On the following day, 10 ng/ml IFN-γ or PBS as the control was added into each well, and treated cultures were incubated at 37°C, 5% CO2 incubator. During the culture, media were changed and fresh hormones and IFN-γ were added every other day. After 6–7 days, BrdU incorporation assay was performed with control and treated secondary neurospheres.
For UV irradiation, dissociated cells from primary neurospheres were plated in coated eight-well chamber slides in differentiation medium: DMEM/F12 containing 10% of fetal bovine serum (Gibco) supplemented with 2 mM L-glutamine and 25 mM glucose in the absence and presence of 100 nM estrogen or testosterone. Cells were cultured for 24 hr and then cells exposed to 200 J/m2 UV with Stratalinker UV cross-linker 1800 (Strata-gene, Los Angeles, CA). Twenty-four hours after UV irradiation, TUNEL assay was performed.
Immunohistochemistry and Immunocytochemistry
For immunohistochemistry, female and male wild-type and p53 null mice, were perfused with 4% paraformaldehyde (PFA) on postnatal day 8 (prepubertal group) and on postnatal day 60 (postpubertal group; young adult) and cut coronally. For immunocytochemistry, cells were fixed with 4% PFA for 20 min at room temperature. Sections and cells were incubated in blocking buffer PGBA (0.1 M phosphate buffer, 0.1% gelatin, 1% bovine serum albumin, 0.002% sodium azide) containing 10% normal goat serum and 0.5% Triton X-100 for 30 min and then stained overnight with primary antibodies in the same blocking buffer. The following primary antibodies were used: anti-Sox2 (1:1,000; catalog No. AB5603; Chemicon, Temecula, CA), BrdU (1:100; catalog No. M0744; Dako, Glostrup, Denmark), and p53 (1:200; catalog No. NCL-p53-CM5p; Novocastra, Newcastle upon Tyne, United Kingdom). After incubation with primary antibodies, sections and cells were incubated with the appropriate secondary antibodies conjugated to fluorescein or rhodamine (Southern Bio-technologies, Birmingham, AL; Amersham Biosciences, Arlington Heights, IL; Jackson Immunoresearch, West Grove, PA; and Vector Laboratories, Bulingame, CA). DAPI (Molecular Probes, Eugene, OR) was used as nuclear counter stain. Images were analyzed using fluorescence microscopy (DM RA; Leica, Heerbrugg, Switzerland) and captured with a Hamamatsu (Hamamatsu City, Japan) CCD camera interfaced with a G4 computer.
BrdU Incorporation Assay and TUNEL Assay
To detect proliferating cells based on their incorporation of BrdU, we labeled secondary neurospheres with 10 μM BrdU (Sigma) for 6 hr, then fixed them with 4% PFA, incubated them in 2 N HCl for 30 min, and washed them with borate buffer (pH 8.5) for 15 min at 37°C. Thereafter, we applied a mouse monoclonal BrdU-specific antibody (1:100; catalog No. M0744; Dako), followed by the appropriate secondary antibody conjugated with fluorophores. Images were captured with a Hamamatsu CCD camera interfaced with a G4 computer. To identify the apoptotic cells, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP end labeling (TUNEL) assay was performed using the Apop-Tag plus kit (Chemicon), following the manufacturer’s instruction. In brief, the cultures were postfixed in pre-cooled ethanol:acetic acid 2:1 for 5 min at −20°C, then incubated with TdT in a humidified chamber at 37°C for 1 hr, followed by the incubation with antidigoxigenin conjugate (fluorescein) at room temperature for 30 min.
Quantification
For quantification of the in vivo experiments, at least three sections per mouse and three mice for each group were evaluated. The total number of Sox2+ cells in the SVZ was counted in both genders on postnatal day 8 and on postnatal day 60. The percentage of TUNEL+ cells was calculated by counting the number of TUNEL+ cells and then dividing this number by the total number of DAPI+ cells. For the count of BrdU+ cells in neurospheres, the total number of BrdU+ cells was counted in a fixed area regardless of the size of the neurosphere. Results were expressed as average ± SD and statistically analyzed via two-tailed Student’s tests.
