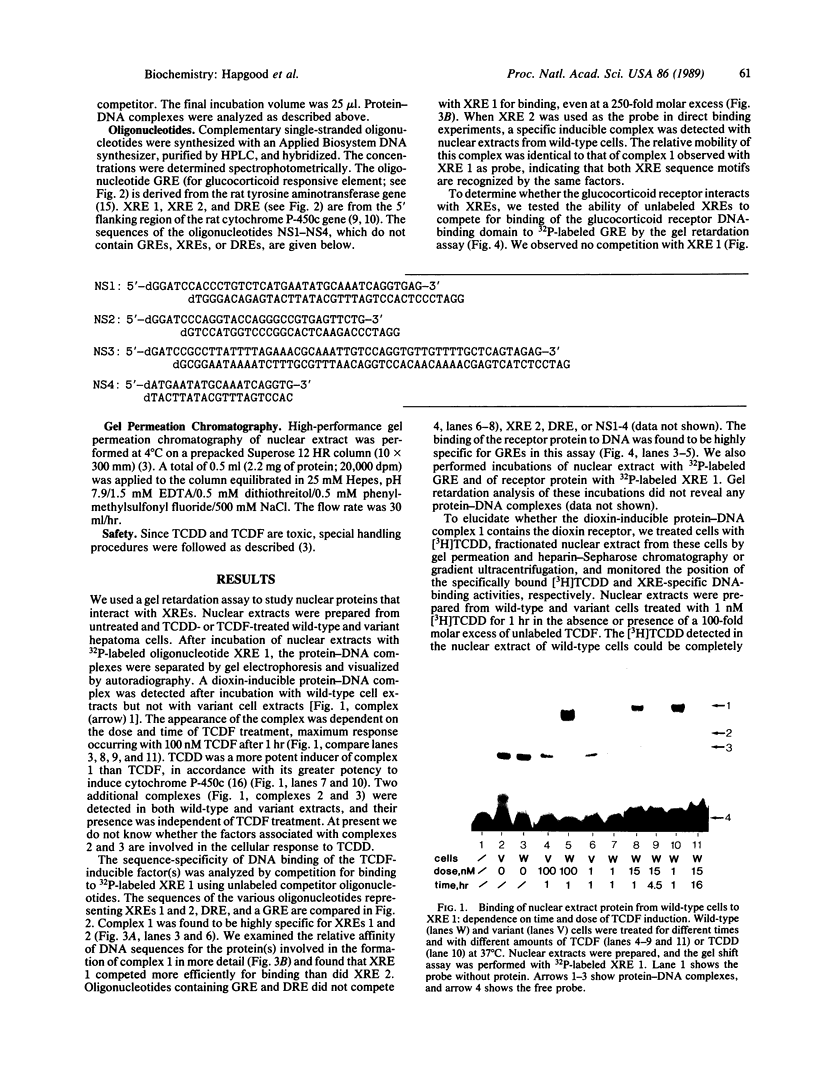
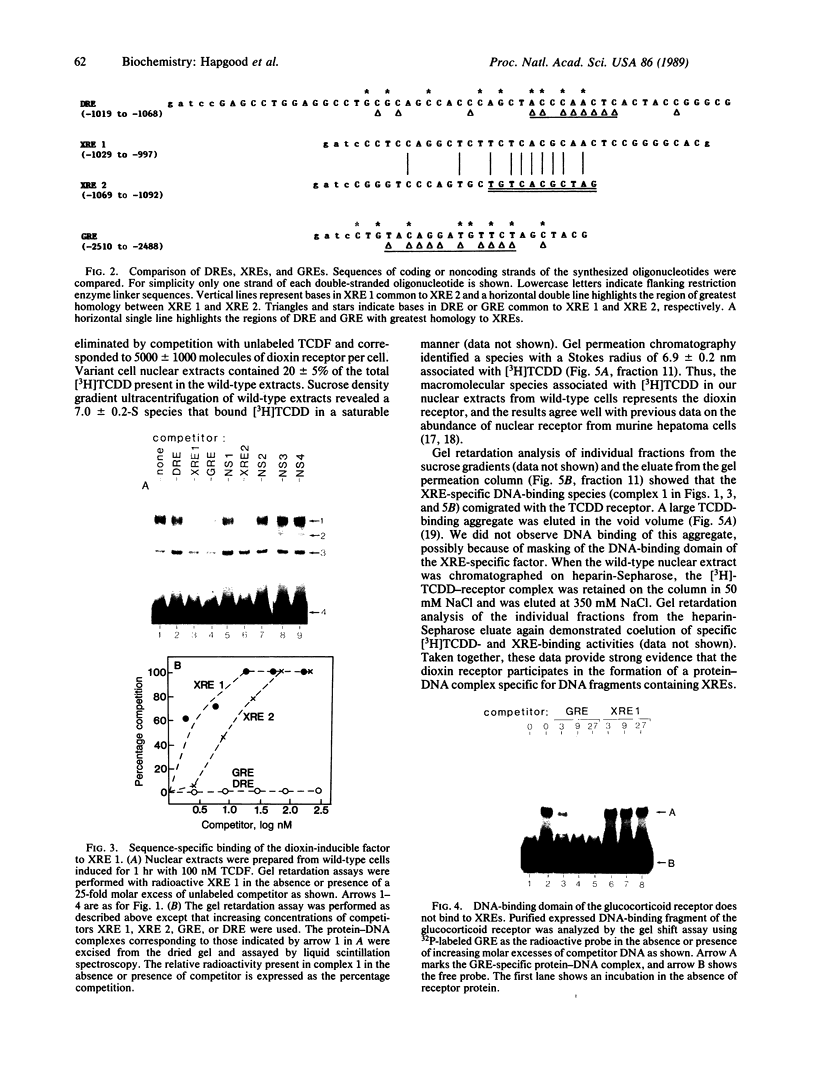
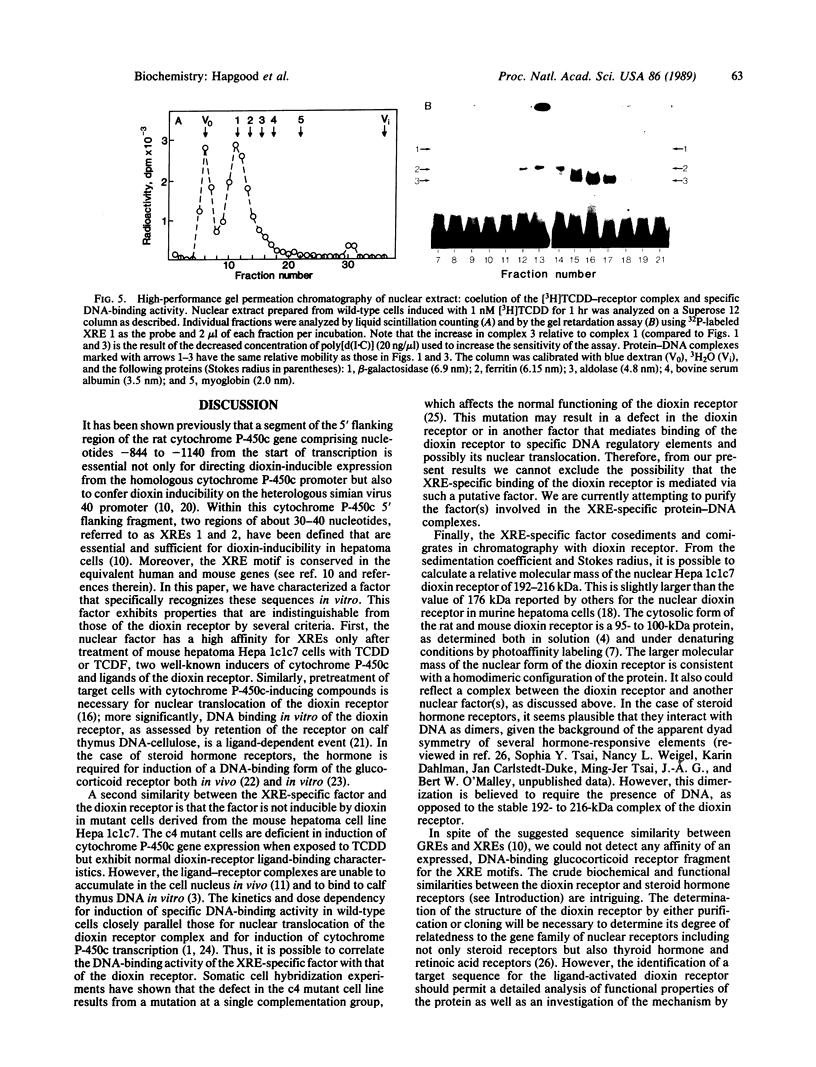
Abstract
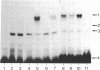
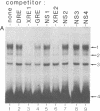
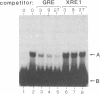
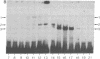
Upon binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin (called dioxin or TCDD), the dioxin receptor exhibits increased affinity for the cell nucleus in vivo and for DNA in vitro. To define the recognition sequence of the dioxin receptor and its relationship with that of the glucocorticoid receptor, oligonucleotides derived from dioxin-responsive elements of the rat cytochrome P-450c gene were tested for their ability to form specific protein-DNA complexes in a gel retardation assay. We found that a previously defined sequence motif that is similar to the glucocorticoid-responsive element and exhibits strong enhancer activity in response to dioxin receptor ligands bound a dioxin-inducible factor with high specificity but was not recognized by the DNA-binding domain of the glucocorticoid receptor. Binding to this element was only observed in nuclear extracts of wild-type mouse hepatoma cells in a time- and dose-dependent manner and not in nuclear extracts from a nonresponsive mutant cell line deficient in DNA binding of the dioxin receptor. The specific DNA-binding activity in wild-type nuclear extracts comigrated in a Superose size-exclusion column and cosedimented on sucrose gradients with the in vivo labeled dioxin receptor. These experiments strongly suggest that the dioxin receptor is a sequence-specific DNA-binding protein and is not only biochemically but also functionally similar to the steroid receptor family.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. B., Gloss B., Schmid W., Strähle U., Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986 Dec 18;324(6098):686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cuthill S., Poellinger L. DNA binding properties of dioxin receptors in wild-type and mutant mouse hepatoma cells. Biochemistry. 1988 Apr 19;27(8):2978–2982. doi: 10.1021/bi00408a047. [DOI] [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Denis M., Poellinger L., Wikstöm A. C., Gustafsson J. A. Requirement of hormone for thermal conversion of the glucocorticoid receptor to a DNA-binding state. Nature. 1988 Jun 16;333(6174):686–688. doi: 10.1038/333686a0. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Nishi C., Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986 Feb 11;14(3):1465–1477. doi: 10.1093/nar/14.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Carlstedt-Duke J., Poellinger L., Okret S., Wikström A. C., Brönnegård M., Gillner M., Dong Y., Fuxe K., Cintra A. Biochemistry, molecular biology, and physiology of the glucocorticoid receptor. Endocr Rev. 1987 May;8(2):185–234. doi: 10.1210/edrv-8-2-185. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Dominant and recessive aryl hydrocarbon hydroxylase-deficient mutants of mouse hepatoma line, Hepa-1, and assignment of recessive mutants to three complementation groups. Somatic Cell Genet. 1983 Jul;9(4):497–514. doi: 10.1007/BF01543050. [DOI] [PubMed] [Google Scholar]
- Hannah R. R., Lund J., Poellinger L., Gillner M., Gustafsson J. A. Characterization of the DNA-binding properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Biochem. 1986 Apr 15;156(2):237–242. doi: 10.1111/j.1432-1033.1986.tb09573.x. [DOI] [PubMed] [Google Scholar]