RESULTS
Gender Dimorphism in the Number of SVZ Cells
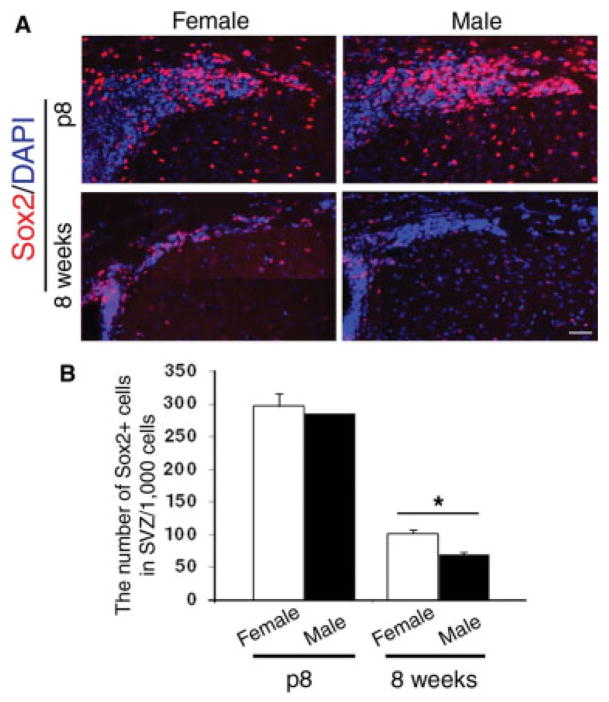
To define whether a gender bias characterizes the number of SVZ cells that have been implicated in repair (Nait-Oumesmar et al., 1999), we counted the number of multipotential cells identified by Sox2 immunoreactivity in male and female mice before and after puberty. Coronal sections of brains from C57BL/6J female and male mice before (i.e., postnatal day 8) and after (i.e., postnatal day 60) puberty were processed for immunohistochemistry using an antibody specific for Sox2. In the SVZ of prepubertal mice, a similar number of Sox2+ cells was detected (Fig. 1A). In contrast, gender-based differences were observed in the postpubertal group, because the number of Sox2+ cells in the SVZ of young adult females (n = 97.3 ± 11.8) was greater than that in young adult males (n = 70 ± 3.3; Fig. 1B). Together these data suggest the existence of gender dimorphism in the number of SVZ cells that manifests itself only after the hormonal surge.
Fig. 1.
Gender dimorphism in the number of multipotential SVZ cells. A: Composite image of multiple photograms of coronal sections of wild-type male (n = 3) and female (n = 3) mice at postnatal days 8 (p8) and p60 (8 weeks). Immunohistochemistry for Sox2 (red) and DAPI (blue) was used to identify the multipotential cells in the SVZ. Note that the number of Sox2+ cells clearly shows an age-dependent decline that is much more pronounced in males. B: Bar graphs indicate the average number of Sox2+ cells counted per 1,000 cells in the SVZ of mice of the indicated gender and age. The error bars indicate SD. ★P < 0.05. Scale bar = 100 μm.
Effect of Sex Steroids on the Proliferation of SVZ Cells
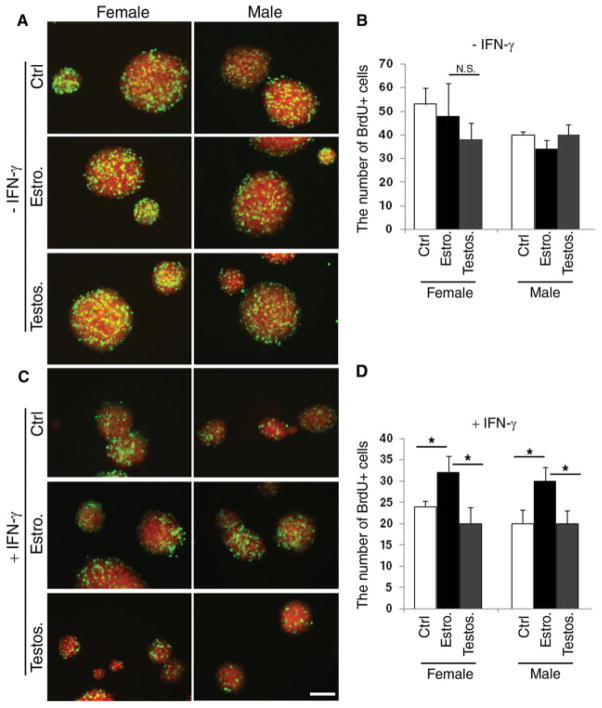
The fact that the difference in the number of Sox2+ cells between males and females was observed only after puberty implied the potential role of sex steroids in modulating proliferation or apoptosis of SVZ cells. We therefore hypothesized that the gender dimorphism in the number of Sox2+ cells could be attributed to a differential effect of sex hormones in the regulation of proliferation or apoptosis. To test this hypothesis, SVZ cells derived from adult C57BL/6J male or female mice were cultured in suspension as primary neurospheres in phenol-red-free medium, to avoid the potential interference of phenol red, which has been reported to have weak estrogenic activity (Berthois et al., 1986; Bindal et al., 1988). The primary neurospheres were mechanically dissociated, and an equal number of cells was plated in uncoated 24-well plates and cultured in proliferation medium containing either EGF alone or EGF supplemented with 100 nM estrogen or testosterone. After 7 days, cells in S-phase were identified by 6-hr labeling with BrdU (10 μM) followed by immunocytochemistry with antibody against BrdU (Fig. 2A) and cell counting (Fig. 2B). Although we noticed a slightly higher number of BrdU+ cells in female-derived cultures compared with male-derived ones, the difference was not statistically significant (Fig. 2B). We therefore concluded that steroid hormones do not play a major role in modulating the proliferative behavior of SVZ cells under physiological conditions.
Fig. 2.
The proliferative response of SVZ cells to hormonal treatment is enhanced by the presence of cytotoxic cytokines. A: Confocal image of secondary neurospheres generated by mechanically dissociating primary neurospheres and growing the same numbers of cells in medium supplemented with EGF alone (ctrl) or with 100 nM estrogen (Estro) or testosterone (Testos) for 7 days. The cells in S-phase were labeled with a 6-hr pulse of BrdU and identified by immunocytochemistry using specific antibodies for BrdU (green) and Sox2 (red). B: Bar graphs represent the results of the quantification of BrdU+ cells in secondary neurospheres cultured as described in A. Bar shows average of three experiments ± SD. C: Confocal image of secondary neurospheres generated by mechanically dissociating primary neurospheres and growing the same numbers of cells in medium supplemented with EGF + 10 ng/ml IFN-γ alone (ctrl) or with 100 nM estrogen (Estro) or testosterone (Testos) for 7 days. Note the dramatic effect of IFN treatment on the size of the male-derived neurospheres. D: Bar graphs represent the results of the quantification of BrdU+ cells in secondary neurospheres cultured as described in C. Bar shows average of three experiments ± SD. *P < 0.005. Scale bar = 100 μm.
Because gender dimorphism has been reported in human and animal models of demyelination characterized by the presence of inflammatory cytokines, we asked whether the subtle differences detected in physiological conditions could be enhanced by the exposure to cytotoxic cytokines. Neurospheres from female- or male-derived SVZ cells were cultured in the presence of a toxic cytokine (IFN-γ, 10 ng/ml) alone or in the presence of 100 nM estrogen or testosterone (Fig. 2C). The negative effect of IFN-γ treatment on the growth of SVZ cells was indicated by the smaller size of treated neurospheres compared with untreated controls (Fig. 2C). Estrogen treatment counteracted the IFN-γ effect on proliferation, whereas testosterone had no effect (Fig. 2D). BrdU labeling followed by cell counts further revealed a statistically significant difference in the number of proliferating cells between the estrogen-treated and testosterone-treated groups (Fig. 2D), independent of the gender of origin of the culture. Thus, IFN-γ treatment unmasked critical differences of sex steroids in the modulation of SVZ proliferative behavior.