- Hannah R. R., Nebert D. W., Eisen H. J. Regulatory gene product of the Ah complex. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3-methylcholanthrene binding to several moieties in mouse liver cytosol. J Biol Chem. 1981 May 10;256(9):4584–4590. [PubMed] [Google Scholar]
- Legraverend C., Hannah R. R., Eisen H. J., Owens I. S., Nebert D. W., Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982 Jun 10;257(11):6402–6407. [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Nebert D. W., Forster-Gibson C. J., Muncan J., Dufresne M. J. Temperature-dependent cytosol-to-nucleus translocation of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in continuous cell culture lines. J Biol Chem. 1980 Dec 10;255(23):11415–11422. [PubMed] [Google Scholar]
- Perdew G. H., Poland A. Purification of the Ah receptor from C57BL/6J mouse liver. J Biol Chem. 1988 Jul 15;263(20):9848–9852. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. Location of regulatory elements responsible for drug induction in the rat cytochrome P-450c gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8044–8048. doi: 10.1073/pnas.83.21.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Klock G., Schütz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr The regulation of cytochrome P-450 gene expression. Annu Rev Pharmacol Toxicol. 1986;26:333–369. doi: 10.1146/annurev.pa.26.040186.002001. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Wikström A. C., Poellinger L. Polyanionic-binding properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. A comparison with the glucocorticoid receptor. J Biol Chem. 1986 Oct 15;261(29):13456–13463. [PubMed] [Google Scholar]