Sex Steroids Modulate the Apoptotic Response of SVZ Cells to UV Irradiation
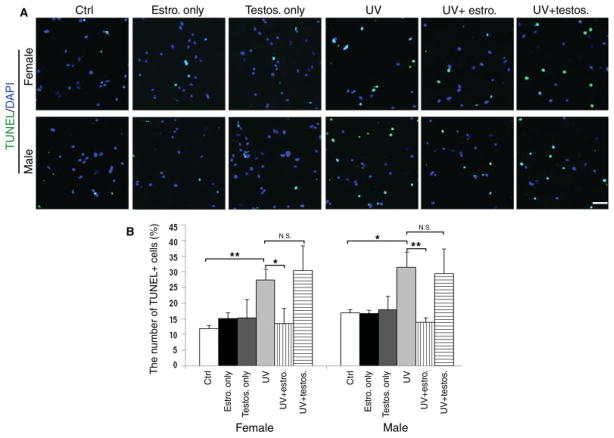
To further define whether gender and/or hormonal treatment affected the apoptotic susceptibility of SVZ cells, we adopted a model of irradiation, because this stimulus has been widely used to induce apoptosis of SVZ cells in vivo (Amano et al., 2002; Fukuda et al., 2005) and a p53-dependent mechanism of death in several cell types in vitro (Bellamy et al., 1997; Lu et al., 1998; Tang et al., 1998). Briefly, male- and female-derived dissociated SVZ cells pretreated with 100 nM estrogen or testosterone for 24 hr were exposed to UV irradiation [200 J/m2 UV with Stratalinker UV, as previously reported (Tang et al., 1998)]. After an additional 24 hr in medium supplemented with hormones, apoptotic cells were visualized using the TUNEL assay (Fig. 3A). UV induced apoptosis in 27.5% ± 3.5% of the cells in female-derived cultures and in 31.5% ± 4.9% of the cells in male-derived cultures. Pretreatment with estrogen protected SVZ cells from apoptosis induced by UV irradiation regardless of gender, whereas testosterone had no effect (Fig. 3B). These results indicated a protective effect of estrogen treatment on SVZ cells.
Fig. 3.
Sex steroid hormones affect apoptotic response to UV irradiation in SVZ cells in vitro. A: Immunofluorescence of dissociated SVZ cells that were either treated with hormones or first pretreated with 100 nM estrogen or testosterone for 24 hr and then UV irradiated (200 J/m2). Apoptosis was assessed by TUNEL (green) after an additional 24 hr under the same culture conditions. Nuclei identified by DAPI (blue). B: Bar graph indicates the percentage of apoptotic cells relative to the total number of DAPI+ cells in each condition. Bar graphs show average of three experiments ± SD. ★P < 0.1, ★ ★P < 0.05; N.S., not significant. Scale bar = 100 μm.
Sex Steroids Modulate the Expression of p53 in UV-Irradiated SVZ Cells
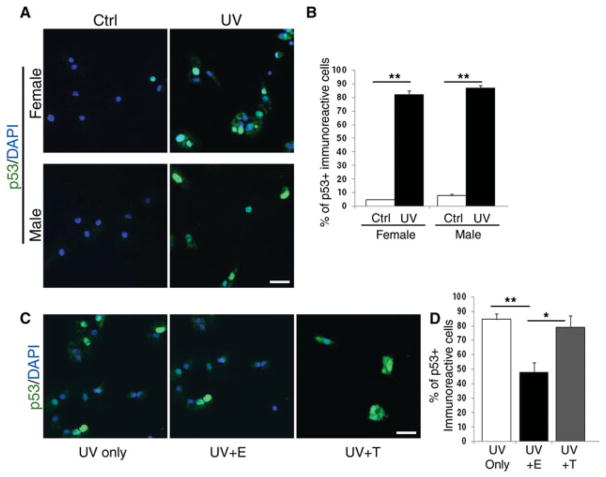
Induction of p53 after UV irradiation is a well-established event in many cell types (Sanchez and Elledge, 1995; Borovitskaya et al., 1996), so we first asked whether a similar phenomenon occurred in SVZ-derived cells. For this reason, dissociated cells derived from the male or female SVZ were exposed to UV irradiation and then processed for immunocytochemistry using antibodies specific for p53 (Fig. 4A). A similar percentage of cells expressed p53 in the absence of any hormonal treatment (Fig. 4B). However, if cells were first pretreated with estrogen or testosterone and then exposed to UV irradiation (Fig. 4C), the percentage of p53-expressing cells was decreased in the estrogen-treated group (Fig. 4D). We conclude that sex steroid, but not gender, differentially modulates the expression of the proapoptotic molecule p53 in response to UV irradiation.
Fig. 4.
Differential modulation of UV-induced p53 expression in SVZ cells in response to hormonal treatment. A: Immunofluorescence micrograph of dissociated SVZ cells either untreated (Ctrl) or exposed to 200 J/m2 UV irradiation (UV) and processed for immunocytochemistry with antibodies recognizing p53 (green). DAPI (blue) was used as nuclear counterstain. B: Bar graph indicates the percentage of p53+ cells relative to the total number of DAPI+ nuclei. Bar graphs show mean of three experiments ± SD. C: Cells were then irradiated either in the absence (UV only) or in the presence of either 100 nm estrogen (UV + E) or 100 nm testosterone (UV + T) and then stained with p53-specific antibodies. D: Bar graphs indicate the percentage of p53+ cells relative to the total number of DAPI+ nuclei in each condition. Bar graphs show average of three experiments ± SD. ★ P < 0.05, ★ ★P < 0.005. Scale bars = 50 μm.
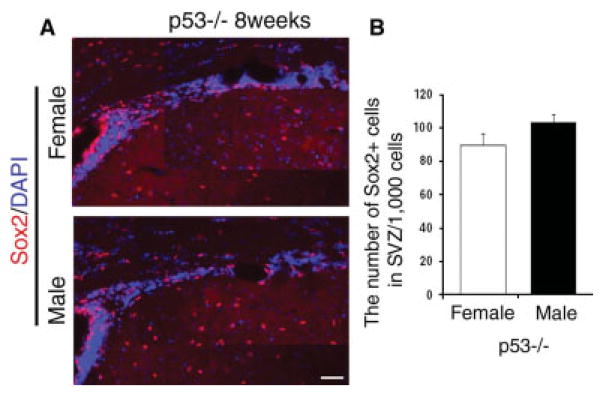
Lack of p53 Abolishes the Gender Dimorphism in the Adult SVZ
We reasoned, based on the differential effect of steroids on apoptosis induced by UV irradiation, that p53 could be a critical determinant of the gender dimorphism detected in the adult SVZ. In other words, we hypothesized that the different numbers of Sox2+ cells in male and female after puberty could be a consequence of the differential activation of p53-dependent pathways of apoptosis in SVZ cells that are differentially affected by sex steroids. To test this hypothesis, male and female p53 null mice were perfused on postnatal day 60 (post-pubertal group), and coronal sections of the brains were processed for immunohistochemistry using an antibody against Sox2 (Fig. 5A). In agreement with the original prediction, immunohistochemical analysis of sections from male and female p53 null mice revealed no difference in the number of Sox2+ cells between the two genders (Fig. 5B). Thus, the difference in Sox2+ cells between males and females that was observed in young adult mice was completely abolished in p53 knockout mice. Taken together, our results suggest an important role of sex steroid and p53 in modulating gender dimorphism of SVZ cell number.
Fig. 5.
The gender dimorphism in the number of Sox2+ cells in the adult SVZ is not detected in the absence of p53. A: Composite image of multiple photograms of coronal sections of p53 knockout male (n = 3) and female (n = 3) mice at postnatal day 60. The brain sections were processed for immunohistochemistry using an antibody specific for Sox2 (red). DAPI (blue) was used as nuclear counterstain. B: Bar graphs indicate the average number of Sox2+ cells counted per 1,000 cells in the SVZ of p53 null mice of the indicated gender. The error bars indicate SD. Scale bar = 100 μm.
DISCUSSION
This study presents new data supporting a proliferative and protective role of estrogens in SVZ cells and suggests that the gender dimorphism in SVZ cell number likely is the result of the interplay between estrogen and negative testosterone effects on p53-mediated pathways in the SVZ. Several studies have supported the importance of SVZ cells in remyelination after demyelination induced by lysolecithin injection (Nait-Oumesmar et al., 1999), cuprizone diet (Mason et al., 2000Mason et al., 2004), and EAE (Picard-Riera et al., 2002). SVZ cells isolated from the neonatal and adult brain have also been shown successfully to remyelinate demyelinated lesions after transplantation in the brain and the spinal cord of mice with EAE (Ben-Hur et al., 2003; Einstein et al., 2003; Pluchino et al., 2003) or in proximity to lesions induced by ethidium bromide injection (Keirstead et al., 1999; Smith and Blakemore 2000). In all these instances, neonatal neurospheres were able to differentiate into OLs and make new myelin, although at low efficiency (Keirstead et al., 1999). Similarly, adult neurospheres, obtained from either human or rodent tissue, have been shown to generate new myelin when transplanted into the nonmyelinated brain of the mutant “shiverer” mouse (Lachapelle et al., 2002) or the spinal cord of ethidium-bromide injected mice (Akiyama et al., 2001) or when injected intracerebrally or into the blood stream of mice with EAE (Pluchino et al., 2003).
In this study, we have identified the existence of a gender-related difference in the SVZ cell number, which is unmasked by the presence of inflammatory cytokines. We report here that postpubertal female C57Bl/6 mice have a greater number of Sox2+ multipotential progenitors than male siblings and that this difference is the likely consequence of hormonal effects, insofar as it is detected only in young adults and not in prepubertal animals. Our data are consistent with previous reports suggesting that administration of estrogens enhances proliferation of SVZ (Saravia et al., 2004; Lee et al., 2007; Suzuki et al., 2007). However, our research differs from the conclusions of other studies in estrogen-treated adult neurospheres, which suggested that 17β-estradiol treatment decreased proliferation in vitro (Brannvall et al., 2002). We believe that the discrepancy with the latter study is the consequence of the distinct experimental conditions. It is important to mention that in our study the effect of estrogen treatment in vitro was not detected when the cells were maintained under physiological conditions but only after the cells were challenged with damaging stimuli (i.e., IFN-γ treatment or UV irradiation). This is consistent with the in vivo reports for rodents, when proliferation was measured in mice or rats after injury (Saravia et al., 2004; Suzuki et al., 2007). Also, in zebra finch brain, the increased level of cell proliferation was detected in the SVZ only after injury, and this proliferative response was suppressed by ovariectomy and recovered with the replacement of estrogen (Lee et al., 2007).
Estrogens have also been shown to protect various cell types from distinct types of death. In oligodendrocytes, for instance, 17β-estradiol protects from death induced by oxidative stress (Takao et al., 2004; Cantarella et al., 2004), whereas testosterone has been shown to potentiate death pathways, by synergistic activation of p53-dependent gene expression (Caruso et al., 2004). Our results clearly indicate that a similar phenomenon occurs in SVZ cells after UV irradiation. Whereas estrogen pretreatment protects from apoptosis, testosterone has no affect and the death correlates with the expression of the proapoptotic molecule p53.
We have previously reported the importance of p53 in regulating the cell cycle of SVZ cells (Gil-Perotin et al., 2006) and in modulating the response to cuprizone-induced demyelination (Li et al., 2008). In this study, we have identified p53 as an important downstream target of testosterone and therefore as a critical modulator of the gender-based difference in SVZ cell behavior. The role of p53 in gender dimorphism has been proposed in the maturation of bone marrow cells (Gupta and Singh, 2007) and in tumor growth of lymphoma cells (Gupta and Singh, 2008) but not in the central nervous system. In addition, anecdotal information on the viability of p53 null embryos has indicated that females have a higher propensity to die from exencephaly. Together, these data support the idea that cell number regulation is the result of a complex interplay between extrinsic influences (i.e., hormones, growth factors) and intrinsic determinants (i.e., cell cycle molecules, such as p53).
Acknowledgments
Contract grant sponsor: NIHNINDS; Contract grant number: RO1-52738; Contract grant sponsor: National Multiple Sclerosis Society; Contract grant number: RG-3553.
This paper is dedicated to Dr. Steven Pfeiffer. P.C.-B. is grateful to him for his insightful comments and his constant support, especially during the early phases of her career.
References
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- Amano T, Inamura T, Wu CM, Kura S, Nakamizo A, Inoha S, Miyazono M, Ikezaki K. Effects pf single low dose irradiation on subventricular zone cells in juvenile rat brain. Neurol Res. 2002;24:809–816. doi: 10.1179/016164102101200771. [DOI] [PubMed] [Google Scholar]
- Bellamy CO, Prost S, Wyllie AH, Harrison DJ. UV but not gamma-irradiationg induces specific transcriptional activity of p53 in primary hepatocytes. J Pathol. 1997;183:177–181. doi: 10.1002/(SICI)1096-9896(199710)183:2<177::AID-PATH909>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindal RD, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. Lipophilic impurities, not phenolsulfonphthalein, account for the estrogenic activity in commercial preparations of phenol red. J Steroid Biochem. 1988;31:287–293. doi: 10.1016/0022-4731(88)90352-4. [DOI] [PubMed] [Google Scholar]
- Borovitskaya AE, Evtushenko VI, Sabol SL. Gamma-radiation-induced cell death in the fetal rat brain possesses molecular characteristics of apoptosis and is associated with specific messenger RNA elevations. Brain Res Mol Brain Res. 1996;35:19–30. doi: 10.1016/0169-328x(95)00177-t. [DOI] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Risuglia N, Lombardo G, Lempereur L, Nicoletti F, Memo M, Bernardini R. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligodendrocytic cell line: evidence for multiple sites of interactions. Cell Death Differ. 2004;11:503–511. doi: 10.1038/sj.cdd.4401367. [DOI] [PubMed] [Google Scholar]
- Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen–specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- Duquette P, Girard M. Hormonal factors in susceptibility to multiple sclerosis. Curr Opin Neurol Neurosurg. 1993;6:195–201. [PubMed] [Google Scholar]
- Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci. 1992;19:466–471. [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- El-Etr M, Vukusic S, Gignoux L, Durand-Dubief F, Achiti I, Baulieu EE, Confavreux C. Steroid hormones in multiple sclerosis. J Neurol Sci. 2005;233:49–54. doi: 10.1016/j.jns.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Fillmore PD, Blankenhorn EP, Zachary JF, Teuscher C. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am J Pathol. 2004;164:167–175. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Fukuda H, Swanpalmer J, Hertzman S, Lannering B, Marly I, Bjork-Eriksson Blomgren K. Age-dependent senisititvity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem. 2005;92:569–584. doi: 10.1111/j.1471-4159.2004.02894.x. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Singh SM. Gender dimorphism in the myeloid differentiation of bone marrow precursor cells in a murine host bearing a T cell lymphoma. J Reprod Immunol. 2007;74:90–102. doi: 10.1016/j.jri.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Gupta V, Singh SM. Gender dimorphism of tumor growth: role of gonadal hormones in differential regulation of apoptosis of a murine T cell lymphoma. J Biomed Sci. 2008;15:147–162. doi: 10.1007/s11373-007-9220-0. [DOI] [PubMed] [Google Scholar]
- Hawkins SA, McDonnell GV. Benign multiple sclerosis? Clinical course, long term follow up, and assessment of prognostic factors. J Neurol Neurosurg Psychiatry. 1999;67:148–152. doi: 10.1136/jnnp.67.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Morgan SV, Wilby MJ, Fawcett JW. Enhanced axonal regeneration following combined demyelination plus schwann cell transplantation therapy in the injured adult spinal cord. Exp Neurol. 1999;159:225–236. doi: 10.1006/exnr.1999.7100. [DOI] [PubMed] [Google Scholar]
- Kim Y, Szele FG. Activation of subventricular zone stem cells after neuronal injury. Cell Tissue Res. 2008;331:337–345. doi: 10.1007/s00441-007-0451-1. [DOI] [PubMed] [Google Scholar]
- Lachapelle F, Avellana-Adalid V, Nait-Oumesmar B, Baron-Van Evercooren A. Fibroblast growth factor-2 (FGF-2) and platelet-derived growth factor AB (PDGF AB) promote adult SVZ-derived oligodendrogenesis in vivo. Mol Cell Neurosci. 2002;20:390–403. doi: 10.1006/mcne.2002.1124. [DOI] [PubMed] [Google Scholar]
- Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobiol. 2007;67:1107–1117. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- Li J, Ghiani CA, Kim JY, Liu A, Sandoval J, DeVellis J, Casaccia-Bonnefil P. Inhibition of p53 transcriptional activity: a potential target for future development of therapeutic strategies for primary demyelination. J Neurosci. 2008;28:6118–6127. doi: 10.1523/JNEUROSCI.0184-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Penderis J, Zhao C, Schumacher M, Franklin RJ. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Exp Neurol. 2006;202:250–254. doi: 10.1016/j.expneurol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi AR, Ford HL. Multiple sclerosis and pregnancy. Postgrad Med J. 2002;78:460–464. doi: 10.1136/pmj.78.922.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Taya Y, Ikeda M, Levine AJ. UV radiation, but not gamma radiation or etoposide induced DNA damage results in the phosphorylation of the murine p53 protein at serine 389. Proc Natl Acad Sci U S A. 1998;95:6399. doi: 10.1073/pnas.95.11.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Liu H, Look K, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental allergic encephalomyelitis: implications for multiple sclerosis. J Neuroimmunology. 2004;149:84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Cotleur AC, Lee JC, Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J Neuroimmunol. 2002;130:211–223. doi: 10.1016/s0165-5728(02)00224-2. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Tomassini V, Marinelli F, Paolillo A, Gasperini C, Bastianello S. “Gender gap” in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol. 2003;10:95–97. doi: 10.1046/j.1468-1331.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Elledge SJ. Stopped for repairs. Bioessays. 1995;17:545–548. doi: 10.1002/bies.950170611. [DOI] [PubMed] [Google Scholar]
- Saravia F, Revsin Y, Lux-Lantos V, Beauquis J, Homo-Delarche F, De Nicola AF. Oestradiol restores cell proliferation in dentate gyrus and subventricular zone of streptozotocin-diabetic mice. J Neuroendocrinol. 2004;16:704–710. doi: 10.1111/j.1365-2826.2004.01223.x. [DOI] [PubMed] [Google Scholar]
- Smith PM, Blakemore WF. Porcine neural progenitors require commitment to the oligodendrocyte lineage prior to transplantation in order to achieve significant remyelination of demyelinated lesions in the adult CNS. Eur J Neurosci. 2000;12:2414–2424. doi: 10.1046/j.1460-9568.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17Beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89:660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- Tang TK, Chang W-C, Chan W-H, Yang S-D, Ni MH, Yu Y-S. Proteolytic cleavage and activation of PAK2 during UV irradiation-induced apoptosis in A431 cells. J Cell Biochem. 1998;70:442–454. [PubMed] [Google Scholar]
- Voskuhl RR, Palaszynski K. Sex hormones in experimental auto-immune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in auto-immunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Wilcoxen SC, Kirkman E, Dowdell KC, Stohlman SA. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J Immunol. 2000;164:6237–6243. doi: 10.4049/jimmunol.164.12.6237. [DOI] [PubMed] [Google Scholar]
- Zhu TS, Glaser M. Neuroprotection and enhancement of remyelination by estradiol and dexamethasone in cocultures of rat DRG neurons and Schwann cells. Brain Res. 2008;1206:20–32. doi: 10.1016/j.brainres.2008.02.051. [DOI] [PubMed] [Google Scholar]